
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sandile Phinda Songca | -- | 1562 | 2022-04-12 13:06:41 | | | |
| 2 | Peter Tang | -107 word(s) | 1453 | 2022-04-13 03:47:32 | | |
Video Upload Options
Originally discovered as a relatively new anticancer therapeutic technology, photodynamic therapy (PDT) has since evolved and is now used in many therapeutic technologies. For example, it is used against viruses, such as the recently reported antiviral activity against COVID-19, bacteria, fungi, and parasites, in treating neovascular disease, in environmental sanitation and pest control, and in many other applications.
1. Introduction
2. The Microbial Biofilm Structural Challenge
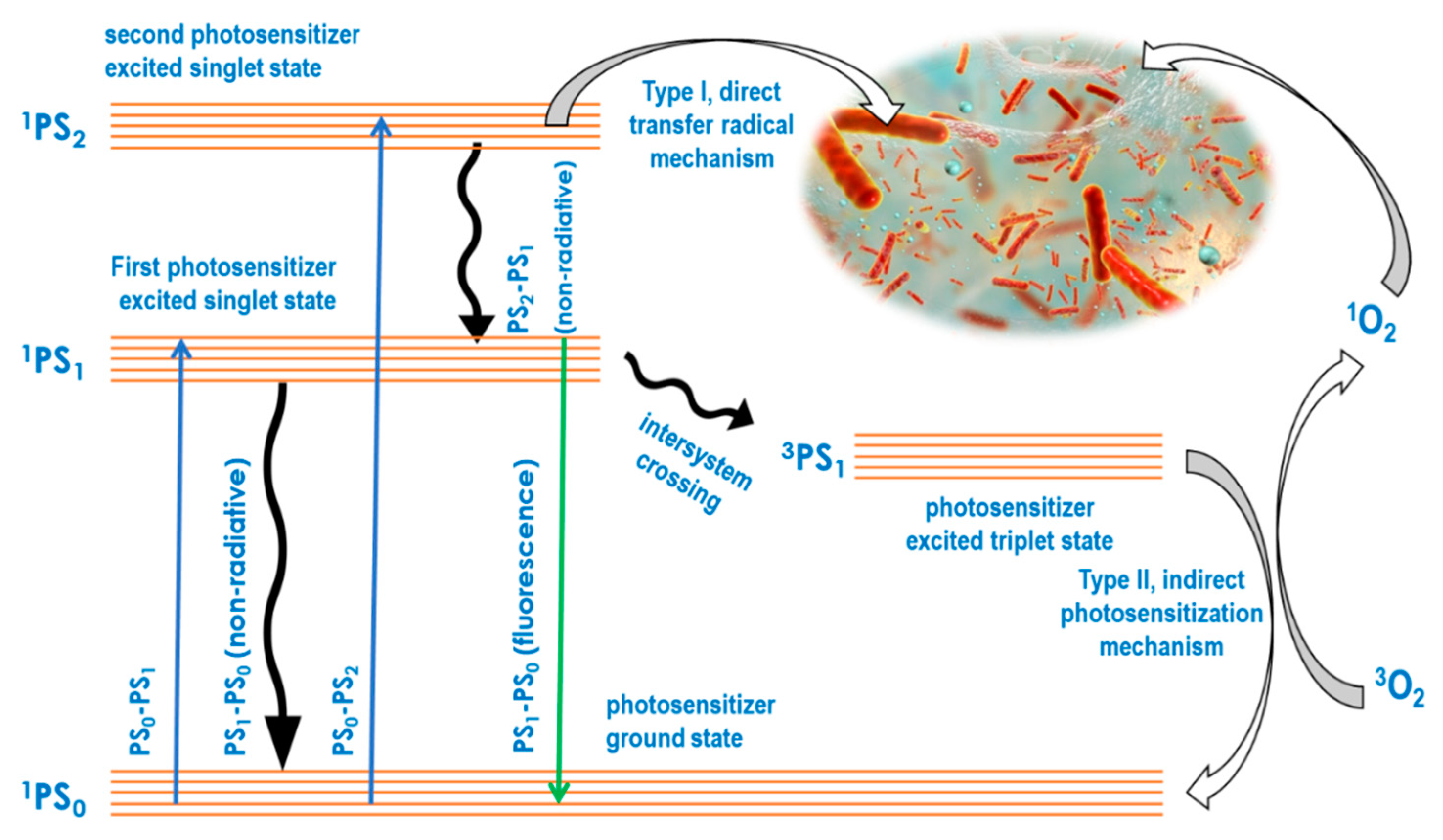
3. Antimicrobial Photodynamic Therapy
|
Photosensitizer |
Nanoconjugate System Used |
Gram Negative |
Gram Positive |
Study Phase |
Impact Target |
Ref |
|---|---|---|---|---|---|---|
|
polyethylenimine-chlorin(e6) and tris-cationic-buckminsterfullerene |
dendrimer nanoconjugate |
E. coli P. mirabilis P. aeruginosa |
S. aureus E. fecalis |
in vitro in vivo |
biofilm + planktonic |
[53] |
|
1-oxo-1H-phenalen-2-yl methyl pyridinium chloride (SAPYR) and 1-oxo-1H-phenalen- 2-yl-methyl-dodecan-1-aminium chloride (SA-PN-05) |
None |
E. coli |
A. naeslundii S. mutans |
in vitro |
biofilm + planktonic |
[54] |
|
porfimer sodium (hematoporphyrin derivative) |
None |
F. nucleatuma |
in vitro |
biofilm |
[57] |
|
|
Methylene blue |
None |
E. coli, K. pneumoniae, P. aeruginosa, S. marcescens, H. influenzae |
C. albicans, E. faecalis, S. aureus, S. pneumoniae |
in vitro in vivo |
biofilm |
[57] |
|
Chlorin-e6 |
None |
H. influenzae |
M. catarrhalis, S. pneumoniae |
in vitro |
biofilm + planktonic |
[59] |
|
Protoporphyrin IX and Methylene blue |
None |
A. baumannii |
in vitro |
biofilm + planktonic |
[60] |
References
- Diamond, I.; Granelli, S.G.; McDonagh, A.F.; Nielsen, S.; Wilson, C.B.; Jaenicke, R. Photodynamic therapy of malignant tumours. Lancet 1972, 2, 1175–1177.
- Svyatchenko, V.A.; Nikonov, S.D.; Mayorov, A.P.; Gelfond, M.L.; Loktev, V.B. Antiviral photodynamic therapy: Inactivation and inhibition of SARS-CoV-2 in vitro using methylene blue and Radachlorin. Photodiagn. Photodyn. Ther. 2021, 33, 102112.
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302.
- Liang, Y.I.; Lu, L.M.; Chen, Y.; Lin, Y.K. Photodynamic therapy as an antifungal treatment. Exp. Ther. Med. 2016, 12, 23–27.
- Debora, P.; Aureliano, D.P.; Ribeiro, M.S.; Lindoso, J.A.L.; Pogliani, F.C.; Sellera, F.P.; Song, D.; Mauricio, S.; Baptista, M.S. Treatment and Control of Leishmaniasis Using Photodynamic Therapy, Leishmaniasis-Trends in Epidemiology, Diagnosis and Treatment; Claborn, D.M., Ed.; IntechOpen: London, UK, 2014.
- Newman, D.K. Photodynamic therapy: Current role in the treatment of chorioretinal conditions. Eye 2016, 30, 202–210.
- Brovko, L.Y.; Meyer, A.; Tiwana, A.S.; Chen, W.; Liu, H.; Filipe, C.D.; Griffiths, M.W. Photodynamic treatment: A novel method for sanitation of food handling and food processing surfaces. J. Food Prot. 2009, 72, 1020–1024.
- Shiao, S.-H.; Weng, S.-C.; Luan, L.; Vicente, M.D.G.H.; Jiang, X.-J.; Ng, D.K.P.; Kolli, B.K.; Chang, K.P. Novel phthalocyanines activated by dim light for mosquito larva- and cell-inactivation with inference for their potential as broad-spectrum photodynamic insecticides. PLoS ONE 2019, 14, e0217355.
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy-what we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589.
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28.
- Farrer, N.J.; Salassa, L.; Sadler, P.J. Photoactivated chemotherapy (PACT): The potential of excited-state d-block metals in medicine. Dalton Trans. 2009, 48, 10690–10701.
- Cieplik, F.; Tabenski, L.; Buchalla, W.; Maisch, T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front. Microbiol. 2014, 5, 405.
- Vollmer, A.; Al-Ahmad, A.; Argyropoulou, A.; Thurnheer, T.; Hellwig, E.; Attin, T.; Vach, K.; Wittmer, A.; Ferguson, K.; Skaltsounis, A.L.; et al. Antimicrobial Photoinactivation Using Visible Light Plus Water-Filtered Infrared-A (VIS + wIRA) and Hypericum Perforatum Modifies In Situ Oral Biofilms. Sci. Rep. 2019, 9, 20325.
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Transl. Res. 2015, 1, 140–167.
- Sabino, C.P.; Wainwright, M.; Ribeiro, M.S.; Sellera, F.P.; dos Anjos, C.; Baptista, M.S.; Lincopan, N. Global priority multidrug-resistant pathogens do not resist photodynamic therapy. J. Photochem. Photobiol. B Biol. 2020, 208, 111893.
- Al-Mutairi, R.; Tovmasyan, A.; Batinic-Haberle, I.; Benov, L. Sublethal Photodynamic Treatment Does Not Lead to Development of Resistance. Front. Microbiol. 2018, 9, 1699.
- Casas, A.; Di Venosa, G.; Hasan, T.; Batlle, A. Mechanisms of Resistance to Photodynamic Therapy. Curr. Med. Chem. 2011, 18, 2486–2515.
- Hughes, G.; Webber, M.A. Novel approaches to the treatment of bacterial biofilm infections. Br. J. Pharmacol. 2017, 174, 2237–2246.
- de Melo, W.C.; Celiešiūtė-Germanienė, R.; Šimonis, P.; Arūnas, S.A. Antimicrobial photodynamic therapy (aPDT) for biofilm treatments. Possible synergy between aPDT and pulsed electric fields. Virulence 2021, 12, 2247–2272.
- Hu, X.; Huang, Y.Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299.
- Pérez-Laguna, V.; Gilaberte, Y.; Millán-Lou, M.I.; Agut, M.; Nonell, S.; Rezusta, A.; Hamblin, M.R.A. A combination of photodynamic therapy and antimicrobial compounds to treat skin and mucosal infections: A systematic review. Photochem. Photobiol. Sci. 2019, 18, 1020–1029.
- Vieira, C.; Gomes, A.T.P.C.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.; Graça, P.M.S.; Faustino, M.A.F.; Almeida, A. An Insight Into the Potentiation Effect of Potassium Iodide, on aPDT Efficacy. Front. Microbiol. 2018, 9, 2665.
- Lee, H.J.; Kang, S.M.; Jeong, S.H.; Chung, K.H.; Kim, B.I. Antibacterial photodynamic therapy with curcumin and Curcuma xanthorrhiza extract against Streptococcus mutans. Photodiagn. Photodyn. Ther. 2017, 20, 116–119.
- Lee, H.; Han, J.; Shin, H.; Han, H.; Na, K.; Kim, H. Combination of chemotherapy and photodynamic therapy for cancer treatment with sonoporation effects. J. Control. Release 2018, 283, 190–199.
- Li, X.; Lovell, J.F.; Yoon, J.; Che, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674.
- Bilici, K.; Atac, N.; Muti, A.; del Valle, C.A.; Miravet, J.F.; Rezusta, A.; Galindo, F. Broad spectrum antibacterial photodynamic and photothermal therapy achieved with indocyanine green loaded SPIONs under near infrared irradiation. Biomater. Sci. 2020, 8, 4616–4625.
- Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Fabbrocini, G.; Sanchez-Blanco, B.; López-Barcenas, A.; EL-Samahy, M.; Juárez-Durán, E.R.; González-Cespón, J.L. New Applications of Photodynamic Therapy in the Management of Candidiasis. J. Fungi 2021, 7, 1025.
- Cramer, S.W.; Chen, C.C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2020, 6, 81.
- Matsubara, T.; Kusuzaki, K.; Matsumine, A.; Asanuma, K.; Nakamura, T.; Uchida, A.; Sudo, A. A Minimally Invasive Surgery for Bone Metastases Using the Combination of Photodynamic Therapy and Hyperthermia Treatment. Int. J. Clin. Med. 2013, 4, 357–363.
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815.
- Karami-Gadallo, L.; Ghoranneviss, M.; Ataie-Fashtami, L.; Pouladian, M.; Sardari, D. Enhancement of cancerous cells treatment by applying cold atmospheric plasma and photo dynamic therapy simultaneously. Clin. Plasma Med. 2017, 7, 46–51.
- Wang, M.; Geilich, B.M.; Keidar, M.; Webster, T.J. Killing malignant melanoma cells with protoporphyrin IX-loaded polymersome-mediated photodynamic therapy and cold atmospheric plasma. Int. J. Nanomed. 2017, 12, 4117–4127.
- Yan, D.; Xu, W.; Yao, X.; Lin, L.; Sherman, J.H.; Keidar, M. The Cell Activation Phenomena in the Cold Atmospheric Plasma Cancer Treatment. Sci. Rep. 2018, 8, 15418.
- Balzer, J.; Heuer, K.; Demir, E.; Hoffmanns, M.A.; Baldus, S.; Fuchs, P.C.; Awakowicz, P.; Suschek, C.V.; Opländer, C. Non-Thermal Dielectric Barrier Discharge (DBD) Effects on Proliferation and Differentiation of Human Fibroblasts Are Primary Mediated by Hydrogen Peroxide. PLoS ONE 2015, 10, e0144968.
- Brehmer, F.; Haenssle, H.A.; Daeschlein, G.; Ahmed, R.; Pfeiffer, S.; Görlitz, A.; Simon, D.; Schön, M.P.; Wandke, D.; Emmert, S. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm(®) VU-2010): Results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT01415622). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 148–155.
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; von Woedtke, T. The plasma jet kINPen–A powerful tool for wound healing. Clin. Plasma Med. 2016, 4, 19–28.
- Arndt, S.; Schmidt, A.; Karrer, S.; von Woedtke, T. Comparing two different plasma devices kINPen and Adtec SteriPlas regarding their molecular and cellular effects on wound healing. Clin. Plasma Med. 2018, 9, 24–33.
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512.
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928.
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681.
- Amankwah, S.; Abdella, K.; Kassa, T. Bacterial Biofilm Destruction: A Focused Review On The Recent Use of Phage-Based Strategies With Other Antibiofilm Agents. Nanotechnol. Sci. Appl. 2021, 14, 161–177.
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The house of biofilm cells. J. Bacteriol. 2007, 189, 7945–7947.
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218.
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11.
- Dickschat, J.S. Quorum sensing and bacterial biofilms. Nat. Prod. Rep. 2010, 27, 343–369.
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355.
- Billings, N.; Birjiniuk, A.; Samad, T.S.; Doyle, P.S.; Ribbeck, K. Material properties of biofilms—A review of methods for understanding permeability and mechanics. Rep. Prog. Phys. 2015, 78, 036601.
- Kesel, S.; Grumbein, S.; Gümperlein, I.; Tallawi, M.; Marel, A.K.; Lieleg, O.; Opitz, M. Direct Comparison of Physical Properties of Bacillus subtilis NCIB 3610 and B-1 Biofilms. Appl. Environ. Microbiol. 2016, 82, 2424–2432.
- Feng, J.; Lamour, G.; Xue, R.; Mirvakliki, M.N.; Hatzikiriakos, S.G.; Xu, J.; Li, H.; Wang, S.; Lu, X. Chemical, physical and morphological properties of bacterial biofilms affect survival of encased Campylobacter jejuni F38011 under aerobic stress. Int. J. Food Microbiol. 2016, 238, 172–182.
- Wu, Y.; Klapper, I.; Stewart, P.S. Hypoxia arising from concerted oxygen consumption by neutrophils and microorganisms in biofilms. Pathog. Dis. 2018, 76, fty043.
- Shrestha, A.; Kishen, A. Polycationic chitosan-conjugated photosensitizer for antibacterial photodynamic therapy. Photochem. Photobiol. 2012, 88, 577–583.
- Calixto, G.M.F.; Bernegossi, J.; De Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342.
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490–499.
- Muehler, D.; Rupp, C.M.; Keceli, S.; Brochhausen, C.; Siegmund, H.; Maisch, T.; Hiller, K.A.; Buchalla, W.; Cieplik, F. Insights Into Mechanisms of Antimicrobial Photodynamic Action Toward Biofilms Using Phenalen-1-One Derivatives as Photosensitizers. Front. Microbiol. 2020, 11, 589364.
- Kikuchi, T.; Mogi, M.; Okabe, I.; Okada, K.; Goto, H.; Sasaki, Y.; Fujimura, T.; Fukuda, M.; Mitani, A. Adjunctive Application of Antimicrobial Photodynamic Therapy in Nonsurgical Periodontal Treatment: A Review of Literature. Int. J. Mol. Sci. 2015, 16, 24111–24126.
- Sharab, L.; Baier, R.E.; Ciancio, S.; Mang, T. Influence of Photodynamic Therapy on Bacterial Attachment to Titanium Surface. J. Oral Implantol. 2021, 47, 427–435.
- Mang, T.; Rogers, S.; Keinan, D.; Honma, K.; Baier, R. Antimicrobial photodynamic therapy (aPDT) induction of biofilm matrix architectural and bioadhesive modifications. Photodiagn. Photodyn. Ther. 2016, 13, 22–28.
- Biel, M.A.; Sievert, C.; Usacheva, M.; Teichert, M.; Balcom, J. Antimicrobial photodynamic therapy treatment of chronic recurrent sinusitis biofilms. Int. Forum Allergy Rhinol. 2011, 1, 329–334.
- Luke-Marshall, N.R.; Hansen, L.A.; Shafirstein, G.; Campagnari, A.A. Antimicrobial Photodynamic Therapy with Chlorin e6 Is Bactericidal against Biofilms of the Primary Human Otopathogens. mSphere 2020, 5, e00492-20.
- Anane, Y.A.; Apalata, T.; Vasaikar, S.; Okuthe, G.E.; Songca, S.P. In vitro antimicrobial photodynamic inactivation of multidrug-resistant Acinetobacter baumannii biofilm using Protoporphyrin IX and Methylene blue. Photodiagn. Photodyn. Ther. 2020, 30, 101752.




