
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nikola Skoro | -- | 3181 | 2022-04-11 15:48:21 | | | |
| 2 | Beatrix Zheng | + 23 word(s) | 3204 | 2022-04-12 03:56:18 | | | | |
| 3 | Beatrix Zheng | + 4 word(s) | 3208 | 2022-04-12 04:10:06 | | | | |
| 4 | Beatrix Zheng | + 31 word(s) | 3239 | 2022-04-12 05:50:21 | | | | |
| 5 | Beatrix Zheng | Meta information modification | 3239 | 2022-04-12 05:54:53 | | |
Video Upload Options
Implementation of the surface dielectric barrier discharge (SDBD) plasma treatment before sowing represents a promising strategy for future investigations and sustainable use of cold plasma in synseed biotechnology. Plasma-treated chrysanthemum synseeds showed a better survival rate and overall plantlet growth under greenhouse conditions in comparison to untreated synseeds.
1. Introduction
| Cultivar | Plant Material | Beads | Sowing | Germination (%) |
Flowering | References |
|---|---|---|---|---|---|---|
| Clone ‘PS 27’ | Nodal segments (in vitro) |
Monolayered (3% Na-alginate + 0.1 mg/L IAA) |
Sterile, water–sand |
50 | no | [29] |
| Double-layered (beads: 3% Na alginate, second layer: water) |
Non-sterile, water–perlite | 45 | no | |||
| Lady group | Shoot tips (in vitro) |
Monolayered (3% Na-alginate) |
Sterile, agar | 52 | no | [19] |
| cv. ‘Royal Purple’ | Shoot tips (ex vitro) |
Monolayered (2.5% Na-alginate, sucrose, vitamin free) |
Non-sterile, vermiculite | 34 | no | [30] |
2. Current Insights
| Electrode Distance-d [mm] | Stray Capacitance—Cp [pF] |
|---|---|
| 2 | 20 |
| 3 | 35 |
| 4 | 41 |
| 5 | 45 |
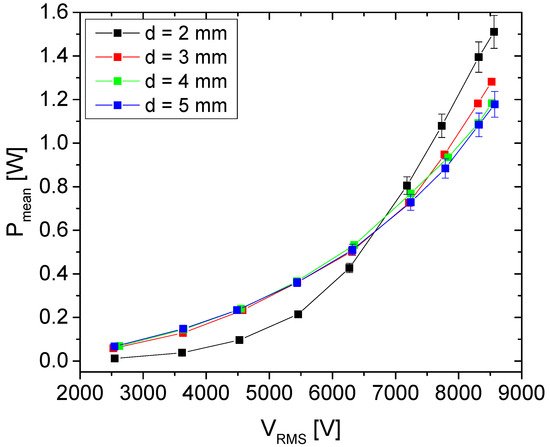
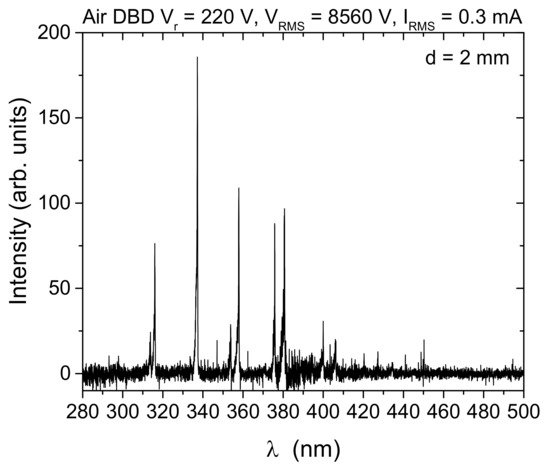
The effect of plasma treatment (10 min) on synseed germination of different chrysanthemum cultivars was evaluated (Table 3). The researchers have found that the plasma treatment significantly enhance the process of synseeds germination and conversion to plantlet after direct sowing in soil for all tested cultivars. This effect of plasma was cultivar dependent (Table 4). Without plasma treatment of chrysanthemum synseeds, frequency of whole plantlet regeneration varied from 6-28% depending on the cultivar. On the other hand, plantlet regeneration from plasma-treated synseeds were 22-49%. The highest treatment effect on whole plantlet development were recorded for cultivars BC and PP (~370% and ~350%, respectively) in comparison to control synseeds, while the lowest values were obtained for the PP cultivar (~160%).
| Plasma Treatment (min) | Synseed Germination | ||
|---|---|---|---|
| Leaf Emergence * (%) |
Shoot Regrowth * (%) |
Plantlet * (%) |
|
|
0 |
60 ± 7 a ** |
33 ± 7 a |
17 ± 5 a |
|
10 |
66 ± 5 a |
60 ± 5 b |
41 ± 5 b |
| Plasma Treatment (min) | Synseed Germination | ||
|---|---|---|---|
| Leaf Emergence * (%) |
Shoot Regrowth * (%) |
Plantlet * (%) |
|
| 0 | 60 ± 7 a ** | 33 ± 7 a | 17 ± 5 a |
| 10 | 66 ± 5 a | 60 ± 5 b | 41 ± 5 b |
References
- Rihan, H.Z.; Kareem, F.; El-Mahrouk, E.; Fuller, M.P. Artificial seeds (Principle, aspects and applications). Agronomy 2017, 7, 71.
- Yücesan, B. Synseed: A new trend in seed technology. In Synthetic Seeds, Germplasm Regeneration, Preservation and Prospects; Faisal, M., Alatar, A.A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 61–75.
- Murashige, T. Plant cell and organ culture as horticultural practice. Acta Hortic. 1977, 78, 17–30.
- Standardi, A.; Picconi, E. Recent perspectives on the synthetic seed technology using non–embryogenic in vitro-derived explants. Int. J. Plant Sci. 1998, 159, 968–978.
- Ara, H.; Jaiswal, U.; Jaiswal, V.S. Synthetic seed: Prospects and limitations. Curr. Sci. 2000, 78, 1438–1444.
- Benelli, C.; Micheli, M.; De Carlo, A. An improved encapsulation protocol for regrowth and conservation of four ornamental species. Acta Soc. Bot. Pol. 2017, 86, 3559.
- Phanornchai, S.; Bodhipadma, K.; Noichnda, S.; Leung, D.W.M. Short-term storability of alginate-encapsulated Persian violet microshoots for germplasm exchange. Plants 2022, 11, 185.
- Sakai, A.; Engelmann, F. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. Cryo Lett. 2007, 28, 151–172.
- Sharma, S.; Shahza, A.; Teixeira da Silva, J.A. Synseed technology—A complete synthesis. Biotechnol. Adv. 2013, 31, 186–207.
- Saxena, A.; Shukla, M.; Saxena, P. Synthetic seeds: Relevance to endangered germplasm conservation in vitro. In Synthetics Seeds, Germplasm Regeneration, Preservation and Prospects; Faisal, M., Alatar, A.A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 21–59.
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 7697031.
- Wiegand, C.; Heinze, T.; Hipler, U.C. Comparative in vitro study on cytotoxicity, antimicrobial activity, and binding capacity for pathophysiological factors in chronic wounds of alginate and silver-containing alginate. Wound Repair Regen. 2009, 17, 511–521.
- Poor, A.E.; Ercan, U.K.; Yost, A.; Brooks, A.D.; Joshi, S.G. Control of multi-drug-resistant pathogens with non-thermal-plasma-treated alginate wound dressing. Surg. Infect. 2014, 15, 233–243.
- Salachna, P.; Grzeszczuk, M.; Miller, E.; Soból, M. Oligo-alginate with low molecular mass improves growth and physiological activity of Eucomisa utumnalis under salinity stress. Molecules 2018, 23, 812.
- Golkar, P.; Taghizadeh, M.; Noormohammadi, A. Effect of sodium alginate on secondary metabolites and antioxidant activity of safflower genotypes under in vitro salinity stress. In Vitro Cell Dev. Biol.-Plant 2019, 55, 527–538.
- Teixeira da Silva, J.A. Ornamental chrysanthemums: Improvement by biotechnology. Plant Cell Tissue Organ Cult. 2004, 79, 1–18.
- Teixeira da Silva, J.A.; Kulus, D. Chrysanthemum biotechnology: Discoveries from the recent literature. Folia Hortic. 2014, 26, 67–77.
- Zalewska, M.; Lema-Rumińska, J.; Miler, N. In vitro propagation using adventitious buds technique as a source of new variability in chrysanthemum. Sci. Hortic. 2007, 113, 70–73.
- Kulus, D.; Zalewska, M. In vitro plant recovery from alginate-encapsulated chrysanthemum x grandiflorum (Ramat.) Kitam. shoot tips. Propag. Ornam. Plants 2014, 14, 3–12.
- Zalewska, M.; Tymoszuk, A.; Miler, N. New Chrysanthemum cultivars as a result of in vitro mutagenesis with the application of different explant types. Acta Sci. Pol. Hort. Cult. 2011, 10, 109–123.
- Hill, G.P. Shoot formation in tissue cultures of chrysanthemum Chrysanthemum morifolium Ramat. JPN J. Breed. 1968, 42, 386–389.
- Jevremović, S.; Radojević, L. In vitro plant regeneration from stem segments of several cultivars of chrysanthemum (Chrysanthemum morifolium Ramat. ) Bull. Inst. Jard. Bot. Univ. Belgrade 1995, 29, 107–114.
- Jevremović, S.; Subotić, A.; Miljković, D.; Trifunović, M.; Petrić, M.; Cingel, A. Clonal fidelity of chrysanthemum cultivars after long term micropropagation by stem culture. Acta Hortic. 2012, 961, 211–216.
- Jevremovic, S.; Subotic, A. Micropropagation of chrysanthemum cultivars in Serbia. In Proceedings of the IX International Scientific Agriculture Symposium, Agrosym 2018, Jahorina, Bosnia and Herzegovina, 4–7 October 2018; Kovacevic, D., Ed.; University of East Sarajevo, Faculty of Agriculture: Sarajevo, Bosnia and Herzegovina, 2018; pp. 408–413.
- Teixeira da Silva, J.A.; Kim, H.; Engelmann, F. Chrysanthemum low-temperature storage and cryopreservation: A review. Plant Cell Tissue Organ Cult. 2015, 120, 423–440.
- Rout, G.R.; Das, P. Recent trends in the biotechnology of Chrysanthemum: A crucial review. Sci. Hortic. 1997, 69, 239–257.
- Teixeira da Silva, J.A. Chrysanthemum: Advances in tissue culture, cryopreservation, postharvest technology, genetics and transgenic biotechnology. Biotechnol. Adv. 2003, 21, 715–766.
- Kulus, D. Application of synthetic seeds in propagation, storage, and preservation of Asteraceae plant species. In Synthetic Seeds, Germplasm Regeneration, Preservation and Prospects; Faisal, M., Alatar, A.A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 155–179.
- Pinker, I.; Abdel-Rahman, S.S. Artificial seeds for propagation of Dendrantema × grandiflora (Ramat.). Propag. Ornam. Plants 2005, 5, 186–191.
- Hung, C.D.; Dung, C.D. Production of chrysanthemum synthetic seeds under non-aseptic conditions for direct transfer to commercial greenhouses. Plant Cell Tissue Organ Cult. 2015, 122, 639–648.
- Reddy, M.C.; Rama Murthy, K.S.; Pullaiah, T. Synthetic seeds: A review in agriculture and forestry. Afr. J. Biotechnol. 2012, 11, 14254–14275.
- Rihan, H.Z.; Al-Issawi, M.; Al-Swedi, F.; Fuller, M.P. The effect of using PPM (Plant preservative mixture) on the development of cauliflower microshoots and the quality of the artificial seed produced. Sci. Hortic. 2012, 141, 47–52.
- Adamovich, I.; Baalrud, S.D.; Bogaerts, A.; Bruggeman, P.J.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.G.; Favia, P.; et al. The Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2017, 50, 323001.
- Weltmann, K.-D.; von Woedtke, T. Plasma medicine—current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031.
- Laroussi, M.; Kong, M.G.; Morfill, G.; Stolz, W. (Eds). Plasma Medicine Applications of Low-Temperature Gas Plasmas in Medicine and Biology; Cambridge University Press: Cambridge, UK, 2012.
- Lazović, S.; Puač, N.; Miletić, M.; Pavlica, D.; Jovanović, M.; Bugarski, D.; Mojsilović, S.; Maletić, D.; Malović, G.; Milenković, P.; et al. The effect of a plasma needle on bacteria in planktonic samples and on peripheral blood mesenchymal stemcells. New J. Phys. 2010, 12, 083037.
- Puac, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Proc. Polym. 2018, 15, 1700174.
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma agriculture from laboratory to farm: A review. Processes 2020, 8, 1002.
- Živković, S.; Puač, N.; Giba, Z.; Grubišić, D.; Petrović, Z.L. The stimulatory effect of non-equilibrium (low temperature) air plasma pretreatment on light-induced germination of Paulownia tomentosa seeds. Seed Sci. Tech. 2004, 32, 693–701.
- Sera, B.; Stranák, V.; Serý, M.; Tichý, M.; Spatenka, P. Germination of Chenopodium album in response to microwave plasma treatment. Plasma Sci. Technol. 2008, 10, 506.
- Lu, X.; Reuter, S.; Laroussi, M.; Liu, D. Non Equilibrium Atmospheric Pressure Plasma Jets: Fundamentals, Diagnostics, and Medical Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–426.
- Waskow, A.; Avino, F.; Howling, A.; Furno, I. Entering the plasma agriculture field: An attempt to standardize protocols for plasma treatment of seeds. Plasma Process Polym. 2022, 19, e2100152.
- Motyka-Pomagruk, A.; Dzimitrowicz, A.; Orlowski, J.; Babinska, W.; Terefinko, D.; Rychlowski, M.; Prusinski, M.; Pohl, P.; Lojkowska, E.; Jamroz, P.; et al. Implementation of a non-thermal atmospheric pressure plasma for eradication of plant pathogens from a surface of economically important seeds. Int. J. Mol. Sci. 2021, 22, 79256.
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84.
- Bradu, C.; Kutasi, K.; Magureanu, M.; Puač, N.; Živković, S. Reactive nitrogen species in plasma-activated water: Generation, chemistry and application in agriculture. J. Phys. D Appl. Phys. 2020, 53, 223001.
- Matthes, R.; Bender, C.; Schlüter, R.; Koban, I.; Bussiahn, R.; Reuter, S.; Lademann, J.; Weltmann, K.D.; Kramer, A. Antimicrobial efficacy of two surface barrier discharges with air plasma against in vitro biofilms. PLoS ONE 2013, 8, 70462.
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Driori, E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci. Rep. 2012, 2, 741.
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832.
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Giedrė, P.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of Common Sunflower (Helianthus annus L.) Seeds with radio-frequency electromagnetic field and cold plasma induces changes in seed phytohormone balance, seedling development and leaf protein expression. Sci. Rep. 2019, 9, 6437.
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kalináková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem. Plasma Process. 2016, 36, 398–414.
- Waskow, A.; Betschart, J.; Butscher, D.; Oberbossel, G.; Klöti, D.; Büttner-Mainik, A.; Adamcik, J.; von Rohr, P.R.; Schuppler, M. Characterization of efficiency and mechanisms of cold atmospheric pressure plasma decontamination of seeds for sprout sroduction. Front. Microbiol. 2018, 9, 3164.
- Liu, B.; Honnorat, B.; Yang, H.; Arancibia, J.; Rajjou, L.; Rousseau, A. Non-thermal DBD plasma array on seed germination of different plant species. J. Phys. D Appl. Phys. 2019, 52, 025401.
- Chen, H.H.; Chang, H.C.; Chen, Y.K.; Hung, C.L.; Lin, S.Y.; Chen, Y.S. An improved process for high nutrition of germinated brown rice production: Low-pressure plasma. Food Chem. 2016, 191, 120–127.
- Brandenburg, R. Dielectric barrier discharges: Progress on plasma. Plasma Sources Sci. Technol. 2017, 26, 053001.
- Winter, J.; Brandenburg, R.; Weltmann, K.D. Atmospheric pressure plasma jets: An overview of devices and new directions. Plasma Sources Sci. Technol. 2015, 24, 064001.
- Bruggeman, P.; Brandenburg, R. Atmospheric pressure discharge filaments and microplasmas: Physics, chemistry and diagnostics. J. Phys. D Appl. Phys. 2013, 46, 464001.
- Janić Hajnal, E.; Vukić, M.; Pezo, L.; Orčić, D.; Puač, N.; Škoro, N.; Milidrag, A.; Šoronja Simović, D. Effect of atmospheric cold plasma treatments on reduction of alternaria toxins content in wheat flour. Toxins 2019, 11, 704.
- Sakiyama, Y.; Graves, D.B.; Chang, H.-W.; Shimizu, T.; Morfill, G.E. Plasma chemistry model of surface microdischarge in humid air and dynamics of reactive neutral species. J. Phys. D Appl. Phys. 2012, 45, 425201.
- Tang, Q.; Jiang, W.; Cheng, Y.; Lin, S.; Lim, T.M.; Xiong, J. Generation of Reactive Species by Gas-Phase Dielectric Barrier Discharges. Ind. Eng. Chem. Res. 2011, 50, 9839–9846.
- Kozlov, K.V.; Brandenburg, R.; Wagner, H.-E.; Morozov, A.M.; Michel, P. Investigation of the filamentary and diffuse mode of barrier discharges in N2/O2 mixtures at atmospheric pressure by cross-correlation spectroscopy. J. Phys. D Appl. Phys. 2005, 38, 518–529.
- Shang, K.; Wang, M.; Peng, B.; Li, J.; Lu, N.; Jiang, N.; Wu, Y. Characterization of a novel volume-surface DBD reactor: Discharge characteristics, ozone production and benzene degradation. J. Phys. D Appl. Phys. 2020, 53, 065201.
- Jiang, N.; Guo, L.; Qiu, C.; Zhang, Y.; Shang, K.; Lu, N.; Li, J.; Wu, Y. Reactive species distribution characteristics and toluene destruction in the three-electrode DBD reactor energized by different pulsed modes. Chem. Eng. J. 2018, 350, 12–19.
- Liberatore, C.M.; Rodolfi Beghèa, D.; Fabbri, A.; Ganino, T.; Chianconea, B. Adventitious shoot organogenesis and encapsulation technology in hop (Humulus lupulus L.). Sci. Hortic. 2020, 270, 109416.
- Benelli, C. Encapsulation of shoot tips and nodal segments for in vitro storage of “kober 5BB” grapevine rootstock. Horticulturae 2016, 10, 185.
- Hung, C.D.; Trueman, S.J. Encapsulation technology for short term preservation and germplasm distribution of the African mahogany Khaya senegalensis. Plant Cell Tissue Organ. Cult. 2011, 107, 397–405.
- Hung, C.D.; Trueman, S.J. Alginate encapsulation of shoot tips and nodal segments for short term storage and distribution of the eucalypt Corymbia torelliana × C. citriodora. Acta Physiol. Plant. 2012, 34, 117–128.
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020.




