Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 3 by Conner Chen.
Soil is the main component in the agroecosystem besides water, microbial communities, and cultivated plants. Several problems face soil, including soil pollution, erosion, salinization, and degradation on a global level. Many approaches have been applied to overcome these issues, such as phyto-, bio-, and nanoremediation through different soil management tools. Mushrooms can play a vital role in the soil through bio-nanoremediation, especially under the biological synthesis of nanoparticles, which could be used in the bioremediation process.
- soil
- mushroom
1. Green Synthesis of Nanoparticles by Mushrooms
It is well-known that nanoparticles (converted to atomic or molecular scales ranging from 1 and 100 nm) could be produced by three main methods, including physical (using mechanical tools such as for grinding or milling), chemical (using some chemicals as deducting agents), and biological tools (using organisms such as plants, bacteria, fungi, actinomycetes, and algae). The production process or the synthesis of stable nanoparticles (NPs) through biological routes could be referred to as green nano-biotechnology [1][25]. The green chemistry of nanotechnology has mainly 12 principles, including (1) minimizing the production of wastes and reducing pollution, (2) the manufacturing of products in safer manner, (3) lowering toxicity due to less hazardous chemical synthesis, (4) using renewable feedstocks, (5) utilizing effective catalysts, (6) reducing unnecessary derivatives, (7) producing economically greener products, (8) controlling and reducing pollution, (9) increasing the efficiency of used energy, (10) using safer solvents for safer reaction conditions, (11) using degradable and recyclable materials, and (12) minimizing the possibility of accidents [1][25]. The biosynthesis of nanoparticles using fungi has been reported by several researchers, focusing on the genera of fungi used in NPs biosynthesis, the bio-activity of this biosynthesis, and the main applications resulting from this biosynthesis (Figure 1), as adapted from Al-Bahrani et al. [2][132].
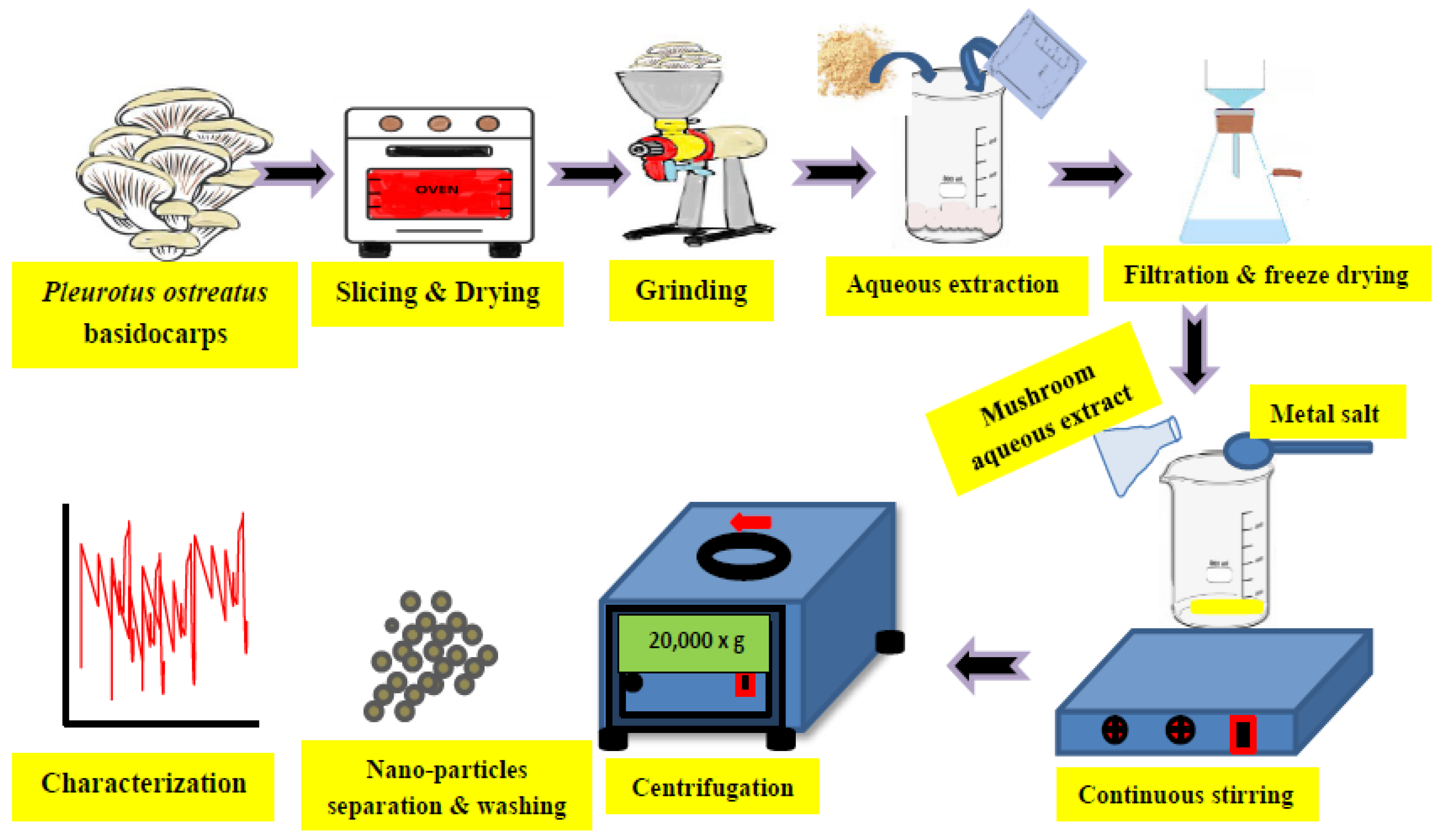
Figure 1. Graphical illustration for the green synthesis of metal nanoparticles using aqueous extract of the fresh basidiocarps of Pleurotus ostreatus. The steps include grinding the collected mushrooms, boiling within aqueous extraction, and then after filtration, the aqueous extraction is added to metal salt, which is needed to produce its nanoparticles (such as Ag, Zn, Cu, etc.).
Concerning the fungal genera used for NPs-biosynthesis, there are several genera of fungi like Aspergillus (27%), Fusarium (21%), Verticillium (9%), Trichoderma, Rhizopus and Penicillium about 7% and some other mushrooms like Pleurotus about 1% [3][133]. Several species of cultivated and wild mushrooms have been successfully used for the green- or bio- or myco-synthesis of many kinds of nanoparticles particularly gold (Au) [4][5][6][134,135,136], silver (Ag) [7][8][9][10][137,138,139,140], magnesium oxide (MgO) [11][141], titanium oxide (TiO2) [12][142], copper (Cu) [13][143], zinc (Zn) [14][15][144,145], and cadmium sulfide (CdS) [3][133]. The mechanism of nanoparticle myco-synthesis by mushrooms seems to be simple, but many factors control the stability, and biocompatibility of produced nanoparticles. These factors mainly include temperature (up to 40 ℃ for Trichoderma harzianum, or 90 ℃ for Aspergillus oryzae), pH of the reaction medium (a higher pH is preferable), the used amount of fungal biomass, and the medium composition [3][133]. The mechanism may return to various types of enzymes, mainly reductases, that can direct the intracellular or extracellular reduction and stabilization of NPs by the fungal exudates of biomolecules [16][146] and [17][18][27,147]. These biomolecules may contain amino acids, alkaloids, carboxylic acids, enzymes, flavonoids, peptides, phenols, polysaccharides, saponins, steroids, tannins, vitamins, and other secondary metabolites [3][9][19][20][21][22][23][133,139,148,149,150,151,152]. Biosynthesis of many metal or metal oxide-NPs using mushrooms like Pleurotus sp. and Pleurotus sajorcaju was reported (Table 1). However, the specific mode of action behind NP-myco-synthesis based on biological materials needs to be fully elucidated [17][27].
Table 1. Green synthesis of some nanoparticles by fundi or mushrooms and their bioactivity.
| Mushroom Species | Nanoparticle Kind and Its Size (nm) | Bioactivity or the Reaction | Reference |
|---|---|---|---|
| Agaricus bisporus | Ag-NPs (80–100) | Antibacterial activities | [24][153] |
| Amanita muscaria | Ag-NPs (5–25) | Anticancer activity | [24][153] |
| Pleurotus ostreatus | Ag-NPs (35) | Antioxidant properties | [25][154] |
| Pleurotus giganteus | Ag-NPs (5–25) | Antimicrobial activity | [26][155] |
| Ganoderma applanatum | Ag-NPs (20–5) | Antioxidant; Antibacterial | [27][156] |
| Ganoderma lucidum | Ag-NPs (15–22) | Antimicrobial activity | [19][148] |
| Pleurotus giganteus | Ag-NPs (2–20) | Antibacterial activity | [26][155] |
| Lentinus tuber-regium | Ag-NPs (5–35) | Antimicrobial activity | [28][157] |
| Pleurotus ostreatus | Ag-NPs (15–45) | Antimicrobial activity | [29][158] |
| Pichia pastoris | Ag-NPs (6.63) | Antioxidant and antimicrobial | [30][159] |
| Inonotus hispidus | Ag-NPs (69.24) | Antibacterial and antifungal | [21][150] |
| Ramaria botrytis | Ag@AuNPs (200) | Antioxidant and antibacterial | [20][149] |
| Flammulina velutipes | Ag-NPs (22) | Antibacterial activities | [31][160] |
| Boletus edulis Coriolus versicolor |
Ag-NPs (87.7) Ag-NPs (86.0) |
Anticancer of breast, colon; liver, and antimicrobial | [7][137] |
| Pleurotus ostreatus Pleurotus djamor |
Ag-NPs (28.44) Ag-NPs (55.76) |
Antioxidant activities and antimicrobial agent | [23][152] |
| Agaricus arvensis | Ag-NPs (20) | Anticancer, Antimicrobial | [8][138] |
| Ganoderma lucidum | Ag-NPs (50) | Antibacterial activities | [10][140] |
| Agaricus bisporus | Ag-NPs (50.44) | Antibacterial activity | [9][139] |
| Pleurotus sajor-caju | Au-NPs (16–18) | Cancer cell inhibition | [5][135] |
| Ganoderma applanatum | Au-NPs (18.7) | Dye decolorization | [32][161] |
| Lentinula edodes | Au-NPs (5–15) | Anticancer activity | [33][162] |
| Ganoderma lucidium | Au-NPs (5–15) | Anticancer activity | [33][162] |
| Agaricus bisporus | Cu-NPs (10) | Antibactericidal activity | [13][143] |
| Pleurotus tuber-regium | Se-NPs (50) | Anticancer activity | [34][163] |
| Pleurotus djamor | TiO2-NPs (31) | Antilarval properties | [12][142] |
| Pleurotuss ostreatu | ZnS-NPs (2–5) | Biomedical; food packaging | [35][164] |
| Agaricus bisporus | Zn-NPs (12–17) | Antirenal cancer | [36][165] |
| Candida albicans | ZnO-NPs (10.2) | Antimicrobial activities | [15][145] |
| Pleurotus floridanus | ZnO-NPs (34.98) | Biomedical applications | [14][144] |
| Pleurotus djamor | ZnO-NPs (38.73) | Antibacterial and anticancer | [37][166] |
| Agarius bisporus | ZnO-NPs (20) | Antibacterial activity | [38][167] |
2. Soil Nanomanagement and Mushrooms
The cultivation of mushrooms is known to have been performed since 600, 1100, and 1650 AD, for the species Auricularia auricula-judae, Lentinula edodes, and Agaricus bisporus, respectively [39][53]. Mushrooms as fungi can play different roles in soil, including promoting soil fertility, biodegrading agrowastes, or removing pollutants from soil, as mentioned in the previous section. According to their main source of carbon for nutrition, the most cultivated mushrooms in the soil are considered saprophyte and heterotrophic fungi. Mushrooms can biosynthesize their own foods from the agroresidues of different crops by converting these by-products and wastes and preventing the accumulation of them as health hazards. Mushrooms cannot synthesize their food through photosynthesis because they are devoid of chlorophylls [39][53]. The vascular xylem and phloem are also absent in mushrooms, but they can absorb O2 and release CO2 [39][53].
The quality of cultivated mushrooms may depend on the concertation of pollutants such as heavy metals and their accumulation in the fruit bodies of mushrooms, which may migrate from the growing substrates [40][168]. The selected proper substrate for mushroom cultivation is considered a limiting factor, which depends on the price and lignocellulosic compounds in used agrowastes [41][169]. Although the use of spent mushroom substrate as raw materials remains a formidable challenge, it could produce complexes of chitin-cellulose nanofiber, which promote plant growth through increasing plant disease resistance [42][170]. An increased concern on the manufacture of nanomaterials derived from mushrooms recently has been noticed (Table 2). These nanomaterials include chitin-cellulose nanofiber from Lentinula edodes substrate [42][170] and chitin nanopaper from Lentinula edodes, Flammulina velutipes, and Pleurotus ostreatus [43][171]. Mushrooms are useful for soil when their wastes from cultivation could be used as fodder for livestock, as a soil conditioner, as organic fertilizers, and/or for environmental bioremediation, as discussed in the next sections.
Table 2. List of some important materials-derived from mushrooms and their applications.
| Mushroom Species | Potential Materials | The Main Application/Main Findings | Refs. |
| Agaricus bisporus | Nanoencapsulation of rutin in β-glucan matrix | Encapsulation by green technology for nutraceutical activities | [18][147] |
| Lentinula edodes | Chitin/cellulose nanofiber | Growth promotion and disease resistance | [42][170] |
| Lentinula edodes, Pleurotus ostreatus | Chitin nanopaper derived from mushroom | Extraction of chitin from the mushrooms to produce nanopaper | [43][171] |
| Pleurotus ostreatus | Chitin–glucan complex | Producing eco-friendly polymers | [44][172] |
| Agaricus bisporus, Pleurotus ostreatus | Biocompatible fluorescent carbon-based nanomaterials | Producing live cells by fluorescent carbon quantum dot derived from mushrooms | [45][173] |
| Lentinus edodes | Nanoemulsion | Nanoemulsion derived from mushroom polysaccharide for the antitumor activity | [46][174] |
| Agaricus bisporus | Chitin nanopaper derived from mushroom | Production of chitin nanopaper from an extract of mushrooms | [47][175] |
| Agaricus bisporus | Fraction of chitin/glucan | Producing glycosidases (e.g., chitinases), which immobilize on nanoparticles and spray for biocontrol of insect pests | [48][176] |
| Lactarius volemus | Modified chitosan with nano-Fe3O4 nanoparticles | Purification of phytase enzymes and their potential in cereal industries | [49][177] |
| Lentinus edodes | Cellulose nanofibers | The highest yield of the film was produced using 0.18 g NaClO per 1.0 g of waste mushroom bed (71% cellulose) | [50][178] |
