Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andreas Tsakalof | -- | 779 | 2022-04-12 18:36:57 | | | |
| 2 | Catherine Yang | Meta information modification | 779 | 2022-04-13 03:05:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tsakalof, A.; Gkotinakou, I.M.; Mylonis, I. Vitamin D Synthesis and Metabolism. Encyclopedia. Available online: https://encyclopedia.pub/entry/21668 (accessed on 10 March 2026).
Tsakalof A, Gkotinakou IM, Mylonis I. Vitamin D Synthesis and Metabolism. Encyclopedia. Available at: https://encyclopedia.pub/entry/21668. Accessed March 10, 2026.
Tsakalof, Andreas, Ioanna Maria Gkotinakou, Ilias Mylonis. "Vitamin D Synthesis and Metabolism" Encyclopedia, https://encyclopedia.pub/entry/21668 (accessed March 10, 2026).
Tsakalof, A., Gkotinakou, I.M., & Mylonis, I. (2022, April 12). Vitamin D Synthesis and Metabolism. In Encyclopedia. https://encyclopedia.pub/entry/21668
Tsakalof, Andreas, et al. "Vitamin D Synthesis and Metabolism." Encyclopedia. Web. 12 April, 2022.
Copy Citation
Vitamin D, conventionally considered a nutrient, is a potent hormone regulating many physiological functions. Vitamin D exists as a prohormone that needs to be transformed into biologically active products that bind to their cognate nuclear receptors to regulate diverse physiological processes.
Vitamin D
metabolic pathways
1. Canonical Vitamin D Metabolic Pathway
There are two major isoforms of vitamin D, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) [1][2]. Both vitamin D2/3 need exposure to sunlight’s UVB radiation to be synthesized from ergosterol and 7-dehydrocholesterol, respectively. Vitamin D (both vitamin D2 and D3, calciol) originating from diet or endogenous skin synthesis is delivered to the liver by vitamin D-binding protein (VDBP). There, vitamin D is metabolized by vitamin D 25-hydroxylase (CYP2R1 and CYP27A1) to 25(OH)D (calcidiol), which is the major circulating form of vitamin D in the serum, and its circulating concentration is accepted as an indicator of vitamin D status in a human organism [3][4]. 25(OH)D is further metabolized by 25(OH)D 1α-hydroxylase (CYP27B1) mainly in the proximal tubule of the kidney to 1α,25-dihydroxyvitamin D (1α,25(OH)2D, calcitriol), which is the recognized biologically active form of vitamin D (Figure 1) [3][4]. Calcitriol then enters the circulation and, after binding to VDBP, is delivered to target tissues such as the intestine, bone, and kidney, where vitamin D is known to regulate absorption, mobilization, and reabsorption, respectively, of calcium and phosphate [1]. After being produced, the levels of both calcidiol and calcitriol are tightly regulated by 25(OH)D 24-hydroxylase (CYP24A1), which is the primary vitamin D inactivating enzyme catalyzing hydroxylation at C-24 and C-23 of both calcidiol and calcitriol [3][4]. This 24-hydroxylation pathway produces the biologically inactive calcitroic acid excreted in the bile [5]. The importance of this inactivation step, mediated by CYP24A1, was highlighted in CYP24A1 knockout mice showing impaired intramembranous bone mineralization and hypercalcemia, leading to a lethal phenotype in 50% of the mice [6][7]. However, this defect was rescued in CYP24A1 and VDR double-knockout mice, which suggested that it is the increased calcitriol levels and not the absence of downstream metabolites that were responsible for the flawed phenotype [7].
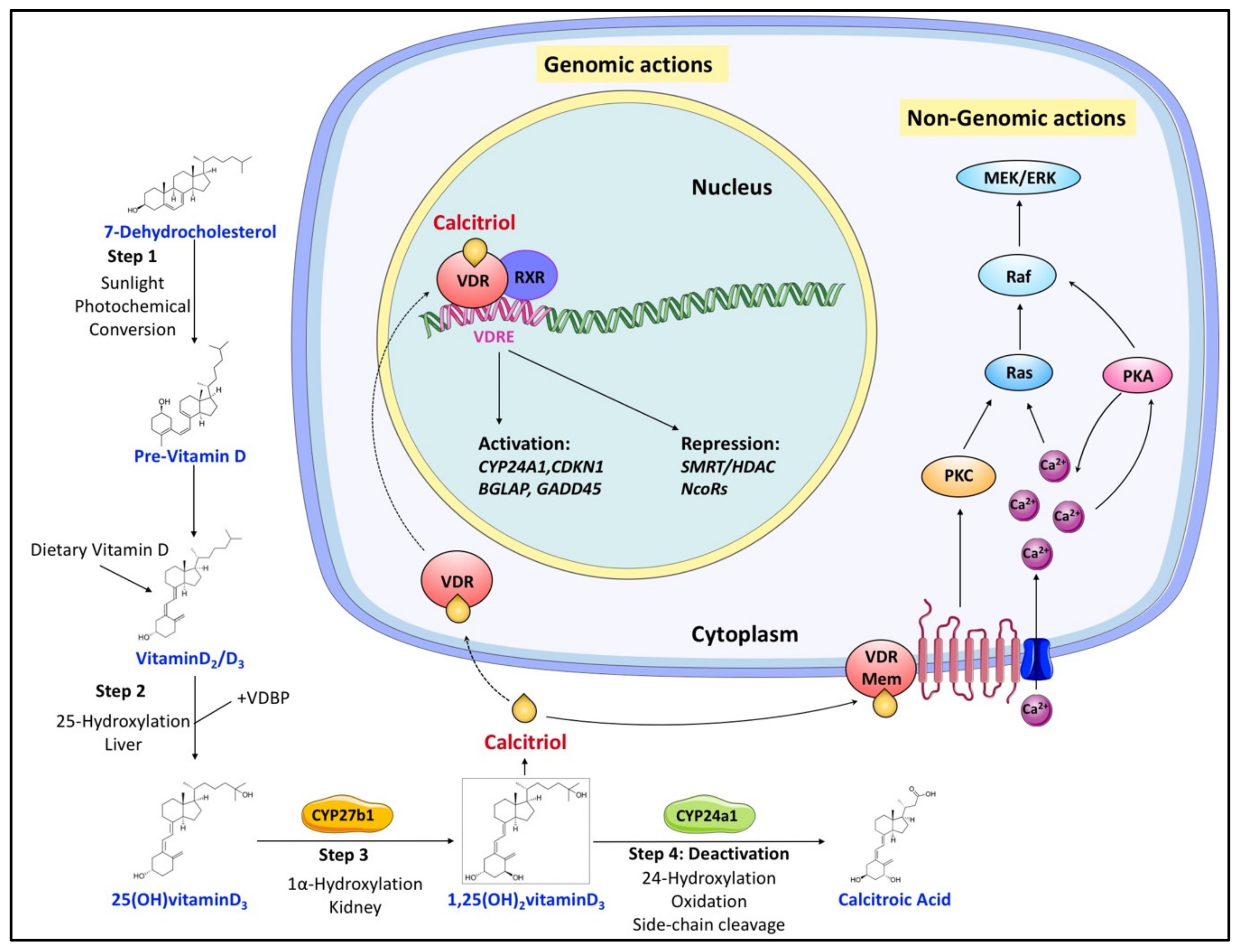
Figure 1. Overview of vitamin D canonical metabolism and its genomic or nongenomic effects. Dietary or cutaneously synthesized vitamin D undergoes two subsequent hydroxylation steps in the liver and kidney to produce active calcitriol (1,25(OH)2 vitaminD3). Calcitriol exerts its functions either by binding to VDR to regulate gene expression or by associating with extracellular binding sites to modulate signaling pathways that influence various cellular processes. Regulation of calcitriol levels also requires inactivation steps mainly involving its hydroxylation by CYP24a1.
2. Noncanonical Vitamin D Metabolic Pathway
Alternatively, vitamin D metabolism is mediated by CYP11A1 (known as a cytochrome P450 side-chain cleavage (P450scc) enzyme) [8]. Vitamin D serves as an alternative substrate for CYP11A1 instead of cholesterol and is sequentially hydroxylated, predominantly at C-20 or C-22, without the cleavage of the side chain producing a multitude of metabolites [9]. Overall, it is estimated that this alternative path produces more than 21 hydroxy-metabolites of vitamin D [8]. Summarily, CYP11A1 products exhibit: (i) antiproliferative, differentiating, and anti-inflammatory abilities in skin cells comparable to that of calcitriol [10][11], (ii) are involved in defense pathways against UVB-induced damage and oxidative stress, and (iii) elicit anticancer abilities in a cell-specific manner [12]. As a point of interest, these alternative metabolites and normal 1,24,25-(OH)3 vitamin D3 do not activate VDR. Thus, the calcemic effects or expression of CYP24A1 can be seen only in response to calcitriol.
3. Hormonal Regulation of the Canonical Vitamin D Metabolic Pathway
As a result of its diverse function, calcitriol is tightly regulated in a negative feedback mechanism [5][13]. Calcitriol inactivation primarily involves modification by CYP24A1, which is among the most prominent targets of the calcitriol–VDR–RXR complex (Figure 1) [14]. In addition, calcitriol can also induce CYP24A1 expression by recruiting histone H4 acetyltransferases and RNA polymerase II to a site approximately 50–70 kb downstream of the human CYP24A1 gene [15]. So, calcitriol signaling levels are tightly kept in control by calcitriol-driven expression of CYP24A1.
Independently, vitamin D metabolism is regulated by two hormones, parathyroid hormone (PTH) and fibroblast growth factor-23 (FGF-23), both of which maintain the calcium and phosphate homeostasis [16]. PTH, secreted by the parathyroid gland in response to calcium levels, stimulates the expression of CYP27B1, leading to an increase in calcitriol production [17]. Although calcitriol signals its degradation via CYP24A1, PTH sustains calcitriol levels by activating the renal cAMP–PKA pathway and invoking the CYP24A1 mRNA degradation [18]. FGF-23, secreted by osteoblasts and osteocytes in response to both phosphate and calcitriol levels [14], reduces serum calcitriol levels by inhibiting the expression of CYP27B1 and simultaneously enhancing the expression of CYP24A1 in the kidney [19].
References
- Heaney, R.P. Vitamin D in Health and Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541.
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136.
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 1331.
- Schuster, I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim. Biophys. Acta 2011, 1814, 186–199.
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329.
- Masuda, S.; Byford, V.; Arabian, A.; Sakai, Y.; Demay, M.B.; St-Arnaud, R.; Jones, G. Altered Pharmacokinetics of 1α,25-Dihydroxyvitamin D3and 25-Hydroxyvitamin D3in the Blood and Tissues of the 25-Hydroxyvitamin D-24-Hydroxylase (Cyp24a1) Null Mouse. Endocrinology 2005, 146, 825–834.
- St-Arnaud, R.; Arabian, A.; Travers, R.; Barletta, F.; Raval-Pandya, M.; Chapin, K.; Depovere, J.; Mathieu, C.; Christakos, S.; DeMay, M.B.; et al. Deficient Mineralization of Intramembranous Bone in Vitamin D-24-Hydroxylase-Ablated Mice Is Due to Elevated 1,25-Dihydroxyvitamin D and Not to the Absence of 24,25-Dihydroxyvitamin D*. Endocrinology 2000, 141, 2658–2666.
- Slominski, A.; Semak, I.; Zjawiony, J.; Wortsman, J.; Li, W.; Szczesniewski, A.; Tuckey, R.C. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005, 272, 4080–4090.
- Slominski, A.; Zmijewski, M.; Semak, I.; Zbytek, B.; Pisarchik, A.; Li, W.; Zjawiony, J.; Tuckey, R. Cytochromes P450 and Skin Cancer: Role of Local Endocrine Pathways. Anti Cancer Agents Med. Chem. 2014, 14, 77–96.
- Slominski, A.T.; Brozyna, A.; Skobowiat, C.; Zmijewski, M.; Kim, T.-K.; Janjetovic, Z.; Oak, A.S.; Jóźwicki, W.; Jetten, A.; Mason, R.; et al. On the role of classical and novel forms of vitamin D in melanoma progression and management. J. Steroid Biochem. Mol. Biol. 2018, 177, 159–170.
- Slominski, A.T.; Brożyna, A.A.; Zmijewski, M.A.; Janjetovic, Z.; Kim, T.-K.; Slominski, R.M.; Tuckey, R.C.; Mason, R.S.; Jetten, A.M.; Guroji, P.; et al. The Role of Classical and Novel Forms of Vitamin D in the Pathogenesis and Progression of Nonmelanoma Skin Cancers. Adv. Exp. Med. Biol. 2020, 1268, 257–283.
- Tongkao-On, W.; Carter, S.; Reeve, V.E.; Dixon, K.M.; Gordon-Thomson, C.; Halliday, G.M.; Tuckey, R.C.; Mason, R.S. CYP11A1 in skin: An alternative route to photoprotection by vitamin D compounds. J. Steroid Biochem. Mol. Biol. 2015, 148, 72–78.
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012, 523, 9–18.
- Zierold, C.; Darwish, H.M.; DeLuca, H.F. Two vitamin D response elements function in the rat 1,25-dihydroxyvitamin D 24-hydroxylase promoter. J. Biol. Chem. 1995, 270, 1675–1678.
- Meyer, M.B.; Goetsch, P.D.; Pike, J.W. A Downstream Intergenic Cluster of Regulatory Enhancers Contributes to the Induction of CYP24A1 Expression by 1α,25-Dihydroxyvitamin D3. J. Biol. Chem. 2010, 285, 15599–15610.
- Khundmiri, S.J.; Murray, R.D.; Lederer, E. PTH and Vitamin D. Compr. Physiol. 2016, 6, 561–601.
- Rost, C.R.; Bikle, D.D.; Kaplan, R.A. In Vitro Stimulation of 25-Hydroxycholecalciferol lα- Hydroxylation by Parathyroid Hormone in Chick Kidney Slices: Evidence for a Role for Adenosine 3′,5′- Monophosphate *. Endocrinology 1981, 108, 1002–1006.
- Zierold, C.; Mings, J.A.; DeLuca, H.F. Parathyroid hormone regulates 25-hydroxyvitamin D3-24-hydroxylase mRNA by altering its stability. Proc. Natl. Acad. Sci. USA 2001, 98, 13572–13576.
- Perwad, F.; Zhang, M.Y.H.; Tenenhouse, H.S.; Portale, A.A. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1α-hydroxylase expression in vitro. Am. J. Physiol. Physiol. 2007, 293, F1577–F1583.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
10.5K
Revisions:
2 times
(View History)
Update Date:
13 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





