Implementation of the surface dielectric barrier discharge (SDBD) plasma treatment before sowing represents a promising strategy for future investigations and sustainable use of cold plasma in synseed biotechnology. Plasma-treated chrysanthemum synseeds showed a better survival rate and overall plantlet growth under greenhouse conditions in comparison to untreated synseeds.
1. Introduction
Synthetic seed technology is one of the most promising tools in plant biotechnology and may represent an innovative method for massive plant production and sustainable agriculture in the future
[1]. Synthetic seeds (artificial seeds or synseeds) have been defined as artificially encapsulated somatic embryos or other non-embryogenic vegetative parts of plants, mainly in alginate, that may be used for storage or sowing under in vitro or ex vitro conditions
[2]. The term ‘synseeds’ was described by Murashige in 1977
[3] as ‘an encapsulated single somatic embryo’, but later, the definition of artificial seeds was extended to any artificially coated micropropagules that have capability to be sown as a seed and converted into a plant
[4][5]. There is a growing trend in applications of synseed technology for medium- and long-term storage of plant material under aseptic conditions
[6][7] or as an advanced procedure of cryopreservation by encapsulation–dehydration and encapsulation–vitrification method
[8][9]. Synseed technology represents an efficient alternative technique for propagation and germplasm conservation of valuable forest, medicinal and vegetable plant species that reproduce mainly vegetatively or have a problem in seed propagation, i.e., plants that produce non-viable seeds or seedless plants
[10].
In synseed technology, an alginate capsule has two roles: (i) it acts as physical barrier of shoot tips against mechanical damage, and (ii) it serves as an artificial endosperm, carbon source and reservoir of nutrients for better survival and supply of energy
[9]. Alginates are a group of naturally occurring anionic polysaccharides derived from brown algae cell walls (
Macrocystis pyrifera,
Limnaris hyperborea,
Ascophyllum nodosum) and several bacterial strains (
Azotobacter,
Pseudomonas). Sodium alginate is soluble in water, but when the sodium is replaced with calcium, the ionic bond with calcium cross links the polymer chain in alginate, which results in the formation of an insoluble gel. Sodium alginate and calcium salt are reported to be the best combination for encapsulation, representing the most successful and widely accepted approach to synseed production
[9]. Alginates can be formed into diverse semisolid or solid structures because of their ability of sol/gel transition and are commonly used as viscosity-increasing agents, thickeners and suspension and emulsion stabilizers in food and the pharmaceutical industry (code E400-E405)
[11]. In addition, alginate gels are the basis for a variety of wound dressings that have showed variety of therapeutically effects, such as hemostatic and bacteriostatic properties
[12][13]. On the other hand, in plants, sodium alginate is considered a potential elicitor that improves tolerance to plant environmental stresses, such as drought, inhibiting plant infections and reducing the toxic effect of heavy metals
[14][15].
Chrysanthemums (
Chrysanthemum morifolium Ramat. syn.
C. grandiflorum Kitam) are, besides roses, the most important economically ornamental crop in the world
[16]. They originate from east Asia, a center of their biodiversity; however, to date, many horticultural varieties and cultivars of chrysanthemums are produced using different biotechnological tools
[17]. The name chrysanthemum means gold flower, but they are also called “autumn roses” because they were, in the past, used as cut flowers during late summer and autumn. Nowadays, there is constant demand on the market for new cultivars that are available during the whole year. Modern biotechnological tools, such as mutation breeding and micropropagation under in vitro conditions, allow for production of hundreds of new chrysanthemum cultivars every year
[18]. Chrysanthemum cultivars are commonly propagated vegetatively by cuttings and suckers and stored as field, greenhouse or in vitro collections due to high spontaneous mutation rates and high levels of ploidy and self-incompatibility
[19][20]. Micropropagation of chrysanthemum cultivars, as an in vitro way of vegetative multiplication in culture, was reported for the first time more than 50 years ago
[21], and numerous reports about plantlet regeneration from various explants of chrysanthemum have been presented
[16][22][23][24].
The application of synseed technology, accompanied by micropropagation, represents a perfect biotechnological approach that could be used for agricultural improvement of year-round plant production of chrysanthemums. There are several advantages of this approach, including large-scale production; easy handling; short and medium storage (4 °C) or low temperature (−196 °C) storage; easy transportation; and the genetically true-to-type nature of the plants produced from synseeds. On the other hand, there are some limitations of wider usage of synseed technology in commercial applications as published to date, such as implementation of labor-intensive procedures, which include double-layer encapsulation or several media changes to derived plantlets with well-developed shoot and roots at the same time. To date, the application of synseed technology of chrysanthemum cultivars has been investigated for in vitro storage and ex vitro planting (summarized in
Table 1). In addition, synseed technology in chrysanthemums is widely used as a part of encapsulation–dehydration and encapsulation–vitrification protocols for long-term storage of chrysanthemum cultivars by cryopreservation in liquid nitrogen
[25]. Considering the fact that chrysanthemums are susceptible to mutations, meristem explants (i.e., nodal segments or shoot tips) proved to be the best explant choice for the plant propagation of chrysanthemums, with a high degree of clonal fidelity as mother plants, as well as for synseed production
[26][27][28].
Table 1. Application of synseed technology for storage and propagation of chrysanthemum cultivars.
| Cultivar |
Plant Material |
Beads |
Sowing |
Germination
(%) |
Flowering |
References |
| Clone ‘PS 27’ |
Nodal segments
(in vitro) |
Monolayered
(3% Na-alginate + 0.1 mg/L IAA) |
Sterile,
water–sand |
50 |
no |
[29] |
Double-layered
(beads: 3% Na alginate, second layer: water) |
Non-sterile, water–perlite |
45 |
no |
| Lady group |
Shoot tips
(in vitro) |
Monolayered
(3% Na-alginate) |
Sterile, agar |
52 |
no |
[19] |
| cv. ‘Royal Purple’ |
Shoot tips
(ex vitro) |
Monolayered
(2.5% Na-alginate, sucrose, vitamin free) |
Non-sterile, vermiculite |
34 |
no |
[30] |
Chrysanthemum synseeds are mainly produced under sterile conditions for short- and long-term storage
[19], but sowing of chrysanthemum synthetic seeds under non-aseptic conditions has been also reported
[29][30]. Chrysanthemum synseeds easily regrow from Na-alginate beads under sterile conditions, whereas for complete germination and whole-plantlet development (shoot and root), it is necessary to add indole-acetic acid to the encapsulation matrix
[29] or, as separate step, in the medium for rooting after shoot regrowth
[19]. For sowing under unsterile conditions, the results showed that presence of organic compounds in the gelling matrix and commercial substrates caused microbial contamination in all synseeds and complete inhibition of further regrowth of the shoots or whole plantlet development. In general, difficulties of sowing artificial seeds directly in soil or in commercial substrates, such as compost, vermiculite, etc., under non-sterile conditions are considered to be one of the main limitations for the widescale practical application of synseed technology
[2][31]. Some progress has been achieved by using chemical mixtures and antibiotics for preservation of synseeds before sowing
[32], but more investigations and novel approaches are still needed to improve the capacity of synseed cultivation under non-sterile conditions.
Atmospheric pressure plasma (non-thermal, “cold”) systems have been extensively used in biomedical applications for almost two decades
[33][34][35][36]. In parallel, another field of plasma applications has been growing-plasma agriculture
[37][38]. One of the first applications of cold plasmas was the treatments of conventional seeds
[39][40]. This includes various applications in seed treatment with several purposes
[41][42][43]. Many authors have shown that plasma treatments can increase seed germination and speed up the whole process of plantlet development
[37][38][39][40]. The rich plasma chemistry (with reactive oxygen and nitrogen species) interacts with the seed coating and triggers various responses, such as increasing water uptake, changes in the surface of seeds’ coating and elimination of pathogens on seed surfaces
[44][45][46][47][48][49][50][51][52][53]. In this sense, cold plasma treatment can have a multiply positive impact on seed germination and subsequent plant development of conventional seeds without the addition of chemicals that can be harmful for the environment.
Nowadays, there is a plethora of plasma sources that operate at atmospheric pressure
[54][55][56][57]. They differ in electrode design, type of applied voltage, feeding gas, etc. The variety of atmospheric pressure plasma sources enables a large number of possible applications, but at the same time, comparison between the results of treatment is difficult. Therefore, it is of the utmost importance to obtain detailed characteristics of the plasma device that is used in experiments. One of the first steps that is usually performed includes electrical characterization of the discharge, accompanied by optical emission spectroscopy, which can give insight into the plasma-excited species. These diagnostic techniques represent only the starting point for a detailed description of plasma characteristics and include mass spectrometry, fast imaging, laser-induced fluorescence, etc.
[56].
2. Current Insights
The researchers used air
surface dielectric barrier discharge (SDBD
) because of the plane-parallel geometry, large effective plasma surface and possibility to operate with only air as a feeding gas. When the discharge was ignited, streamers were formed at temporary random points in the form of microfilaments, and they conducted higher discharge current than the rest of the discharge. Because the researchers were not treating plant cells directly but the sodium-alginate-encapsulated plant material, the samples could withstand these local inhomogeneities in the active plasma volume without any damage. Nevertheless, due to the nature of an SDBD source, the total current of filaments was limited, preventing formation of current hot-spots. Another reason for choosing this type of plasma source was its simplicity, both in design and in application. It did not require the addition of feeding gas, and, as an important feature for the future technology, it has a potential for scaling up. Detailed electrical characterization of the SDBD and optical emission spectra was presented where the plasma source, with its plan-parallel geometry, served as a capacitance in the electrical circuit. Regarding this, the researchers used a simple and reliable method to measure the stray capacitance, C
p, for different distances,
d, between upper and lower electrode segments. Results showed that with the increase in the distance between the powered electrode and grounded bottom electrode, the researchers had an increase in the system capacitance, which was expected (
Table 2). The obtained C
p values allowed for determination of the displacement current of each input voltage. The measured current signals included both the displacement and discharge current. Thus, after subtracting the displacement current, all current waveforms represented only current through the discharge. The capacitance determined for each
d enabled calculation of the discharge current for all possible configurations of the plasma source.
Table 2. Values of stray capacitance in the SDBD system.
| Electrode Distance-d [mm] |
Stray Capacitance—Cp [pF] |
| 2 |
20 |
| 3 |
35 |
| 4 |
41 |
| 5 |
45 |
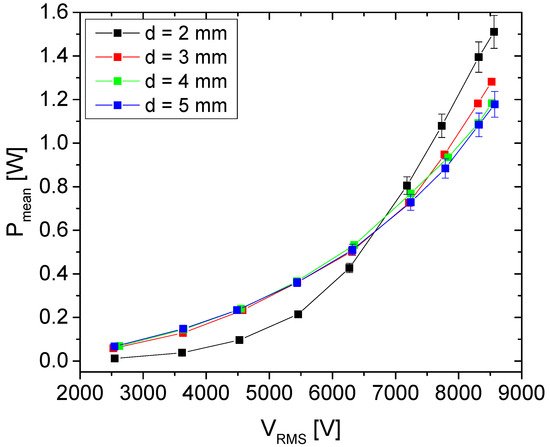
With respect to application, one of the most important macro parameters in plasma treatments is power deposited to the discharge. This macro parameter that can be easily monitored; it reflects the electron density and temperature and, to some extent, through these two parameters, plasma chemistry
[58]. P
mean (
Figure 1) depends on the distance between electrodes, as well as formation of microfilaments. Thus, the highest increase and mean power values were achieved for d = 2 mm. For this distance, the researchers chose to treat the synseeds at a power of 1.1 W. This showed to be the optimal value with respect to the effect on the seeds for the three treatment times that the researchers used.
Figure 1. Mean power transmitted to the discharge as a function of the voltage at the powered electrode.
Apart from power, the feeding gas (in this case air) and humidity can also play an important role in plasma chemistry
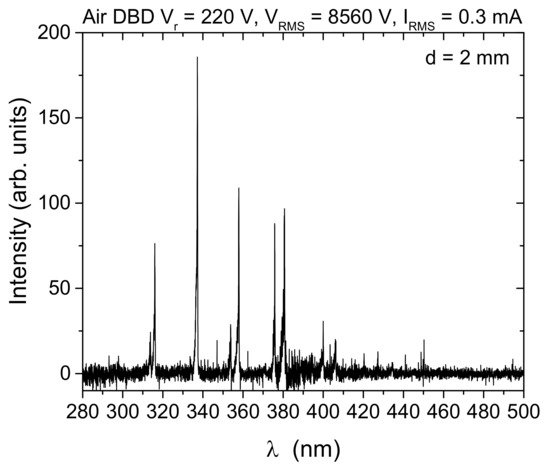
[59]. Although humidity in the treatment environment was not controlled, all experiments were performed in a room with constant humidity. Additionally, measurements of discharge characterization, as well as all treatments, were repeated several times in order to verify the reproducibility of the measurements. All measurements were performed with and without synseed samples. One way to obtain an insight into the chemical reactions occurring in the discharge is optical emission spectroscopy, as it can show the existence of certain excited species. In this experiment, the spectra were recorded for different RMS voltages and interelectrode distances without samples, but all have the same lines belonging only to the N
2 Second Positive System, SPS (
Figure 2). Absence of the lines of, e.g., NO, OH and atomic oxygen, from the spectrum of an air DBD has been noted before and is related to dominant excitation and quenching reactions that favor N
2 excitation in filamentary discharges
[11][12][59][60]. Moreover, other important reactive species, such as O
3, do not have emissions in the spectral range investigated. Nevertheless, these kinds of plasma sources generate a large amount of ozone and N
2O that is important for treatments of alginate surfaces of synseeds
[13][14][61][62].
Figure 2. Optical emission spectrum from an air SDBD source obtained from side-on recording of spatially integrated emissions from the whole discharge volume (electrode gap, d = 2 mm). The intensity signal is corrected for spectral efficiency of the optical system.
The effect of plasma treatment (10 min) on synseed germination of different chrysanthemum cultivars was evaluated (Table 3). The researchers have found that the plasma treatment significantly enhance the process of synseeds germination and conversion to plantlet after direct sowing in soil for all tested cultivars. This effect of plasma was cultivar dependent (Table 4). Without plasma treatment of chrysanthemum synseeds, frequency of whole plantlet regeneration varied from 6-28% depending on the cultivar. On the other hand, plantlet regeneration from plasma-treated synseeds were 22-49%. The highest treatment effect on whole plantlet development were recorded for cultivars BC and PP (~370% and ~350%, respectively) in comparison to control synseeds, while the lowest values were obtained for the PP cultivar (~160%).
Table 3. The effect of plasma treatment on synseed germination of and plantlet development of different chrysanthemum synseeds grown ex vitrocultivars.
Plasma Treatment
(min) |
Plantlet (%) |
| BC * |
Q * |
PC * |
PP * |
| 0 |
6 ± 1 a ** |
17 ± 2 a |
28 ± 1 a |
14 ± 1 a |
| 10 |
22 ± 6 b |
40 ± 3 b |
44 ± 2 b |
49 ± 3 b |
| Increment (%) |
~370 |
~230 |
~160 |
~350 |
| Leaf Emergence * |
|---|
(%) |
Shoot Regrowth *
(%) |
Plantlet *
(%) |
|---|
|
0
|
60 ± 7 a **
|
33 ± 7 a
|
17 ± 5 a
|
|
10
|
66 ± 5 a
|
60 ± 5 b
|
41 ± 5 b
|
* Leaf emergence is evaluated as first sign of shoot appearance out of alginate beads one week after sowing; shoot regrowth was evaluated as a fully developed shoot out of the bead three weeks after sowing; plantlet development was recorded as a fully developed plant with well-developed shoot and roots. ** Values represent mean ± standard error. The data signed with a different letter within the same column are significantly different according to Fisher’s LSD test.
The researchers found that the plasma treatment significantly enhanced synseed germination and complete plantlet development for all investigated chrysanthemum cultivars. The response to plasma treatment of chrysanthemum synseeds was cultivar-dependent. Observed differences could be attributed to the fact that different chrysanthemum cultivars have distinct nutritional requirements, as was reported earlier for other chrysanthemum cultivars
[19][30]. According to the results, no morphological disorders were noticed among plantlets derived from untreated and plasma-treated synseeds. The absence of any morphological or flower color alterations in chrysanthemum plants may be explained as a consequence of the regeneration protocol used in this research. First, the researchers used stock shoot cultures derived from one mother plant. In addition, initiation of shoot regeneration was mainly achieved by direct shoot induction on the initial explant, avoiding a callus phase and further shoot multiplication by axillary meristem activation, which minimized possibilities for genetic changes due to somaclonal variations
[16][17]. According to available data, this research represents first data about complete chrysanthemum plantlet regeneration from synseeds to flowering plants.
Data about application of synseed technology for short- and long-term storage in vitro or easy transport of valuable genetic resources are available
[63][64], whereas data regarding synseed manipulations for ex vitro growth are quite scarce
[65][66]. In chrysanthemum, plantlet development from synseeds formed in vitro and sowing ex vitro was successfully achieved from double-layered synseeds
[29]. In the current work, the researchers planted untreated and plasma-treated simple, one-layer chrysanthemum synseeds directly in soil substrate. After three weeks of ex vitro cultivation of chrysanthemum synseeds, two-fold higher shoot development was observed in the case of plasma-treated chrysanthemum synseeds in comparison to untreated synseeds. Additionally, after six weeks of growth under ex vitro conditions, plasma-treated chrysanthemum synseeds showed significantly higher plantlet conversion compared to the untreated control. The enhanced survival, regrowth and further complete plantlet formation of plasma-treated chrysanthemum synseeds shown in the research might be explained, besides by antimicrobial effect, by the prolonged effects of chemical changes in the alginate gels after plasma treatment and their antimicrobial properties. Similar effects were reported for plasma treatment of alginate wound dressings
[12]. Plasma treatment of alginate gels inactivated bacterial and fungal infection for a month, which was a long enough period for successful shoot development and complete conversion of synseeds to plantlets. On the other hand, continued growth of untreated chrysanthemum synseeds was significantly reduced due to contamination and lack of adventitious root formation for other plant species
[9]. The researchers examined the germination of plasma-treated and untreated chrysanthemum synseeds, as well as the subsequent growth and development of plantlets after direct sowing in soil substrate (ex vitro conditions) (
Table 4).
Table 4. The effect of plasma treatment on germination of chrysanthemum synseeds grown ex vitro.
| Plasma Treatment (min) |
Synseed Germination |
Leaf Emergence *
(%) |
Shoot Regrowth *
(%) |
Plantlet *
(%) |
| 0 |
60 ± 7 a ** |
33 ± 7 a |
17 ± 5 a |
| 10 |
66 ± 5 a |
60 ± 5 b |
41 ± 5 b |
| Plasma Treatment (min) |
Synseed Germination |
|---|
Leaf Emergence *
(%) |
Shoot Regrowth *
(%) |
Plantlet *
(%) |
| 0 |
60 ± 7 a ** |
33 ± 7 a |
17 ± 5 a |
| 10 |
66 ± 5 a |
60 ± 5 b |
41 ± 5 b |
* Leaf emergence is evaluated as first sign of shoot appearance out of alginate beads one week after sowing; shoot regrowth was evaluated as a fully developed shoot out of the bead three weeks after sowing; plantlet development was recorded as a fully developed plant with well-developed shoot and roots. ** Values represent mean ± standard error. The data signed with a different letter within the same column are significantly different according to Fisher’s LSD test.
During direct sowing of synseeds, contamination by microorganisms is one of the major hurdles for the commercialization of encapsulation technology for many plant species
[9]. Besides that, one of the main limiting factors for plantlet conversion is low-nutrient availability due to inhibition of root growth. Numerous factors are involved in this process, such as poor rooting ability and survival due to the lack of nutrients and oxygen supply. Organic nutrients released by the beads are mainly responsible for severe contamination of synseeds
[29][30]. Unfortunately, the depletion of nutritional compounds in beads may cause lower shoot regrowth or complete growth inhibition
[28][29][30]. To date, there are two strategies to overcome this problem in chrysanthemum synseeds. The first strategy is to use double-layered synseeds to restrict contamination, where the second layer is formed by Ca-alginate made with water
[29]. A recently reported strategy for both production and sowing of chrysanthemum synseeds in non-aseptic conditions proposes eliminating all carbon sources and organic additives both inside and outside the synseeds
[30]. The reported strategy might be promising, but it was applied to one cultivar only, and it is questionable whether it is applicable to other cultivars. Therefore, it is necessary to build up a system that lowers contamination and keeps a nutrient reservoir within the encapsulated plant tissue, which is necessary for successful rooting. Considering the results of the present research, this problem may be successfully solved by plasma treatment of chrysanthemum synseeds before sowing.
There are many research data that demonstrate that cold plasma has a potent general antimicrobial effect through its generation of free radicals, reactive oxygen species (ROS) and reactive nitrogen species, such as hydrogen peroxide, superoxide, singlet oxygen, nitric oxide and ammonia
[42]. The generated reactive species or their products are responsible for the antimicrobial effect and, in certain situations, have proven to be non-toxic to eukaryotic cells
[67]. The chemical changes produced in the gel are relatively stable, and the anti-microbial properties of such a gel may last for close to one month, as reported after plasma treatment of alginate wound dressings
[13]. The treated alginate gels inactivated all of the Gram-negative, Gram-positive and fungal pathogens by generated ROS inside bacterial cells, leading to their rapid death or triggering programmed cell death exhibiting characteristic features of apoptosis
[12]. According to the results, the researchers can conclude that treatment with non-thermal plasma generates chemical and physical responses in an alginate gel, producing changes that implicate not only biocidal effects but possibly growth-promoting effects.