Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hassan Ragab El-Ramady | -- | 1214 | 2022-04-13 08:17:50 | | | |
| 2 | Conner Chen | Meta information modification | 1214 | 2022-04-13 08:41:34 | | | | |
| 3 | Conner Chen | Meta information modification | 1214 | 2022-04-13 08:42:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
El-Ramady, H. Soil Nanomanagement and Mushrooms. Encyclopedia. Available online: https://encyclopedia.pub/entry/21686 (accessed on 08 February 2026).
El-Ramady H. Soil Nanomanagement and Mushrooms. Encyclopedia. Available at: https://encyclopedia.pub/entry/21686. Accessed February 08, 2026.
El-Ramady, Hassan. "Soil Nanomanagement and Mushrooms" Encyclopedia, https://encyclopedia.pub/entry/21686 (accessed February 08, 2026).
El-Ramady, H. (2022, April 13). Soil Nanomanagement and Mushrooms. In Encyclopedia. https://encyclopedia.pub/entry/21686
El-Ramady, Hassan. "Soil Nanomanagement and Mushrooms." Encyclopedia. Web. 13 April, 2022.
Copy Citation
Soil is the main component in the agroecosystem besides water, microbial communities, and cultivated plants. Several problems face soil, including soil pollution, erosion, salinization, and degradation on a global level. Many approaches have been applied to overcome these issues, such as phyto-, bio-, and nanoremediation through different soil management tools. Mushrooms can play a vital role in the soil through bio-nanoremediation, especially under the biological synthesis of nanoparticles, which could be used in the bioremediation process.
soil
mushroom
1. Green Synthesis of Nanoparticles by Mushrooms
It is well-known that nanoparticles (converted to atomic or molecular scales ranging from 1 and 100 nm) could be produced by three main methods, including physical (using mechanical tools such as for grinding or milling), chemical (using some chemicals as deducting agents), and biological tools (using organisms such as plants, bacteria, fungi, actinomycetes, and algae). The production process or the synthesis of stable nanoparticles (NPs) through biological routes could be referred to as green nano-biotechnology [1]. The green chemistry of nanotechnology has mainly 12 principles, including (1) minimizing the production of wastes and reducing pollution, (2) the manufacturing of products in safer manner, (3) lowering toxicity due to less hazardous chemical synthesis, (4) using renewable feedstocks, (5) utilizing effective catalysts, (6) reducing unnecessary derivatives, (7) producing economically greener products, (8) controlling and reducing pollution, (9) increasing the efficiency of used energy, (10) using safer solvents for safer reaction conditions, (11) using degradable and recyclable materials, and (12) minimizing the possibility of accidents [1]. The biosynthesis of nanoparticles using fungi has been reported by several researchers, focusing on the genera of fungi used in NPs biosynthesis, the bio-activity of this biosynthesis, and the main applications resulting from this biosynthesis (Figure 1), as adapted from Al-Bahrani et al. [2].
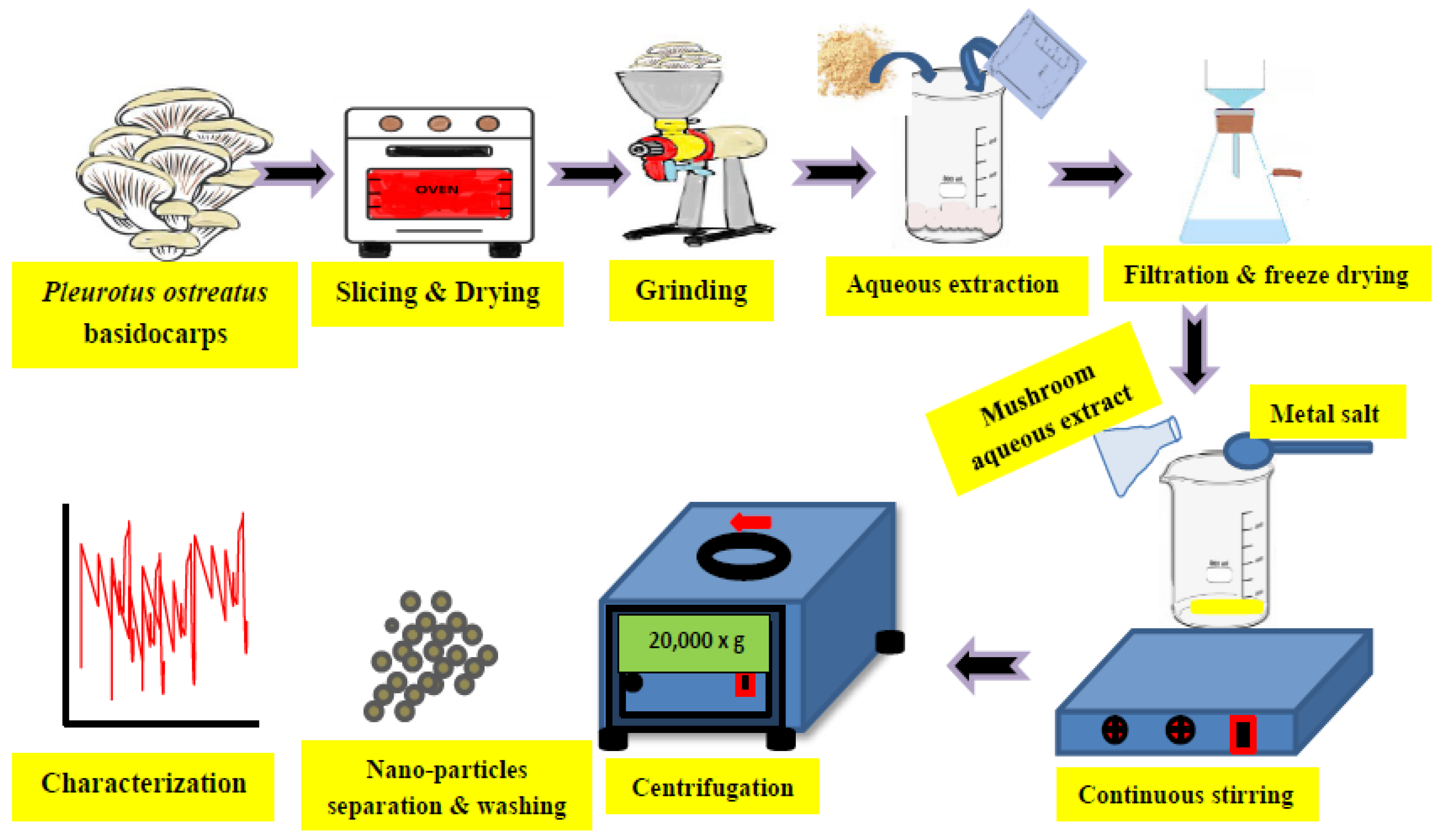
Figure 1. Graphical illustration for the green synthesis of metal nanoparticles using aqueous extract of the fresh basidiocarps of Pleurotus ostreatus. The steps include grinding the collected mushrooms, boiling within aqueous extraction, and then after filtration, the aqueous extraction is added to metal salt, which is needed to produce its nanoparticles (such as Ag, Zn, Cu, etc.).
Concerning the fungal genera used for NPs-biosynthesis, there are several genera of fungi like Aspergillus (27%), Fusarium (21%), Verticillium (9%), Trichoderma, Rhizopus and Penicillium about 7% and some other mushrooms like Pleurotus about 1% [3]. Several species of cultivated and wild mushrooms have been successfully used for the green- or bio- or myco-synthesis of many kinds of nanoparticles particularly gold (Au) [4][5][6], silver (Ag) [7][8][9][10], magnesium oxide (MgO) [11], titanium oxide (TiO2) [12], copper (Cu) [13], zinc (Zn) [14][15], and cadmium sulfide (CdS) [3]. The mechanism of nanoparticle myco-synthesis by mushrooms seems to be simple, but many factors control the stability, and biocompatibility of produced nanoparticles. These factors mainly include temperature (up to 40 ℃ for Trichoderma harzianum, or 90 ℃ for Aspergillus oryzae), pH of the reaction medium (a higher pH is preferable), the used amount of fungal biomass, and the medium composition [3]. The mechanism may return to various types of enzymes, mainly reductases, that can direct the intracellular or extracellular reduction and stabilization of NPs by the fungal exudates of biomolecules [16] and [17][18]. These biomolecules may contain amino acids, alkaloids, carboxylic acids, enzymes, flavonoids, peptides, phenols, polysaccharides, saponins, steroids, tannins, vitamins, and other secondary metabolites [3][9][19][20][21][22][23]. Biosynthesis of many metal or metal oxide-NPs using mushrooms like Pleurotus sp. and Pleurotus sajorcaju was reported (Table 1). However, the specific mode of action behind NP-myco-synthesis based on biological materials needs to be fully elucidated [17].
Table 1. Green synthesis of some nanoparticles by fundi or mushrooms and their bioactivity.
| Mushroom Species | Nanoparticle Kind and Its Size (nm) | Bioactivity or the Reaction | Reference |
|---|---|---|---|
| Agaricus bisporus | Ag-NPs (80–100) | Antibacterial activities | [24] |
| Amanita muscaria | Ag-NPs (5–25) | Anticancer activity | [24] |
| Pleurotus ostreatus | Ag-NPs (35) | Antioxidant properties | [25] |
| Pleurotus giganteus | Ag-NPs (5–25) | Antimicrobial activity | [26] |
| Ganoderma applanatum | Ag-NPs (20–5) | Antioxidant; Antibacterial | [27] |
| Ganoderma lucidum | Ag-NPs (15–22) | Antimicrobial activity | [19] |
| Pleurotus giganteus | Ag-NPs (2–20) | Antibacterial activity | [26] |
| Lentinus tuber-regium | Ag-NPs (5–35) | Antimicrobial activity | [28] |
| Pleurotus ostreatus | Ag-NPs (15–45) | Antimicrobial activity | [29] |
| Pichia pastoris | Ag-NPs (6.63) | Antioxidant and antimicrobial | [30] |
| Inonotus hispidus | Ag-NPs (69.24) | Antibacterial and antifungal | [21] |
| Ramaria botrytis | Ag@AuNPs (200) | Antioxidant and antibacterial | [20] |
| Flammulina velutipes | Ag-NPs (22) | Antibacterial activities | [31] |
| Boletus edulis Coriolus versicolor |
Ag-NPs (87.7) Ag-NPs (86.0) |
Anticancer of breast, colon; liver, and antimicrobial | [7] |
| Pleurotus ostreatus Pleurotus djamor |
Ag-NPs (28.44) Ag-NPs (55.76) |
Antioxidant activities and antimicrobial agent | [23] |
| Agaricus arvensis | Ag-NPs (20) | Anticancer, Antimicrobial | [8] |
| Ganoderma lucidum | Ag-NPs (50) | Antibacterial activities | [10] |
| Agaricus bisporus | Ag-NPs (50.44) | Antibacterial activity | [9] |
| Pleurotus sajor-caju | Au-NPs (16–18) | Cancer cell inhibition | [5] |
| Ganoderma applanatum | Au-NPs (18.7) | Dye decolorization | [32] |
| Lentinula edodes | Au-NPs (5–15) | Anticancer activity | [33] |
| Ganoderma lucidium | Au-NPs (5–15) | Anticancer activity | [33] |
| Agaricus bisporus | Cu-NPs (10) | Antibactericidal activity | [13] |
| Pleurotus tuber-regium | Se-NPs (50) | Anticancer activity | [34] |
| Pleurotus djamor | TiO2-NPs (31) | Antilarval properties | [12] |
| Pleurotuss ostreatu | ZnS-NPs (2–5) | Biomedical; food packaging | [35] |
| Agaricus bisporus | Zn-NPs (12–17) | Antirenal cancer | [36] |
| Candida albicans | ZnO-NPs (10.2) | Antimicrobial activities | [15] |
| Pleurotus floridanus | ZnO-NPs (34.98) | Biomedical applications | [14] |
| Pleurotus djamor | ZnO-NPs (38.73) | Antibacterial and anticancer | [37] |
| Agarius bisporus | ZnO-NPs (20) | Antibacterial activity | [38] |
2. Soil Nanomanagement and Mushrooms
The cultivation of mushrooms is known to have been performed since 600, 1100, and 1650 AD, for the species Auricularia auricula-judae, Lentinula edodes, and Agaricus bisporus, respectively [39]. Mushrooms as fungi can play different roles in soil, including promoting soil fertility, biodegrading agrowastes, or removing pollutants from soil, as mentioned in the previous section. According to their main source of carbon for nutrition, the most cultivated mushrooms in the soil are considered saprophyte and heterotrophic fungi. Mushrooms can biosynthesize their own foods from the agroresidues of different crops by converting these by-products and wastes and preventing the accumulation of them as health hazards. Mushrooms cannot synthesize their food through photosynthesis because they are devoid of chlorophylls [39]. The vascular xylem and phloem are also absent in mushrooms, but they can absorb O2 and release CO2 [39].
The quality of cultivated mushrooms may depend on the concertation of pollutants such as heavy metals and their accumulation in the fruit bodies of mushrooms, which may migrate from the growing substrates [40]. The selected proper substrate for mushroom cultivation is considered a limiting factor, which depends on the price and lignocellulosic compounds in used agrowastes [41]. Although the use of spent mushroom substrate as raw materials remains a formidable challenge, it could produce complexes of chitin-cellulose nanofiber, which promote plant growth through increasing plant disease resistance [42]. An increased concern on the manufacture of nanomaterials derived from mushrooms recently has been noticed (Table 2). These nanomaterials include chitin-cellulose nanofiber from Lentinula edodes substrate [42] and chitin nanopaper from Lentinula edodes, Flammulina velutipes, and Pleurotus ostreatus [43]. Mushrooms are useful for soil when their wastes from cultivation could be used as fodder for livestock, as a soil conditioner, as organic fertilizers, and/or for environmental bioremediation, as discussed in the next sections.
Table 2. List of some important materials-derived from mushrooms and their applications.
| Mushroom Species | Potential Materials | The Main Application/Main Findings | Refs. |
| Agaricus bisporus | Nanoencapsulation of rutin in β-glucan matrix | Encapsulation by green technology for nutraceutical activities | [18] |
| Lentinula edodes | Chitin/cellulose nanofiber | Growth promotion and disease resistance | [42] |
| Lentinula edodes, Pleurotus ostreatus | Chitin nanopaper derived from mushroom | Extraction of chitin from the mushrooms to produce nanopaper | [43] |
| Pleurotus ostreatus | Chitin–glucan complex | Producing eco-friendly polymers | [44] |
| Agaricus bisporus, Pleurotus ostreatus | Biocompatible fluorescent carbon-based nanomaterials | Producing live cells by fluorescent carbon quantum dot derived from mushrooms | [45] |
| Lentinus edodes | Nanoemulsion | Nanoemulsion derived from mushroom polysaccharide for the antitumor activity | [46] |
| Agaricus bisporus | Chitin nanopaper derived from mushroom | Production of chitin nanopaper from an extract of mushrooms | [47] |
| Agaricus bisporus | Fraction of chitin/glucan | Producing glycosidases (e.g., chitinases), which immobilize on nanoparticles and spray for biocontrol of insect pests | [48] |
| Lactarius volemus | Modified chitosan with nano-Fe3O4 nanoparticles | Purification of phytase enzymes and their potential in cereal industries | [49] |
| Lentinus edodes | Cellulose nanofibers | The highest yield of the film was produced using 0.18 g NaClO per 1.0 g of waste mushroom bed (71% cellulose) | [50] |
References
- Nair, G.M.; Sajini, T.; Mathew, B. Advanced Green Approaches for Metal and Metal Oxide Nanoparticles Synthesis and Their Environmental Applications. Talanta Open 2022, 5, 100080.
- Al-Bahrani, R.; Raman, J.; Lakshmanan, H.; Hassan, A.A.; Sabaratnam, V. Green Synthesis of Silver Nanoparticles Using Tree Oyster Mushroom Pleurotus Ostreatus and Its Inhibitory Activity against Pathogenic Bacteria. Mater. Lett. 2017, 186, 21–25.
- Srivastava, S.; Bhargava, A. Biological Synthesis of Nanoparticles: Fungi. In Green Nanoparticles: The Future of Nanobiotechnology; Springer: Singapore, 2022; pp. 101–137. ISBN 9789811671050.
- Owaid, M.N.; Rabeea, M.A.; Abdul Aziz, A.; Jameel, M.S.; Dheyab, M.A. Mushroom-Assisted Synthesis of Triangle Gold Nanoparticles Using the Aqueous Extract of Fresh Lentinula edodes (Shiitake), Omphalotaceae. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100270.
- Chaturvedi, V.K.; Yadav, N.; Rai, N.K.; Bohara, R.A.; Rai, S.N.; Aleya, L.; Singh, M.P. Two Birds with One Stone: Oyster Mushroom Mediated Bimetallic Au-Pt Nanoparticles for Agro-Waste Management and Anticancer Activity. Environ. Sci. Pollut. Res. 2021, 28, 13761–13775.
- Krishnamoorthi, R.; Bharathakumar, S.; Malaikozhundan, B.; Mahalingam, P.U. Mycofabrication of Gold Nanoparticles: Optimization, Characterization, Stabilization and Evaluation of Its Antimicrobial Potential on Selected Human Pathogens. Biocatal. Agric. Biotechnol. 2021, 35, 102107.
- Kaplan, Ö.; Gökşen Tosun, N.; Özgür, A.; Erden Tayhan, S.; Bilgin, S.; Türkekul, İ.; Gökce, İ. Microwave-Assisted Green Synthesis of Silver Nanoparticles Using Crude Extracts of Boletus edulis and Coriolus versicolor: Characterization, Anticancer, Antimicrobial and Wound Healing Activities. J. Drug Deliv. Sci. Technol. 2021, 64, 102641.
- Kaplan, Ö.; Gökşen Tosun, N.; İmamoğlu, R.; Türkekul, İ.; Gökçe, İ.; Özgür, A. Biosynthesis and Characterization of Silver Nanoparticles from Tricholoma ustale and Agaricus arvensis Extracts and Investigation of Their Antimicrobial, Cytotoxic, and Apoptotic Potentials. J. Drug Deliv. Sci. Technol. 2022, 69, 103178.
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A.; Khaniabadi, P.M.; Kareem, A.A.; Alrosan, M.; Ali, A.T.; Rabeea, M.A.; Mehrdel, B. Mycosynthesis of Ultrasonically-Assisted Uniform Cubic Silver Nanoparticles by Isolated Phenols from Agaricus bisporus and Its Antibacterial Activity. Surf. Interfaces 2022, 29, 101774.
- Nguyen, T.H.A.; Nguyen, V.-C.; Phan, T.N.H.; Le, V.T.; Vasseghian, Y.; Trubitsyn, M.A.; Nguyen, A.-T.; Chau, T.P.; Doan, V.-D. Novel Biogenic Silver and Gold Nanoparticles for Multifunctional Applications: Green Synthesis, Catalytic and Antibacterial Activity, and Colorimetric Detection of Fe(III) Ions. Chemosphere 2022, 287, 132271.
- Jhansi, K.; Jayarambabu, N.; Reddy, K.P.; Reddy, N.M.; Suvarna, R.P.; Rao, K.V.; Kumar, V.R.; Rajendar, V. Biosynthesis of MgO Nanoparticles Using Mushroom Extract: Effect on Peanut (Arachis hypogaea L.) Seed Germination. 3 Biotech 2017, 7, 263.
- Manimaran, K.; Natarajan, D.; Balasubramani, G.; Murugesan, S. Pleurotus sajor caju Mediated TiO2 Nanoparticles: A Novel Source for Control of Mosquito Larvae, Human Pathogenic Bacteria and Bone Cancer Cells. J. Clust. Sci. 2021.
- Sriramulu, M.; Shanmugam, S.; Ponnusamy, V.K. Agaricus Bisporus Mediated Biosynthesis of Copper Nanoparticles and Its Biological Effects: An in-Vitro Study. Colloid Interface Sci. Commun. 2020, 35, 100254.
- Rafeeq, C.M.; Paul, E.; Vidya Saagar, E.; Manzur Ali, P.P. Mycosynthesis of Zinc Oxide Nanoparticles Using Pleurotus floridanus and Optimization of Process Parameters. Ceram. Int. 2021, 47, 12375–12380.
- Gur, T.; Meydan, I.; Seckin, H.; Bekmezci, M.; Sen, F. Green Synthesis, Characterization and Bioactivity of Biogenic Zinc Oxide Nanoparticles. Environ. Res. 2022, 204, 111897.
- Khandel, P.; Shahi, S.K. Mycogenic Nanoparticles and Their Bio-Prospective Applications: Current Status and Future Challenges. J. Nanostruct. Chem. 2018, 8, 369–391.
- Sudheer, S.; Bai, R.G.; Muthoosamy, K.; Tuvikene, R.; Gupta, V.K.; Manickam, S. Biosustainable Production of Nanoparticles via Mycogenesis for Biotechnological Applications: A Critical Review. Environ. Res. 2022, 204, 111963.
- Shah, A.; ul Ashraf, Z.; Gani, A.; Masoodi, F.A.; Gani, A. β-Glucan from Mushrooms and Dates as a Wall Material for Targeted Delivery of Model Bioactive Compound: Nutraceutical Profiling and Bioavailability. Ultrason. Sonochem. 2022, 82, 105884.
- Aygün, A.; Özdemir, S.; Gülcan, M.; Cellat, K.; Şen, F. Synthesis and Characterization of Reishi Mushroom-Mediated Green Synthesis of Silver Nanoparticles for the Biochemical Applications. J. Pharm. Biomed. Anal. 2020, 178, 112970.
- Bhanja, S.K.; Samanta, S.K.; Mondal, B.; Jana, S.; Ray, J.; Pandey, A.; Tripathy, T. Green Synthesis of Bimetallic Composite Nanoparticles Using a Polysaccharide Extracted from Ramaria botrytis Mushroom and Performance in Catalytic Reduction of 4-Nitrophenol and Antioxidant, Antibacterial Activity. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100341.
- Jaloot, A.S.; Owaid, M.N.; Naeem, G.A.; Muslim, R.F. Mycosynthesizing and Characterizing Silver Nanoparticles from the Mushroom Inonotus hispidus (Hymenochaetaceae), and Their Antibacterial and Antifungal Activities. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100313.
- Kurhade, P.; Kodape, S.; Choudhury, R. Overview on Green Synthesis of Metallic Nanoparticles. Chem. Pap. 2021, 75, 5187–5222.
- Martínez-Flores, H.E.; Contreras-Chávez, R.; Garnica-Romo, M.G. Effect of Extraction Processes on Bioactive Compounds from Pleurotus ostreatus and Pleurotus djamor: Their Applications in the Synthesis of Silver Nanoparticles. J. Inorg. Organomet Polym. 2021, 31, 1406–1418.
- Loshchinina, E.A.; Vetchinkina, E.P.; Kupryashina, M.A.; Kursky, V.F.; Nikitina, V.E. Nanoparticles Synthesis by Agaricus Soil Basidiomycetes. J. Biosci. Bioeng. 2018, 126, 44–52.
- Musa, S.F.; Yeat, T.S.; Kamal, L.Z.M.; Tabana, Y.M.; Ahmed, M.A.; El Ouweini, A.; Lim, V.; Keong, L.C.; Sandai, D. Pleurotus sajor-caju Can Be Used to Synthesize Silver Nanoparticles with Antifungal Activity against Candida albicans. J. Sci. Food Agric. 2018, 98, 1197–1207.
- Debnath, G.; Das, P.; Saha, A.K. Green Synthesis of Silver Nanoparticles Using Mushroom Extract of Pleurotus Giganteus: Characterization, Antimicrobial, and α-Amylase Inhibitory Activity. Bionanoscience 2019, 9, 611–619.
- Suleman Ismail Abdalla, S.; Katas, H.; Chan, J.Y.; Ganasan, P.; Azmi, F.; Fauzi Mh Busra, M. Antimicrobial Activity of Multifaceted Lactoferrin or Graphene Oxide Functionalized Silver Nanocomposites Biosynthesized Using Mushroom Waste and Chitosan. RSC Adv. 2020, 10, 4969–4983.
- Debnath, G.; Das, P.; Saha, A.K. Characterization, Antimicrobial and α-Amylase Inhibitory Activity of Silver Nanoparticles Synthesized by Using Mushroom Extract of Lentinus tuber-regium. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 37–45.
- Irshad, A.; Sarwar, N.; Sadia, H.; Riaz, M.; Sharif, S.; Shahid, M.; Khan, J.A. Silver Nano-Particles: Synthesis and Characterization by Using Glucans Extracted from Pleurotus ostreatus. Appl. Nanosci. 2020, 10, 3205–3214.
- Eze, F.N.; Nwabor, O.F. Valorization of Pichia Spent Medium via One-Pot Synthesis of Biocompatible Silver Nanoparticles with Potent Antioxidant, Antimicrobial, Tyrosinase Inhibitory and Reusable Catalytic Activities. Mater. Sci. Eng. C 2020, 115, 111104.
- Zhang, L.; Wei, Y.; Wang, H.; Wu, F.; Zhao, Y.; Liu, X.; Wu, H.; Wang, L.; Su, H. Green Synthesis of Silver Nanoparticles Using Mushroom Flammulina velutipes Extract and Their Antibacterial Activity Against Aquatic Pathogens. Food Bioprocess Technol. 2020, 13, 1908–1917.
- Abdul-Hadi, S.Y.; Owaid, M.N.; Rabeea, M.A.; Abdul Aziz, A.; Jameel, M.S. Rapid Mycosynthesis and Characterization of Phenols-Capped Crystal Gold Nanoparticles from Ganoderma applanatum, Ganodermataceae. Biocatal. Agric. Biotechnol. 2020, 27, 101683.
- Vetchinkina, E.P.; Loshchinina, E.A.; Vodolazov, I.R.; Kursky, V.F.; Dykman, L.A.; Nikitina, V.E. Biosynthesis of Nanoparticles of Metals and Metalloids by Basidiomycetes. Preparation of Gold Nanoparticles by Using Purified Fungal Phenol Oxidases. Appl. Microbiol. Biotechnol. 2017, 101, 1047–1062.
- Wong, K.H. Preparation of Novel Selenium Nanoparticles with Strong In Vitro and In Vivo Anticancer Efficacy Using Tiger Milk Mushroom. In Proceedings of the 248th National Meeting of the American-Chemical-Society (ACS), San Francisco, CA, USA, 10–14 August 2014.
- Senapati, U.S.; Sarkar, D. Characterization of Biosynthesized Zinc Sulphide Nanoparticles Using Edible Mushroom Pleurotuss ostreatu. Indian J. Phys. 2014, 88, 557–562.
- El-Sonbaty, S.; Kandil, E.I.; Haroun, R.A.-H. Assessment of the Antitumor Activity of Green Biosynthesized Zinc Nanoparticles as Therapeutic Agent Against Renal Cancer in Rats. Biol. Trace Elem. Res. 2022.
- Manimaran, K.; Balasubramani, G.; Ragavendran, C.; Natarajan, D.; Murugesan, S. Biological Applications of Synthesized ZnO Nanoparticles Using Pleurotus djamor Against Mosquito Larvicidal, Histopathology, Antibacterial, Antioxidant and Anticancer Effect. J. Clust. Sci. 2021, 32, 1635–1647.
- Preethi, P.S.; Narenkumar, J.; Prakash, A.A.; Abilaji, S.; Prakash, C.; Rajasekar, A.; Nanthini, A.U.R.; Valli, G. Myco-Synthesis of Zinc Oxide Nanoparticles as Potent Anti-Corrosion of Copper in Cooling Towers. J. Clust. Sci. 2019, 30, 1583–1590.
- Chang, S.T.; Wasser, S.P. The Cultivation and Environmental Impact of Mushrooms. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: New York, NY, USA, 2017; ISBN 978-0-19-938941-4.
- Ab Rhaman, S.M.S.; Naher, L.; Siddiquee, S. Mushroom Quality Related with Various Substrates’ Bioaccumulation and Translocation of Heavy Metals. J. Fungi 2022, 8, 42.
- Kurd-Anjaraki, S.; Ramezan, D.; Ramezani, S.; Samzadeh-Kermani, A.; Pirnia, M.; Shahi, B.Y. Potential of Waste Reduction of Agro-Biomasses through Reishi Medicinal Mushroom (Ganoderma lucidum) Production Using Different Substrates and Techniques. Acta Ecol. Sin. 2022, 42, 90–101.
- Li, H.; Yoshida, S.; Mitani, N.; Egusa, M.; Takagi, M.; Izawa, H.; Matsumoto, T.; Kaminaka, H.; Ifuku, S. Disease Resistance and Growth Promotion Activities of Chitin/Cellulose Nanofiber from Spent Mushroom Substrate to Plant. Carbohydr. Polym. 2022, 284, 119233.
- Mat Zin, M.I.; Jimat, D.N.; Wan Nawawi, W.M.F. Physicochemical Properties of Fungal Chitin Nanopaper from Shiitake (L. edodes), Enoki (F. velutipes) and Oyster Mushrooms (P. ostreatus). Carbohydr. Polym. 2022, 281, 119038.
- Boureghda, Y.; Satha, H.; Bendebane, F. Chitin–Glucan Complex from Pleurotus Ostreatus Mushroom: Physicochemical Characterization and Comparison of Extraction Methods. Waste Biomass Valor. 2021, 12, 6139–6153.
- Sargin, I.; Karakurt, S.; Alkan, S.; Arslan, G. Live Cell Imaging With Biocompatible Fluorescent Carbon Quantum Dots Derived From Edible Mushrooms Agaricus bisporus, Pleurotus ostreatus, and Suillus luteus. J. Fluoresc. 2021, 31, 1461–1473.
- Li, T.; Han, X.; Bao, R.; Hao, Y.; Li, S. Preparation and Properties of Water-in-Oil Shiitake Mushroom Polysaccharide Nanoemulsion. Int. J. Biol. Macromol. 2019, 140, 343–349.
- Fazli Wan Nawawi, W.M.; Lee, K.-Y.; Kontturi, E.; Murphy, R.J.; Bismarck, A. Chitin Nanopaper from Mushroom Extract: Natural Composite of Nanofibers and Glucan from a Single Biobased Source. ACS Sustain. Chem. Eng. 2019, 7, 6492–6496.
- del Rocío Urbina-Salazar, A.; Inca-Torres, A.R.; Falcón-García, G.; Carbonero-Aguilar, P.; Rodríguez-Morgado, B.; del Campo, J.A.; Parrado, J.; Bautista, J. Chitinase Production by Trichoderma Harzianum Grown on a Chitin-Rich Mushroom Byproduct Formulated Medium. Waste Biomass Valor. 2019, 10, 2915–2923.
- Onem, H.; Nadaroglu, H. Immobilization of Purified Phytase Enzyme from Tirmit (Lactarius volemus) on Coated Chitosan with Iron Nanoparticles and Investigation of Its Usability in Cereal Industry. Iran J. Sci. Technol. Trans. Sci. 2018, 42, 1063–1075.
- Konno, N.; Kimura, M.; Okuzawa, R.; Nakamura, Y.; Ike, M.; Hayashi, N.; Obara, A.; Sakamoto, Y.; Habu, N. Preparation of Cellulose Nanofibers from Waste Mushroom Bed of Shiitake (Lentinus edodes) by TEMPO-Mediated Oxidation. Wood Preserv. 2016, 42, 157–164.
More
Information
Subjects:
Environmental Sciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
3 times
(View History)
Update Date:
13 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




