
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Graça Campos | -- | 2470 | 2022-04-13 06:32:02 | | | |
| 2 | Vivi Li | Meta information modification | 2470 | 2022-04-13 11:47:02 | | |
Video Upload Options
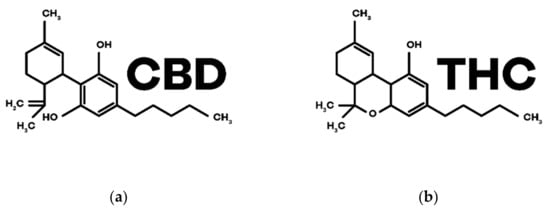
Translational research made with Cannabis sativa L. and its biocompounds provides data for some targeted diseases, as also symptoms associated with Autism Spectrum Disorders (ASDs). The main compounds ∆9-tetrahydrocannabinol (THC) and cannabidiol (CBD), are capable of modulating the endocannabinoid system since its dysregulation interferes with the pathophysiology of ASDs there are clinical evidence for its potential use in the treatment of the disease. Conventional therapy still has limitations, as it does not always treat the central symptoms, and there are many patients who do not respond to treatment, which demands more research on new therapies. Through the analysis of published literature on this topic, it is verified that cannabinoids, in particular CBD, improves symptoms associated with common comorbidities in ASDs. Some studies also demonstrate the therapeutic potential of these compounds in the treatment of central symptoms of autism. In addition, cannabinoid therapy to ASDs is associated with low adverse effects and a reduction in concomitant medication.
1. Introduction
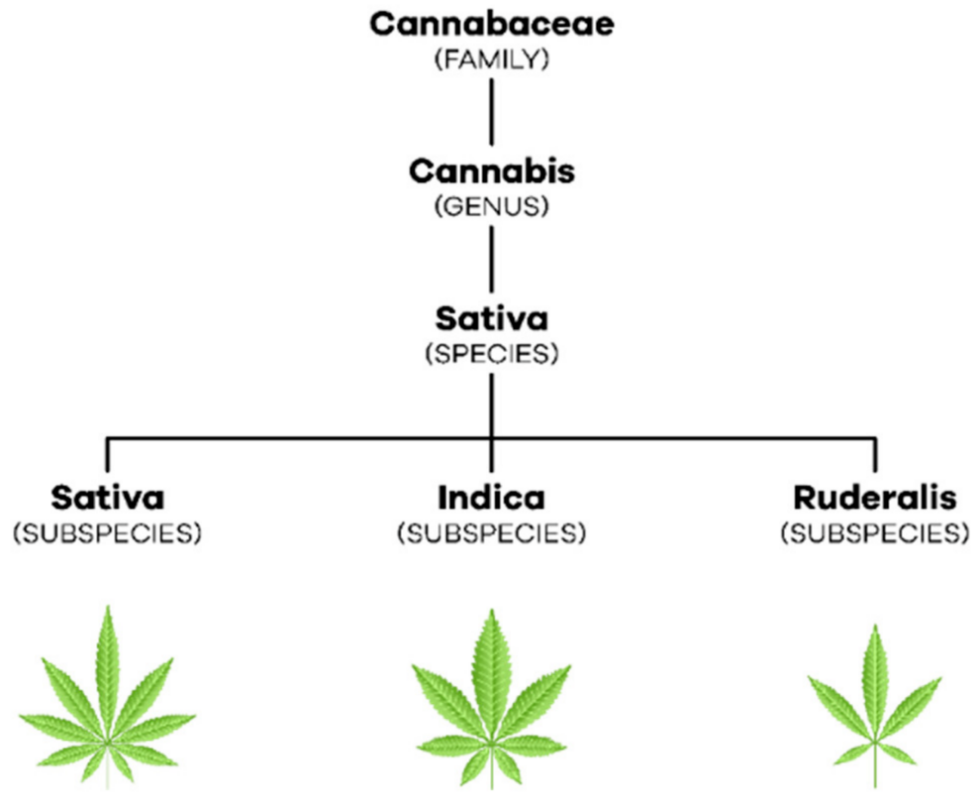
2. Cannabis, Flos
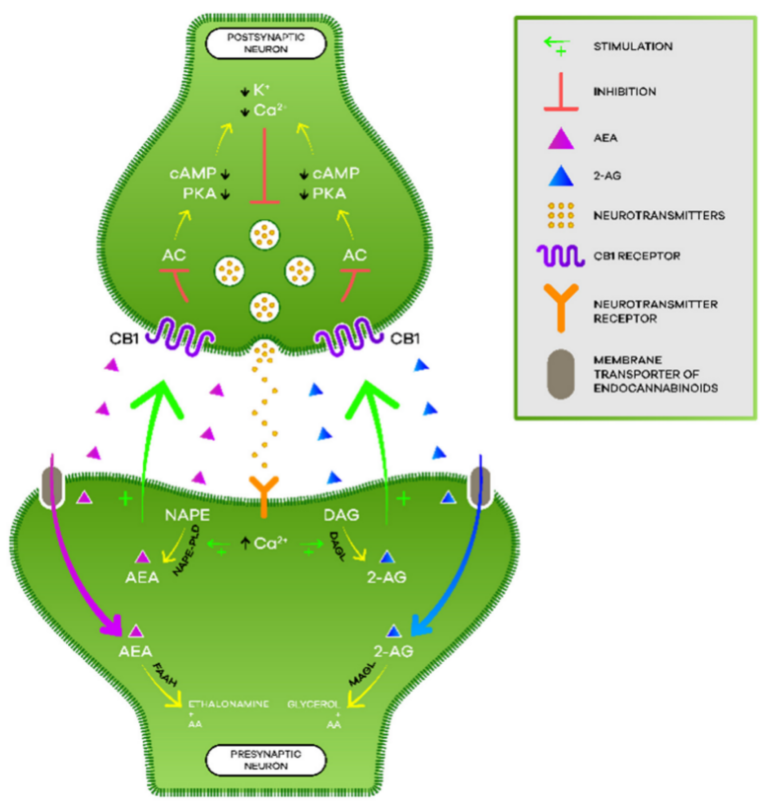
3. Endocannabinoid System
3.1. Cannabinoid Receptors
3.2. Endocannabinoids
3.3. Phytocannabinoids
4. Physiological and Therapeutic Effects of Cannabinoids
| Diseases and Symptoms | Therapeutic Potential of Cannabinoids |
|---|---|
| Alzheimer’s Disease | Anti-inflammatory Neuroprotector Antioxidant |
| Parkinson’s Disease | |
| Huntington’s Disease | |
| Multiple Sclerosis | Antispastic Analgesic |
| Epilepsy | Anticonvulsant |
| Tourette’s Syndrome | Improvement of symptomatology |
| Cancer | Analgesic Antiemetic Appetite stimulator Antitumor |
| Glaucoma | Intraocular pression reduction |
| Inflammatory Bowel Diseases | Anti-inflammatory Healing |
| Schizophrenia | Antipsychotic |
| Sleep Disorders | Decrease sleep latency and nocturnal awakenings Sedative |
| Pain | Analgesic |
| Post-Traumatic Stress Disorder | Anxiolytic |
| Nausea and Vomiting | Antiemetic |
| Anorexia | Appetite stimulator |
References
- Klumpers, L.E.; Thacker, D.L. A brief background on cannabis: From plant to medical indications. J. AOAC Int. 2019, 102, 412–420.
- Pollio, A. The Name of Cannabis: A Short Guide for Nonbotanists. Cannabis Cannabinoid Res. 2016, 1, 234–238.
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315.
- Poleg, S.; Golubchik, P.; Offen, D.; Weizman, A. Cannabidiol as a suggested candidate for treatment of autism spectrum disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 8, 90–96.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; pp. 50–59.
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520.
- Young, N.; Findling, R.L. An update on pharmacotherapy for autism spectrum disorder in children and adolescents. Curr. Opin. Psychiatry 2015, 28, 91–101.
- Sharma, S.R.; Gonda, X.; Tarazi, F.I. Autism Spectrum Disorder: Classification, diagnosis and therapy. Pharmacol. Ther. 2018, 190, 91–104.
- Loss, C.M.; Teodoro, L.; Rodrigues, G.D.; Moreira, L.R.; Peres, F.F.; Zuardi, A.W.; Crippa, J.A.; Hallak, J.E.C.; Abílio, V.C. Is Cannabidiol during Neurodevelopment a Promising Therapy for Schizophrenia and Autism Spectrum Disorders? Front. Pharmacol. 2021, 11, 2461.
- Bundesinstitut für Arzneimittel und Medizinprodukte. German Pharmacopoeia, Cannabis, Flor–Monograph. Cannabisblüten Cannabis Flos; BAnz AT 24.04.2018 B5; Bekanntmachung einer Mitteilung zum Deutschen Arzneibuch; Bundesinstitut für Arzneimittel und Medizinprodukte: Bonn, Germany, 2018.
- Mcpartland, J.M. Cannabis Systematics at the Levels of Family, Genus, and Species. Cannabis Cannabinoid Res. 2018, 3, 203–212.
- Kinghorn, A.D.; Falk, H.; Gibbons, S.; Kobayashi, J. 103-Phytocannabinoids-Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa. In Progress in the Chemistry of Organic Natural Products; Springer Nature: Cham, Switzerland, 2017.
- Gould, J. The Cannabis Crop. Nature 2015, 525, S2–S3.
- European Medicines Agency; Herbal Medicinal Products Committee. Guideline on Good Agricultural and Collection Practice (GACP) for Starting Materials of Herbal Origin. 2006. Available online: https://www.ema.europa.eu/en/good-agricultural-collection-practice-starting-materials-herbal-origin (accessed on 17 January 2022).
- Maroon, J.; Bost, J. Review of the neurological benefits of phytocannabinoids. Surg. Neurol. Int. 2018, 9, 91.
- Lu, D.; Potter, D.E. Cannabinoids and the Cannabinoid Receptors: An Overview. In Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2017.
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833.
- Brigida, A.L.; Schultz, S.; Cascone, M.; Antonucci, N.; Siniscalco, D. Endocannabinod signal dysregulation in autism spectrum disorders: A correlation link between inflammatory state and Neuro-Immune alterations. Int. J. Mol. Sci. 2017, 18, 1425.
- Araujo, D.J.; Tjoa, K.; Saijo, K. The Endocannabinoid System as a Window into Microglial Biology and Its Relationship to Autism. Front. Cell. Neurosci. 2019, 13, 424.
- Cohen, K.; Weizman, A.; Weinstein, A. Positive and Negative Effects of Cannabis and Cannabinoids on Health. Clin. Pharmacol. Ther. 2019, 105, 1139–1147.
- Loprinzi, P.D.; Zou, L.; Li, H. The endocannabinoid system as a potential mechanism through which exercise influences episodic memory function. Brain Sci. 2019, 9, 112.
- Chonhofen, P.; Bristot, I.J.; Crippa, J.A.; Hallak, J.E.C.; Zuardi, A.W.; Parsons, R.B.; Klamt, F. Cannabinoid-Based Therapies and Brain Development: Potential Harmful Effect of Early Modulation of the Endocannabinoid System. CNS Drugs 2018, 32, 697–712.
- Aran, A.; Cayam-Rand, D. Medical cannabis in children. Rambam Maimonides Med. J. 2020, 11, e0003.
- Appendino, G. The early history of cannabinoid research. Rend. Lincei 2020, 31, 919–929.
- Pisanti, S.; Malfitno, M.; Ciaglia, E.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; Laezza, C.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150.
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Marzo, V.D.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802.
- Gu, B. Cannabidiol provides viable treatment opportunity for multiple neurological pathologies of autism spectrum disorder. Glob. Drugs Ther. 2017, 2, 1–4.
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703.
- White, C.M. A Review of Human Studies Assessing Cannabidiol’s (CBD) Therapeutic Actions and Potential. J. Clin. Pharmacol. 2019, 59, 923–934.
- Bridgeman, M.B.; Abazia, D.T. Medicinal Cannabis: History, Pharmacology, and Implications for the Acute Care Setting. Pharm. Ther. 2017, 42, 180–188.
- Stasiłowicz, A.; Tomala, A.; Podolak, I.; Cielecka-Piontek, J. Cannabis sativa L. As a natural drug meeting the criteria of a multitarget approach to treatment. Int. J. Mol. Sci. 2021, 22, 778.
- Breijyeh, Z.; Jubeh, B.; Bufo, S.A.; Karaman, R.; Scrano, L. Cannabis: A Toxin-Producing Plant with Potential Therapeutic Uses. Toxins 2021, 13, 117.
- Oberbarnscheidt, T.; Miller, N.S. The Impact of Cannabidiol on Psychiatric and Medical Conditions. J. Clin. Med. Res. 2020, 12, 393–403.
- Goyal, H.; Singla, U.; Gupta, U.; May, E. Role of cannabis in digestive disorders. Eur. J. Gastroenterol. Hepatol. 2017, 29, 135–142.
- Babson, K.A.; Sottile, J.; Morabito, D. Cannabis, Cannabinoids, and Sleep: A Review of the Literature. Curr. Psychiatry Rep. 2017, 19, 23.




