Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pablo Jose Antunez-Muiños | -- | 2729 | 2022-04-09 15:59:43 | | | |
| 2 | Vivi Li | -1 word(s) | 2728 | 2022-04-11 05:31:36 | | | | |
| 3 | Vivi Li | + 2 word(s) | 2730 | 2022-04-11 08:39:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Antunez-Muiños, P.; Cruz-Gonzalez, I.; López-Tejero, S.; , . Mitral Paravalvular Leak. Encyclopedia. Available online: https://encyclopedia.pub/entry/21532 (accessed on 08 February 2026).
Antunez-Muiños P, Cruz-Gonzalez I, López-Tejero S, . Mitral Paravalvular Leak. Encyclopedia. Available at: https://encyclopedia.pub/entry/21532. Accessed February 08, 2026.
Antunez-Muiños, Pablo, Ignacio Cruz-Gonzalez, Sergio López-Tejero, . "Mitral Paravalvular Leak" Encyclopedia, https://encyclopedia.pub/entry/21532 (accessed February 08, 2026).
Antunez-Muiños, P., Cruz-Gonzalez, I., López-Tejero, S., & , . (2022, April 09). Mitral Paravalvular Leak. In Encyclopedia. https://encyclopedia.pub/entry/21532
Antunez-Muiños, Pablo, et al. "Mitral Paravalvular Leak." Encyclopedia. Web. 09 April, 2022.
Copy Citation
Paravalvular leak incidence after mitral surgical replacement ranges from 7% to 17%. Between 1% and 5% of these are clinically significant. Large PVLs can cause important clinical manifestations such as heart failure or haemolysis. Current guidelines consider that surgical reparation is the gold-standard therapy in symptomatic patients with paravalvular leak. However, these recommendations are based in non-randomized observational registries. On the other hand, transcatheter paravalvular leak closure has shown excellent results with a low rate of complications, and nowadays it is considered the first option in selected patients in some experienced centres.
paravalvular leaks
mitral regurgitation
heart failure and haemolytic anaemia
valvular prosthesis
percutaneous closure
1. Introduction
Paravalvular leak (PVL) is defined as the presence of any channel between the anatomical annulus and the prosthetic valve that causes a regurgitation jet between two chambers of the heart. As life expectancy continues to grow in developed countries, one of the consequences is that valvular heart disease is progressively more and more common. For example, in the United States or Europe, these pathologies affect up to 2.5% of the population, and prevalence is much higher in patients older than 75 years old. When severe valvular disease is established, surgical percutaneous valve replacement is usually needed. In Europe, mitral valve regurgitation and stenosis comprised 21% and 5% of all referrals for valve interventions. On the other hand, over recent years, in the United States more than 120,000 procedures, have been performed including at least 14,000 mitral surgical valve replacements [1]. After surgical intervention, different registries showed rates of PVL between 5% and 17%. Incidence of mitral valve replacement is higher than aortic ones, ranging from 7% to 17% and 2% to 10%, respectively [2][3]. The vast majority of these are diagnosed in the first year after surgery. Different risk factors of PVL have been identified such as heavy calcification of the annulus, the use of mechanical valves, non-pledged or continuous suture, endocarditis infection, larger atria or renal insufficiency. However, most PVLs are small and patients can remain asymptomatic. On the other hand, between 1 and 5% of them are clinically significant. Large PVLs can cause important clinical manifestations such as heart failure in almost 90% of the cases, or haemolysis in one-third of them, approximately.
2. Diagnosis
Diagnosis and characterization of PVLs are challenging. As researchers have discussed, it should be suspected when an onset of abnormal murmur appears at physical examination after a valvular replacement, especially in those patients who were admitted due to heart failure and/or haemolytic anaemia. In this situation, a transthoracic echocardiography (TTE) should be performed firstly. Rergardless, multimodal imaging is instrumental in guiding diagnostic and therapeutic strategies when managing PVL. These imaging techniques are summarized below and in the central illustration (Figure 1).
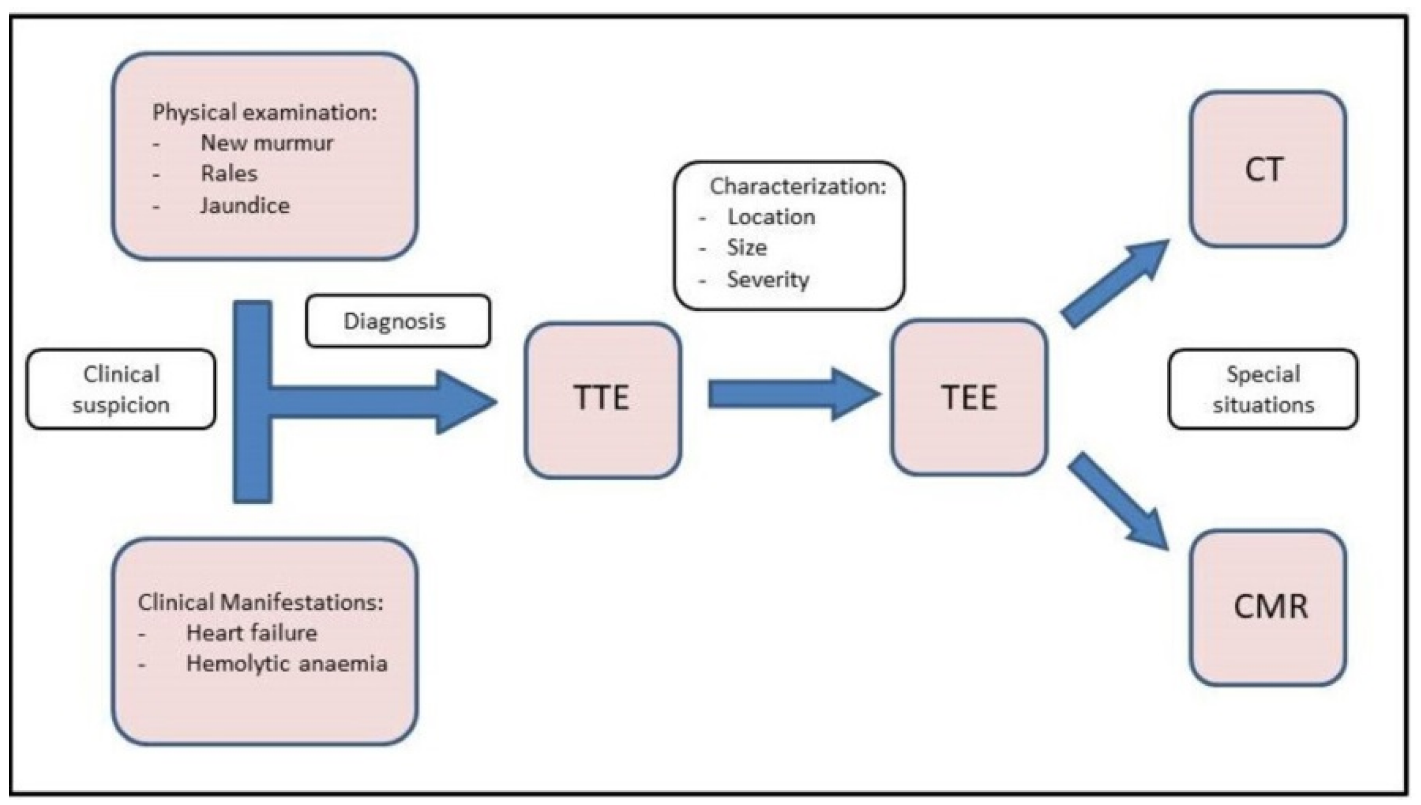
Figure 1. Central Illustration. Diagnostic flow chart. Diagnosis starts with clinical suspicion. TTE and TEE should be performed to confirm the diagnosis and correct characterization. Finally, CMR and CT can be used in special situations.
2.1. TTE
TTE is the initial diagnostic test of choice for all patients with suspected PVL. Although TTE is an excellent method for the assessment of valvular gradients, it is often limited by acoustic shadowing from mechanical components of prosthetic valves, annular calcification or prosthetic valve sewing rings [3]. Acoustic shadowing affects visualization of prosthetic valve components, and it may also result in the absence of colour Doppler signal with potential underestimation of the degree of PVL (Figure 2). This makes more difficult to identify the gradation of PVL [4]. At this point, Doppler evaluation can be a good tool to avoid underestimating PVL. In addition, a cardiac evaluation of atrial and ventricular size and function, pulmonary artery systolic pressure, and concomitant native valvular disease must be performed. It is important to investigate the presence of endocarditis due to its potential association with PVL.
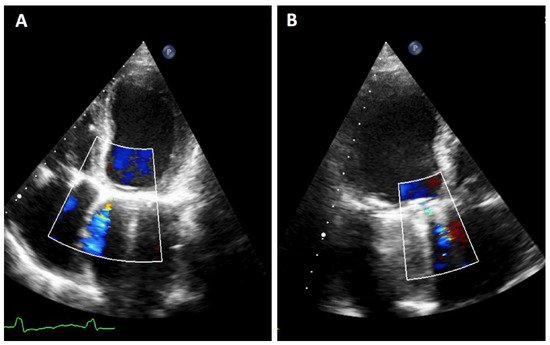
Figure 2. TTE PVL. Mitral regurgitation is detected. It must be noted that it is difficult to quantify the exact proportion of the flow in both planes due to artifacts as acoustic shadow. (A): 4-chamber image showing a mitral PVL with colour Doppler. (B): 3-chamber with acustic shadoiw in the left atrium and an anterior regurgigant jet of a PVL leak.
2.2. TEE
Transoesophageal echocardiography is the gold standard when performing an exhaustive analysis of the PVL that can further characterize the leak regurgitation location, size, and severity [5]. Two-dimensional (2D) TEE is very sensitive in identifying the presence of PVLs; however, assessing the number, shape and location can be difficult in some cases [2]. Three-dimensional (3D) TEE achieves better definition, and it has been shown to be superior to 2D-TEE to study PVLs [6][7][8]. 3D images allow researchers to find out the shape (crescent-shaped vs. round), valve dehiscence, the distance from the sewing ring, the orientation and movement of prosthetic leaflets and the degree of regurgitation as well as helping researchers to improve the identification and quantification of multiple regurgitant jets [9]. Indeed, 3D-TEE is the recommended technique to guide percutaneous PVL closure procedures (especially in mitral location), as well as playing an important role in selection of the most appropriate closure device [5][8][10][11]. Recently, photorealistic rendering views (True- Vue, Philips Healthcare, Best, Netherlands) have been developed to increase 3D perception, making it possible to change the lighting source to improve contrast and enhance details, which would make it easier to identify defects [12][13] (Figure 3).
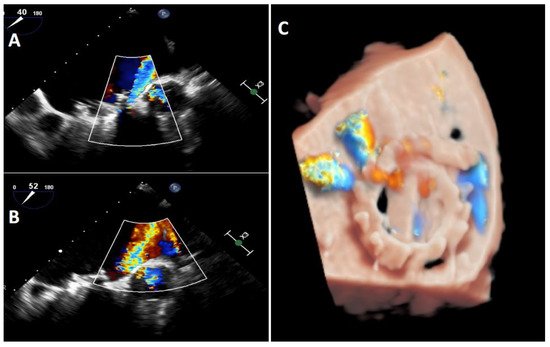
Figure 3. Mitral TEE. (A,B): regurgitation jet more severe that could be seen at TTE on figure (A); (C): Truevue with colour Doppler.
Nowadays, the clockwise format from “surgical view” is used to improve communication between interventional cardiologist and imaging specialists. In this scheme, the 12 o’clock position is at the mitral–aortic continuity, the left atrial appendage corresponds to the 9 o’clock position, and the interatrial septum is adjacent to the 3 o’clock position [14] (Figure 4).
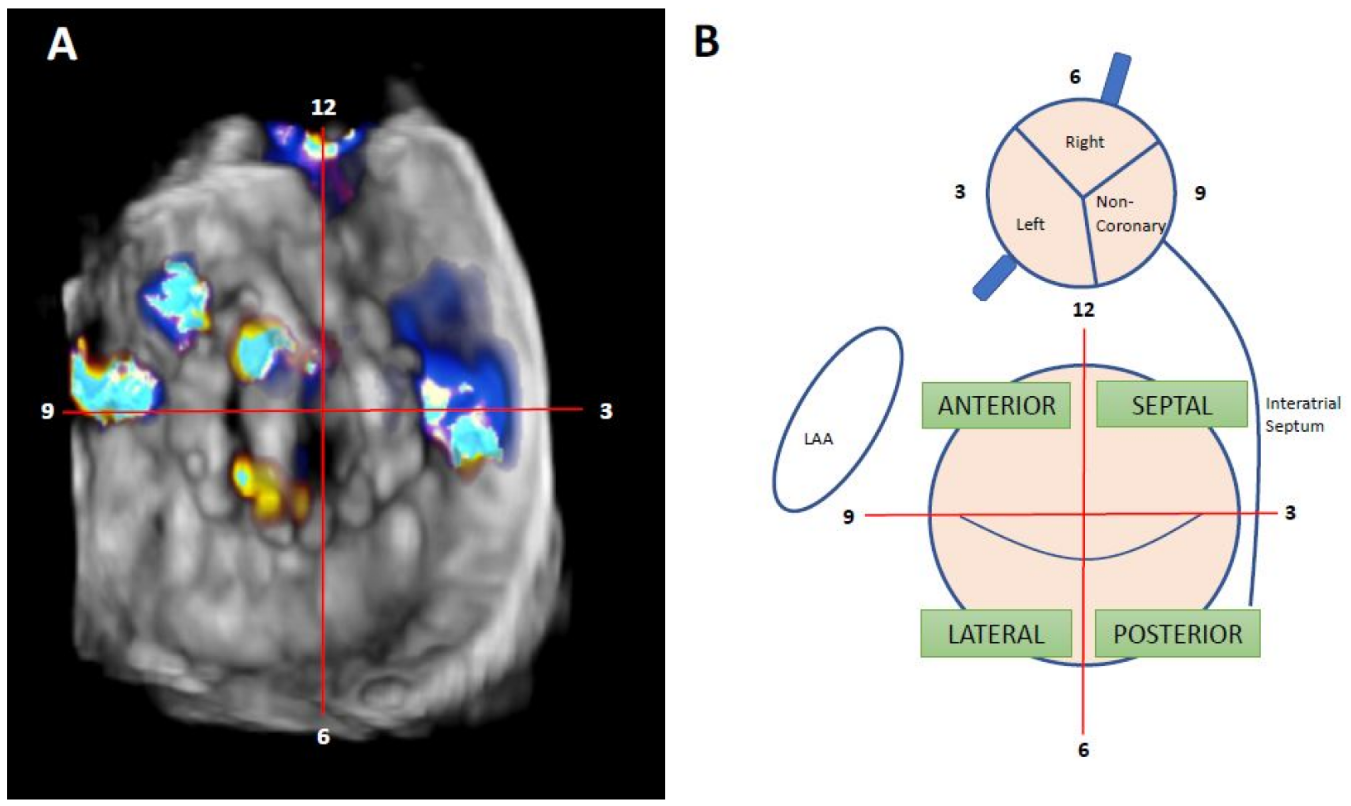
Figure 4. Localization of mitral PVL. (A): Clockwise format. Surgeon’s view. TEE from the same patients; it can be seen the presence of two anterolateral leaks, at 9 and 10 h, and one septal leak at 3 h. (B): mitral and aortic drawing of clockwise format and interactions between different hearts structures—adapted from reference [2].
The approach for detecting and grading prosthesis regurgitation is described in Table 1 and involves the evaluation of several echo parameters [3][14][15]:
Table 1. Assessment of PVL severity.
| MILD | MODERATE | SEVERE | |
|---|---|---|---|
| Colour Flow Area | <4 cm2, <20% LA area | Variable | >8 cm2, >40% LA area |
| Jet Density | Incomplete | Dense | Dense |
| Jet Contour | Parabolic | Variable | Early peaking, triangular, holosystolic |
| Pulmonary Venous Flow | Normal | Systolic blunting | Systolic flow reversal |
| PASP | Normal | Variable | Incremented |
| Vena contracta | <3 mm | 3–6.9 mm | >7 mm |
| Circumferential extent of PVL | <10% | 10–29% | >30% |
| Regurgitant Volume | <30 mL | 30–59 mL | >60 mL |
| Regurgitant Fraction * | <30% | 30–49% | >50% |
| EROA | <20 mm2 | 20–39 mm2 | >40 mm2 |
Mitral PVL quantification criteria. * Cardiac MR has the same values for this parameter. Adapted from reference [3].
Mitral PVL: qualitative parameters are used for mitral paravalvular regurgitation such as colour-flow regurgitant jet area, jet density, and systolic pulmonary venous flow reversal, a specific sign of severe mitral regurgitation. Due to the Coanda effect, the jet may be underestimated by jet area measurement. Quantitative parameters, such as vena contracta diameter and regurgitant volume and fraction, are also helpful. Although the proximal isovelocity surface area (PISA) approach has not been validated in the setting of paravalvular regurgitation, the presence of a large PISA could be consistent with more severe regurgitation.
Intracardiac echography (ICE) is less useful due to presence of acoustic shadowing, but it may be useful in certain instances (for example, patients which cannot be anesthetized, or who have oesophageal problems that forbid TEE, etc.). It can be performed without general anaesthesia, making procedures shorter and safer and further enabling the treatment of patients that may have been turned down for intervention. Ruparelia N. et al. reached acceptable procedural success rates (77.8%) with similar functional improvement and no related complications [16].
2.3. CMR
Cardiac MRI has a limited role within the diagnostic of PVL. Virtually all prosthetic valves (including mechanical valves) can be imaged by CMR [17]. Phase-contrast velocity mapping is performed in the short-axis plane just distal to the prosthetic valve, with subsequent quantitation of regurgitant volume and regurgitant fraction [18]. It can be useful in the presence of multiple and eccentric leaks or acoustic shadows that reduce the accuracy of echocardiography [3].
2.4. Cardiac CT
The most important contribution of cardiac CT is the capacity for anatomical characterization of PVL in patients with significantly limited echocardiographic images, which has an important role in pre-procedural planning [3]. Helical CT acquisition is performed in multiple phases with contrast injection protocols, and a reconstruction of theses phases is then processed. With adjustment of opacity and colour and applying cut-planes it is possible to visualize the PVL in great detail [15]. Despite these advantages, some evidence suggests that cardiac CT has no impact on PVL detection in comparison with 2D TEE [19].
In terms of fusion imaging, development of computed tomography (CT)–fluoroscopy fusion imaging has allowed CT imaging to provide a valuable tool for guidance during percutaneous PVL closure [20]. With this technique, single-phase CT data are reconstructed into 3D images. After that, they is co-registered with fluoroscopy and the relevant structures (such as the cardiac chambers, valves, sternotomy, etc) are overlaid onto the fluoroscopy screen. The CT data remain merged to fluoroscopy with rotation of the C-arm, providing real-time 3D anatomic information during the procedure. CT–fluoroscopy fusion can facilitate access, wire crossing, and device deployment during PVL closure [14]. This tool may be an important way to perform PVL closure in cases with special anatomical considerations, TEE ultrasound disturbance or X-ray translucent prothesis (Figure 5).
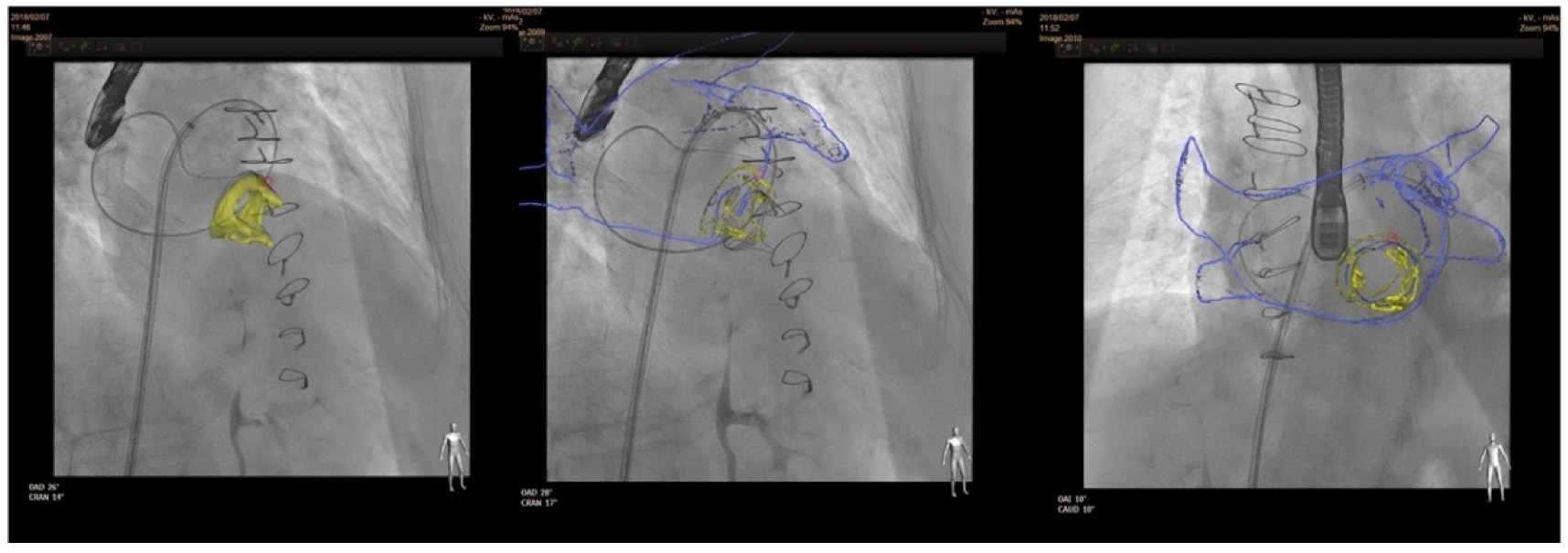
Figure 5. CT–fluoroscopy fusion for mitral PVL closure.
In selected complex cases, 3D printed models could be useful in the pre-procedure planning [21] (Figure 6).
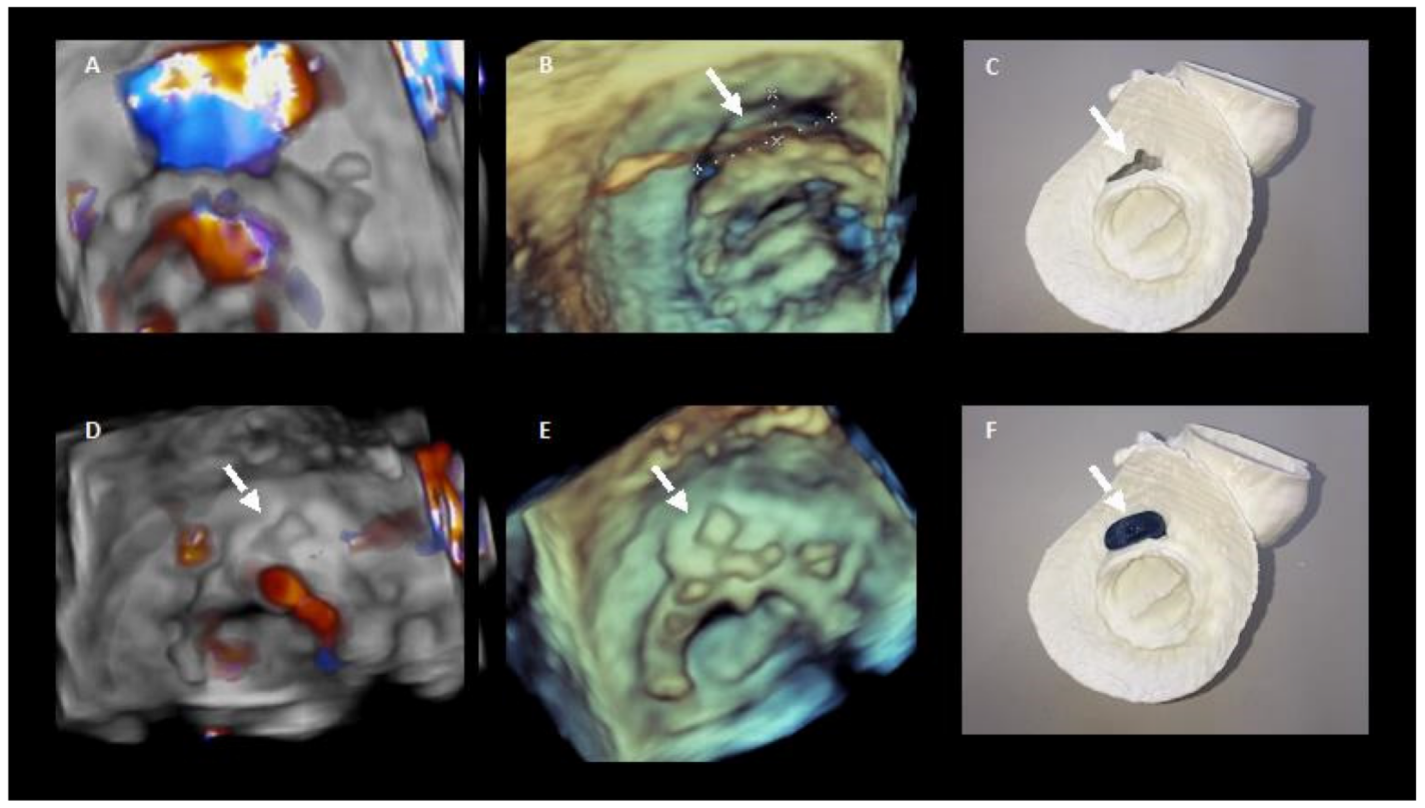
Figure 6. 3D printing in preprocedural planning of paravalvular leak closure. (A,B). 3D echocardiography images of the PVL. (D,E) 3D echocardiography images showing the result after percutaneous closure. (C,F) show the 3D model of the PVL and how it colud be closed with the device.
3. Management
3.1. Treatment
Medical management has a limited role in patients with symptomatic PVLs, as it cannot resolve the underlying cause.
Both ACC/AHA 2020 and ESC 2021 Guidelines consider that surgical reparation is the gold-standard therapy in symptomatic PVL [22][23]. However, the level of evidence in ESC guidelines is only C (expert consensus). Moreover, AHA bases its decision in non-randomized observational registries. On the other hand, transcatheter closure is recommended in those symptomatic cases with a high or prohibitive operatory risk. Both guidelines point out that the percutaneous optionshould be considered depending not only on the risk status of the patient but also in the leak morphology and the local expertise.
Transcatheter closure of PVL has shown excellent results in different cases with a low rate of complications. This approach is contraindicated in the presence of active endocarditis, prosthesis instability or large PVL affecting more than 30% of the circumference [5].
3.2. Devices
For this purpose, different devices can be used. Most of them consist of a waist with two discs at each end [2]. The Occlutech PLD has two different shapes, one is square and another is rectangular [24]. On the other hand, the Amplatzer Vascular Plug (AVP) was once designed to close peripheral vessels, but nowadays is the most frequently used device for PVL closure. The AVPII is the most common in the United States (US) and the AVPIII in Europe [1][25], as the AVPIII has not been approved by the FDA yet. Duct, atrial septal and muscular ventricular septal occluders can be also used for this procedure in selected cases. In this sense, the selection of the device must take in to account the shape and size of the PVL and the operators experience. AVPIII or Occlutech PLD devices may be used in oval leaks due to its rectangular shape. In contrast, square devices such as AVPII or Occlutech PLD can be useful in leaks with a more cylindrical shape. Those with a crescent shape could be closed with a rectangular device. These different devices are shown in Figure 7. In terms of sizing, researchers recommend to use devices at least 1–2 mm larger than the PVL maximum diameter; in some large PVLs, two devices can be deployed simultaneously, as previously described [5].

Figure 7. PVL closure devices. (a): Amplatzer Muscular VSD Occluder. (b): Amplatzer Duct Occluder. (c): Amplatzer Vascular Plug III. (d): Occlutech PLD (square-shaped design). (e): Amplatzer Septal Occluder. (f): Amplatzer Vascular Plug II. (g): Amplatzer Vascular Plug IV. (h): Occlutech PLD (rectangular-shaped design).
3.3. Procedure
Pre-procedural planning is one of the most important steps of a successful procedure. Localization and characterization of the leak are crucial, and the closure strategy must be decided before initiating the procedure based on the previous information. In researchers' opinion, transcatheter closure of mitral PVL should be guided by 3D transoesophageal echocardiography under general anaesthesia
3.3.1. Anterograde Approach
In the antegrade approach, once a successful transeptal puncture is performed, a diagnostic catheter (e.g., Judkins right) is advanced into the left atrium. In most cases, the use of a deflectable catheter (e.g., Agilis, Abbott medical) is recommended [3][5]. After that, a hydrophilic guidewire (e. g., Terumo guidewire, Terumo Medical Corporation) is often used to cross the mitral PVL, and in most cases, an arteriovenous loop is established in the aorta. Alternatively, an extra-support wire can be placed in the left ventricle. Finally, a delivery sheath is advanced from the venous access over the loop or extra-support wire and the device is deployed. Before releasing, the disc movement in mechanical valves should be confirmed (Figure 8).
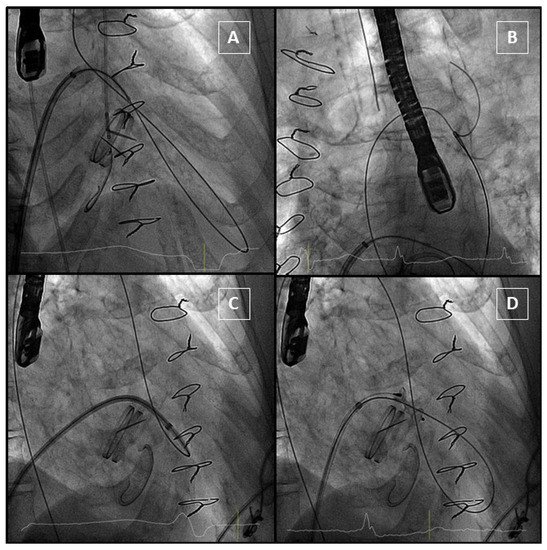
Figure 8. Anterograde approach. (A): Hydrophilic guidewire passes through the PVL and gets into aorta. (B): Guidewire is snared from an arterial access to complete the arteriovenous loop. (C): The delivery sheath crosses the PVL. (D): The device is delivered, and it does not interfere with the mechanical prosthesis.
3.3.2. Retrograde Approach
In the retrograde approach, a hydrophilic guidewire (e.g., Terumo guidewire, Terumo Medical Corporation) over a catheter (e.g., Judkins right) is often used to cross the PVL from the left ventricle to the left atrium. After crossing, an arteriovenous wire loop is often created in the left atrium; therefore, a transeptal puncture is needed. Finally, the delivery sheath is advanced from the venous access and the device is deployed (Figure 9).
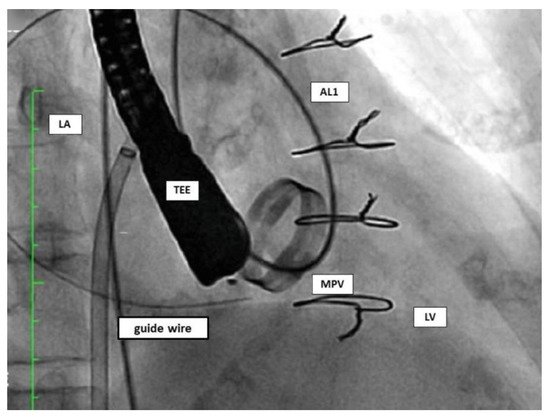
Figure 9. Retrograde approach. AL1: Amplatz Left catheter; LA: left atrium; LV: left ventricle; MPV: mitral prosthetic valve; TEE: transoesophageal echocardiography.
3.3.3. Transapical Approach
Transapical access could be an alternative for mitral PVL closure (especially for posterior or septal defects or patients with mitro-aortic monodisc mechanical valves) [26] (Figure 10). The main advantages of this access are the often less difficult wiring of the PVL and less resistance to cross the PVL, however the rate of complications of this approach is higher than in the retrograde or antegrade approach.
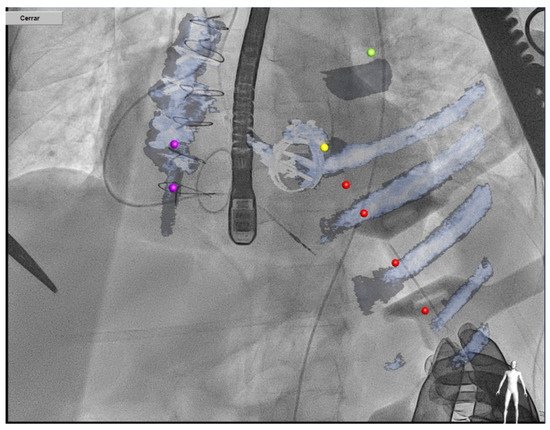
Figure 10. Transapical approach using CT–fluoroscopy fusion.
3.4. Final Result Assesment
Before releasing the device, echocardiography reassessment should be made in order to discard complications, such as disc movement restriction or LVOT (left ventricle outflow tract) obstruction. In anterior and septal PVL, an extremely rare but severe complication could be LVOT obstruction. It should be suspected in patients with septal hypertrophy, and it must be assessed by echocardiography during the procedure. In case this complication occurs, the device should not be released and its orientation or size should be changed (i.e., two smaller devices can be deployed rather than one larger device). In the same way, disc movement blockage should be always checked before device releasing; this is particularly important in monodisc prosthesis. This complication could be prevented using smaller devices, changing the orientation of the device, deploying the “ventricular” disc inside the PVL tunnel or using devices such as the AVPII (the disc size is the same as the body of the device). However, these complications are very rare, but disc blockage is usually the reason for urgent surgery after PVL closure [27].
Furthermore, sometimes a residual leak is detected before releasing. In this situation, a simultaneous deployment of two devices can be performed, or a second device can be deployed sequentially [5]. If the leak is detected after releasing the device or during follow-up, this residual leak can be recrossed, and a second device can be deployed.
3.5. Post-Procedural Medical Therapy and Follow-Up
For patients under anticoagulation therapy, this should be continued after the procedure. Dual antiplatelet therapy for at least 3 months is recommended in non-anticoagulated patients (i.e., biological prostheses). Post-procedural imaging with TEE to assess device position and residual regurgitation is recommended. The timing of a follow-up TEE varies between institutions, but researchers recommend an initial early TEE 3 months after the procedure. If persisting or new PVL are observed, percutaneous or surgical management can be chosen depending on the size of the remaining PVL.
References
- Desai, A.; John, C. Messenger, Robert Quaife, John Carroll. Update in Paravalvular Leak Closure. Curr. Cardiol. Rep. 2021, 23, 122.
- Cruz-Gonzalez, I.; Rama-Merchan, J.C.; Rodríguez-Collado, J.; Martín-Moreiras, J.; Diego-Nieto, A.; Barreiro-Pérez, M.; Sánchez, P.L. Transcatheter closure of paravalvular leaks: State of the art. Neth. Heart J. 2017, 25, 116–124.
- Bernard, S.; Yucel, E. Paravalvular Leaks-From Diagnosis to Management. Curr Treat. Options Cardiovasc. Med. 2019, 21, 67.
- Bertrand, P.B.; Levine, R.A.; Isselbacher, E.M.; Vandervoort, P.M. Fact or artifact in two-dimensional echocardiography: Avoiding misdiagnosis and missed diagnosis. J. Am. Soc. Echocardiogr. 2016, 29, 381–391.
- Cruz-Gonzalez, I.; Rama-Merchan, J.C.; Calvert, P.A.; Rodríguez-Collado, J.; Barreiro-Pérez, M.; Martín-Moreiras, J.; Diego-Nieto, A.; Hildick-Smith, D.; Sánchez, P.L. Percutaneous Closure of Paravalvular Leaks: A Systematic Review. J. Interv. Cardiol. 2016, 29, 382–392.
- Kronzon, I.; Sugeng, L.; Perk, G.; Hirsh, D.; Weinert, L.; Garcia Fernandez, M.A.; Lang, R.M. Real-time 3-dimensional transesophageal echocardiography in the evaluation of post-operative mitral annuloplasty ring and prosthetic valve dehiscence. J. Am. Coll. Cardiol. 2009, 53, 1543–1547.
- García-Fernández, M.A.; Cortés, M.; García-Robles, J.A.; de Diego, J.J.; Perez-David, E.; García, E. Utility of real-time three-dimensional transesophageal echocar- diography in evaluating the success of percutaneous transcatheter closure of mitral paravalvular leaks. J. Am. Soc. Echocardiogr. 2010, 23, 26–32.
- Arribas-Jimenez, A.; Rama-Merchan, J.C.; Barreiro-Pérez, M.; Merchan-Gómez, S.; Iscar-Galán, A.; Martín-García, A.; Nieto-Ballestero, F.; Sánchez-Corral, E.; Rodriguez-Collado, J.; Cruz-González, I.; et al. Utility of Real-Time 3-Dimensional Transesophageal Echocardiography in the Assessment of Mitral Paravalvular Leak. Circ. J. 2016, 80, 738–744.
- Lampropoulos, K.; Aggeli, C.; Megalou, A.; Barbetseas, J.; Budts, W. Diagnosis and Treatment of Left-Sided Prosthetic Paravalvular Regurgitation. Cardiology 2016, 133, 27–34.
- Lazaro, C.; Hinojar, R.; Zamorano, J.L. Cardiac imaging in prosthetic paravalvular leaks. Cardiovasc. Diagn. Ther. 2014, 4, 307–313.
- Faletra, F.F.; Pedrazzini, G.; Pasotti, E.; Muzzarelli, S.; Dequarti, M.C.; Murzilli, R.; Schlossbauer, S.A.; Slater, I.P.; Moccetti, T. 3D TEE during catheter-based interventions. JACC Cardiovasc. Imaging 2014, 7, 292–308.
- Barreiro-Perez, M.; Cruz-González, I.; Gil-Ortega, M.V.; Rasco, A.G.; Sánchez, P.L. Photo-Realistic Echocardiography Imaging During Percutaneous Paravalvular Leak Closure. JACC Cardiovasc. Interv. 2020, 13, e185–e187.
- Karagodin, I.; Shah, A.P.; Lang, R.M. Guided by the Light-Transillumination of a Paravalvular Leak. JAMA Cardiol. 2020, 5, e203260.
- Kumar, R.; Jelnin, V.; Kliger, C.; Ruiz, C.E. Percutaneous paravalvular leak closure. Cardiol. Clin. 2013, 31, 431–440.
- Kliger, C.; Eiros, R.; Isasti, G.; Einhorn, B.; Jelnin, V.; Cohen, H.; Kronzon, I.; Perk, G.; Fontana, G.P.; Ruiz, C.E. Review of surgical prosthetic paravalvular leaks: Diagnosis and catheter-based closure. Eur. Heart J. 2013, 34, 638–649.
- Ruparelia, N.; Cao, J.; Newton, J.D.; Wilson, N.; Daniels, M.J.; Ormerod, O.J. Paravalvular leak closure under intracardiac echocardiographic guidance. Catheter. Cardiovasc. Interv. 2018, 91, 958–965.
- Suchá, D.; Symersky, P.; Tanis, W.; Mali, W.P.; Leiner, T.; van Herwerden, L.A.; Budde, R.P. Multimodality Imaging Assessment of Prosthetic Heart Valves. Circ. Cardiovasc. Imaging 2015, 8, e003703.
- Orwat, S.; Diller, G.P.; Kaleschke, G.; Kerckhoff, G.; Kempny, A.; Radke, R.M.; Buerke, B.; Burg, M.; Schülke, C.; Baumgartner, H. Aortic regurgitation severity after transcatheter aortic valve implantation is underestimated by echocardiography compared with MRI. Heart 2014, 100, 1933–1938.
- Suh, Y.J.; Hong, G.R.; Han, K.; Im, D.J.; Chang, S.; Hong, Y.J.; Lee, H.J.; Hur, J.; Choi, B.W.; Chang, B.C.; et al. Assessment of mitral paravalvular leakage after mitral valve replacement using cardiac computed tomography: Comparison with surgical findings. Circ. Cardiovasc. Imaging 2016, 9, e004153.
- Krishnaswamy, A.; Tuzcu, E.M.; Kapadia, S.R. Three-dimensional computed tomography in the cardiac catheterization laboratory. Catheter. Cardiovasc. Interv. 2011, 77, 860–865.
- Cruz-González, I.; Barreiro-Pérez, M.; Valverde, I. 3D-printing in Preprocedural Planning of Paravalvular Leak Closure: Feasibility/Proof-of-concept. Rev. Esp. Cardiol. Engl. Ed. 2019, 72, 342.
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 172.
- Writing Committee Members; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, I.I.I.J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease. Circulation 2021, 143, e72–e227.
- Onorato, E.M.; Muratori, M.; Smolka, G.; Zakarkaite, D.; Mussayev, A.; Christos, C.P.; Bauer, F.; Gandet, T.; Luca, G.; Martinelli, M.D.; et al. Midterm procedural and clinical outcomes of percutaneous paravalvular leak closure with the Occlutech Paravalvular Leak Device. EuroIntervention 2020, 15, e1251–e1259.
- Sorajja, P. Mitral Paravalvular Leak Closure. Interv. Cardiol. Clin. 2016, 5, 45–54.
- Ruiz, C.E.; Jelnin, V.; Kronzon, I.; Dudiy, Y.; Del Valle-Fernandez, R.; Einhorn, B.N.; Chiam, P.T.; Martinez, C.; Eiros, R.; Roubin, G.; et al. Clinical outcomes in patients undergoing percutaneous closure of periprosthetic paravalvular leaks. J. Am. Coll. Cardiol. 2011, 58, 2210–2217.
- García, E.; Arzamendi, D.; Jimenez-Quevedo, P.; Sarnago, F.; Martí, G.; Sanchez-Recalde, A.; Lasa-Larraya, G.; Sancho, M.; Iñiguez, A.; Goicolea, J.; et al. Outcomes and predictors of success and complicationsfor paravalvular leak closure: An analysis of the SpanisH real-wOrld paravalvular LEaks closure (HOLE)registry. EuroIntervention 2017, 12, 1962–1968.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Entry Collection:
Hypertension and Cardiovascular Diseases
Revisions:
3 times
(View History)
Update Date:
11 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




