
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jaidyn Muhandiramge | -- | 3921 | 2022-04-09 10:17:06 | | | |
| 2 | Peter Tang | Meta information modification | 3921 | 2022-04-09 10:27:54 | | | | |
| 3 | Peter Tang | Meta information modification | 3921 | 2022-04-11 08:54:13 | | |
Video Upload Options
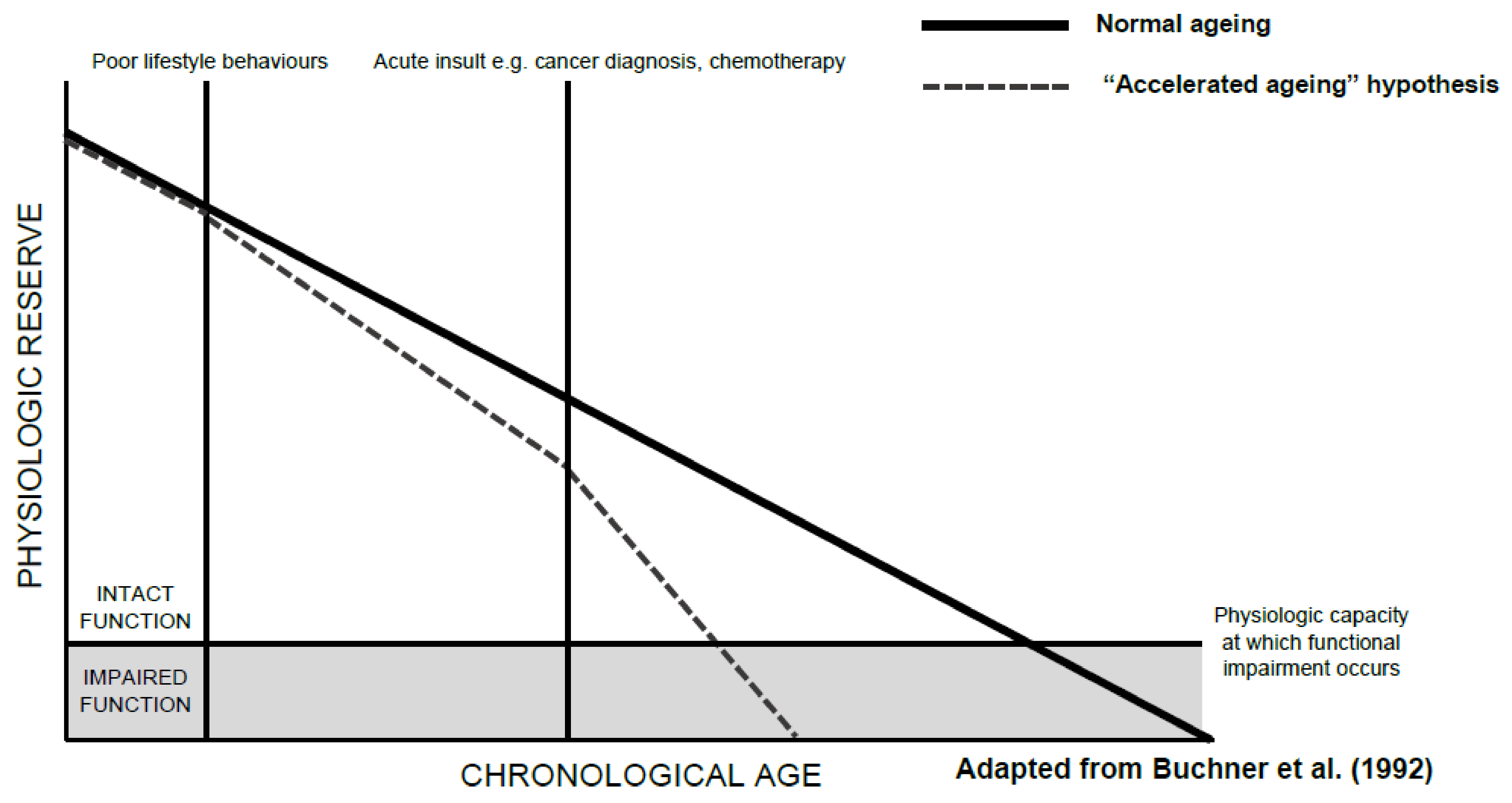
A decline in functional status, an individual’s ability to perform the normal activities required to maintain adequate health and meet basic needs, is part of normal ageing. Functional decline, however, appears to be accelerated in older patients with cancer. Such decline can occur as a result of a cancer itself, cancer treatment-related factors, or a combination of the two. The accelerated decline in function seen in older patients with cancer can be slowed, or even partly mitigated through routine assessments of functional status and timely interventions where appropriate. This is particularly important given the link between functional decline and impaired quality of life, increased mortality, comorbidity burden, and carer dependency.
1. Introduction
2. Assessment of Functional Decline
|
Instrument |
Method of Administration |
Domains Assessed |
Comments |
|---|---|---|---|
|
Functional status |
|||
|
Barthel Index [25] (Basic ADLs) |
Patient-reported or direct observation |
Feeding, toileting, bathing, dressing, and undressing, toilet transfers, incontinence, bed transfers, and ambulation |
Intended for patients with stroke, neuromuscular disorders, musculoskeletal disorders, and cancer. |
|
Eastern Cooperative Oncology Group Performance Status (ECOG) [26] |
Patient-reported |
Percentage of day spent ambulatory or in bed |
5-point scale, where 0 is “Fully active” and independent and 5 is “Dead”. Commonly used in oncology due to its simplicity [27]. Tends to have minimal direct input from the patient. Noted by the International Society of Geriatric Oncology (SIOG) to be a poor marker of function as functional impairment can occur in the presence of good performance status [28]. |
|
Karnofsky Performance Status Scale (KPS) (Both instrumental and basic ADLs) |
Patient-reported |
Activity, work, self-care |
10–100-point scale, gold-standard measurement of performance status in cancer. Thorne-modified KPS better suited to community-based and palliative care settings [29], while Australia-modified KPS is better suited to settings with multiple venues of care across both inpatient and outpatient settings [30]. Noted by SIOG to be a poor marker of function as functional impairment can occur in the presence of good performance status [28]. |
|
Katz Index of Independence in Activities of Daily Living Scale (ADL) [31] (Basic ADLs) |
Patient-reported |
Bathing, dressing, toileting, transferring, continence, and feeding |
Most commonly used instrument in studies assessing activities of daily living in adults with cancer [32]. Shortened versions are often used due to length: modified Katz-1 assesses dressing, bathing, transferring, eating, and toileting, but does not assess continence; modified Katz-2 assesses the original six domains in the Katz ADL scale, as well as walking across a small room [27]. |
|
Lawton Instrumental Activities of Daily Living Scale (IADL) [33] |
Patient-reported |
Ability to use telephone, shopping, food preparation, housekeeping, laundry, transport, responsibility for medications, and finances |
Second-most commonly used instrument used in studies assessing activities of daily living in adults with cancer [32]. |
|
Rosow–Breslau Health Scale [33] |
Patient-reported |
Ability to do heavy housework, walk up and down stairs, and walk half a mile |
Simple 3-point scale that can be easily implemented in the clinical setting. Less commonly used in patients with cancer and in oncology research. |
|
Functional Independence Measure (FIM) [34] |
Direct observation |
Self-care, sphincter control, transfers, locomotion, communication, and social cognition |
Used for evaluation in the rehabilitation of patients post-stroke, traumatic brain injury, spinal cord injury, or cancer. |
|
Frail Elderly Functional Assessment Questionnaire (FEFA) [35] |
Patient-reported |
Mobility, transfers, housework, meal preparation, finances, telephone use, eating, dressing, personal hygiene, and medication management |
Older, less-widely used tool. Validated against Katz ADL, IADL, and Barthel Index [36]. |
|
Elderly Functional Index (ELFI) [37] |
Patient-reported |
Physical functioning, role functioning, social functioning, and mobility |
Newer tool derived from functional domains of common quality of life instrument European Organisation for Research and Treatment (EORTC) Quality of Life Questionnaire Core-30 (QLQ-C30). Suggested for use as an endpoint of functional status in clinical trials or in clinical practice. |
|
Physical performance measures |
|||
|
Grip strength |
Direct observation |
Forearm strength |
Requires a dynamometer for testing. Poorer scores are associated with poorer health-related quality of life [38] and increased mortality [39] in patients with cancer. |
|
Gait speed [40] |
Direct observation |
Walking speed over a short distance, typically 4, 6, 8, or 10 m |
Poorer scores are associated with decreased survival outcomes and treatment-related complications in cancer survivors [41]. Requires a stopwatch, although electronic gait mats or automatic timing devices provide more accurate assessments [40]. |
|
6-Minute Walk Test (6MWT) [17] |
Direct observation |
Aerobic capacity and endurance over six minutes of walking |
Good measure of cardiorespiratory fitness. Validated for use in patients with cancer [42]. Does not require specialised equipment, but does require a stopwatch and a walkway of known length. |
|
Timed Up and Go Test (TUG) [43] |
Direct observation |
Gait speed and mobility: measures the time taken to rise from a chair, walk three meters, turn around, walk back to the chair, and sit down while turning 180 degrees |
Poorer scores are associated with decreased survival outcomes, treatment-related complications, and functional decline in cancer survivors [41]. Can be used as a substitute measure for gait speed. Does not require specialised equipment. |
|
Short Physical Performance Battery (SPPB) [44] |
Direct observation |
Lower limb muscle strength, balance, and mobility |
Poorer scores are associated with decreased survival outcomes, treatment-related complications, and functional decline in cancer survivors [41]. Can be used as a substitute measure for gait speed. Does not require specialised equipment. |
|
Physical Performance Test (PPT) [45] |
Direct observation |
Writing, eating, dressing, grip strength, mobility, dexterity, communication, upper limb function, and balance |
Requires various household items for assessment. Direct comparison with the KPS scale indicates that the PPT is more accurate in measuring functional status in older patients with cancer [46]. |
3. Impact of Cancer on Functional Decline
3.1. Prevalence of Functional Impairment in Older Patients with Cancer
3.2. Cancer-Related Functional Decline
3.3. Cancer Treatment-Related Functional Decline
3.3.1. Systemic Therapy
3.3.2. Radiotherapy
3.3.3. Surgery
4. Mechanisms Driving Functional Decline
|
Patient Characteristics and Social Factors |
Clinical Factors |
|---|---|
|
Cancer-related factors |
Treatment-related factors |
References
- Leidy, N.K. Functional Status and the Forward Progress of Merry-Go-Rounds: Toward a coherent analytical framework. Nurs. Res. 1994, 43, 196–202.
- Wensing, M.; Vingerhoets, E.; Grol, R. Functional status, health problems, age and comorbidity in primary care patients. Qual. Life Res. 2001, 10, 141–148.
- Muhandiramge, J.; Orchard, S.; Haydon, A.; Zalcberg, J. The acceleration of ageing in older patients with cancer. J. Geriatr. Oncol. 2020, 12, 343–351.
- Wedding, U.; Röhrig, B.; Klippstein, A.; Pientka, L.; Höffken, K. Age, severe comorbidity and functional impairment independently contribute to poor survival in cancer patients. J. Cancer Res. Clin. Oncol. 2007, 133, 945–950.
- Repetto, L.; Fratino, L.; Audisio, R.A.; Venturino, A.; Gianni, W.; Vercelli, M.; Parodi, S.; Lago, D.D.; Gioia, F.; Monfardini, S.; et al. Comprehensive Geriatric Assessment Adds Information to Eastern Cooperative Oncology Group Performance Status in Elderly Cancer Patients: An Italian Group for Geriatric Oncology Study. J. Clin. Oncol. 2002, 20, 494–502.
- Jordhoy, M.S.; Fayers, P.; Loge, J.H.; Saltnes, T.; Ahlnerelmqvist, M.; Kaasa, S. Quality of life in advanced cancer patients: The impact of sociodemographic and medical characteristics. Br. J. Cancer 2001, 85, 1478–1485.
- Edemekong, P.F.; Bomgaars, D.L.; Sukumaran, S.; Levy, S.B. Activities of Daily Living. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- De Vries, N.M.; Staal, J.B.; van Ravensberg, C.D.; Hobbelen, J.S.M.; Rikkert, M.G.M.; Nijhuis-van der Sanden, M.W.G. Outcome instruments to measure frailty: A systematic review. Ageing Res. Rev. 2011, 10, 104–114.
- Guida, J.L.; Ahles, T.A.; Belsky, D.; Campisi, J.; Cohen, H.J.; DeGregori, J.; Fuldner, R.; Ferrucci, L.; Gallicchio, L.; Gavrilov, L.; et al. Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors. J. Natl. Cancer Inst. 2019, 111, 1245–1254.
- Petrick, J.L.; Reeve, B.B.; Kucharska-Newton, A.M.; Foraker, R.E.; Platz, E.A.; Stearns, S.C.; Han, X.; Windham, B.G.; Irwin, D.E. Functional status declines among cancer survivors: Trajectory and contributing factors. J. Geriatr. Oncol. 2014, 5, 359–367.
- Buchner, D.M.; Wagner, E.H. Preventing Frail Health. Clin. Geriatr. Med. 1992, 8, 1–18.
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157.
- Bennett, J.A.; Winters-Stone, K.M.; Dobek, J.; Nail, L.M. Frailty in Older Breast Cancer Survivors: Age, Prevalence, and Associated Factors. Oncol. Nurs. Forum 2013, 40, E126–E134.
- Bylow, K.; Hemmerich, J.; Mohile, S.G.; Stadler, W.M.; Sajid, S.; Dale, W. Obese Frailty, Physical Performance Deficits, and Falls in Older Men with Biochemical Recurrence of Prostate Cancer on Androgen Deprivation Therapy: A Case-control Study. Urology 2011, 77, 934–940.
- Winters-Stone, K.M.; Moe, E.; Graff, J.N.; Dieckmann, N.; Ms, S.S.; Borsch, C.; Alumkal, J.J.; Amling, C.L.; Beer, T.M. Falls and Frailty in Prostate Cancer Survivors: Current, Past, and Never Users of Androgen Deprivation Therapy. J. Am. Geriatr. Soc. 2017, 65, 1414–1419.
- Schnipper, L.E.; Smith, T.J.; Raghavan, D.; Blayney, D.W.; Ganz, P.A.; Mulvey, T.M.; Wollins, D.S. American Society of Clinical Oncology Identifies Five Key Opportunities to Improve Care and Reduce Costs: The Top Five List for Oncology. J. Clin. Oncol. 2012, 30, 1715–1724.
- Steffen, T.M.; Hacker, T.A.; Mollinger, L. Age- and Gender-Related Test Performance in Community-Dwelling Elderly People: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and Gait Speeds. Phys. Ther. 2002, 82, 128–137.
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O.; et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J. Clin. Oncol. 2018, 36, 2326–2347.
- Treanor, C.; Donnelly, M. A methodological review of the Short Form Health Survey 36 (SF-36) and its derivatives among breast cancer survivors. Qual. Life Res. 2014, 24, 339–362.
- Garcia, M.V.; Agar, M.R.; Soo, W.-K.; To, T.; Phillips, J.L. Screening Tools for Identifying Older Adults with Cancer Who May Benefit from a Geriatric Assessment: A Systematic Review. JAMA Oncol. 2021, 7, 616–627.
- Kenig, J.; Zychiewicz, B.; Olszewska, U.; Richter, P. Screening for frailty among older patients with cancer that qualify for abdominal surgery. J. Geriatr. Oncol. 2015, 6, 52–59.
- Yokom, D.W.; Alibhai, S.M.; Sattar, S.; Krzyzanowska, M.K.; Puts, M.T. Geriatric oncology screening tools for CGA-based interventions: Results from a phase II study of geriatric assessment and management for older adults with cancer. J. Geriatr. Oncol. 2018, 9, 683–686.
- Russo, C.; Giannotti, C.; Signori, A.; Cea, M.; Murialdo, R.; Ballestrero, A.; Scabini, S.; Romairone, E.; Odetti, P.; Nencioni, A.; et al. Predictive values of two frailty screening tools in older patients with solid cancer: A comparison of SAOP2 and G8. Oncotarget 2018, 9, 35056–35068.
- Geriatric Assessment—Healthcare Professional Tool: Cancer and Aging Research Group. Available online: https://www.mycarg.org/?page_id=1936 (accessed on 9 October 2021).
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65.
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655.
- Liebzeit, D.; King, B.; Bratzke, L. Measurement of function in older adults transitioning from hospital to home: An integrative review. Geriatr. Nurs. 2018, 39, 336–343.
- Wildiers, H.; Heeren, P.; Puts, M.; Topinkova, E.; Janssen-Heijnen, M.L.; Extermann, M.; Falandry, C.; Artz, A.; Brain, E.; Colloca, G.; et al. International Society of Geriatric Oncology Consensus on Geriatric Assessment in Older Patients with Cancer. J. Clin. Oncol. 2014, 32, 2595–2603.
- Nikoletti, S.; Porock, D.; Kristjanson, L.J.; Medigovich, K.; Pedler, P.; Smith, M. Performance Status Assessment in Home Hospice Patients Using a Modified Form of the Karnofsky Performance Status Scale. J. Palliat. Med. 2000, 3, 301–311.
- Abernethy, A.P.; Shelby-James, T.; Fazekas, B.S.; Woods, D.; Currow, D.C. The Australia-modified Karnofsky Performance Status (AKPS) scale: A revised scale for contemporary palliative care clinical practice . BMC Palliat. Care 2005, 4, 7.
- Katz, S. Assessing Self-maintenance: Activities of Daily Living, Mobility, and Instrumental Activities of Daily Living. J. Am. Geriatr. Soc. 1983, 31, 721–727.
- Neo, J.; Fettes, L.; Gao, W.; Higginson, I.J.; Maddocks, M. Disability in activities of daily living among adults with cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2017, 61, 94–106.
- Rosow, P.I.; Breslau, M.N. A Guttman Health Scale for the Aged. J. Gerontol. 1966, 21, 556–559.
- Linacre, J.M.; Heinemann, A.W.; Wright, B.D.; Granger, C.V.; Hamilton, B.B. The structure and stability of the Functional Inde-pendence Measure. Arch. Phys. Me.d Rehabil. 1994, 75, 127–132.
- Gloth, F.; Scheve, A.A.; Shah, S.; Ashton, R.; McKinney, R. The frail elderly functional assessment questionnaire: Its responsiveness and validity in alternative settings. Arch. Phys. Med. Rehabil. 1999, 80, 1572–1576.
- Gloth, F.M., 3rd; Walston, J.; Meyer, J.; Pearson, J. Reliability and validity of the frail elderly functional assessment questionnaire. Am. J. Phys. Med. Rehabil. 1995, 74, 45–53.
- Soo, W.K.; King, M.; Pope, A.; Steer, C.; Devitt, B.; Chua, S.; Parente, P.; Davis, I.D.; Dārziņš, P. The Elderly Functional Index (ELFI), a patient-reported outcome measure of functional status in patients with cancer: A multicentre, prospective validation study. Lancet Health Longev. 2021, 2, e24–e33.
- Paek, J.; Choi, Y.J. Association between hand grip strength and impaired health-related quality of life in Korean cancer survivors: A cross-sectional study. BMJ Open 2019, 9, e030938.
- Zhuang, C.; Zhang, F.; Li, W.; Wang, K.; Xu, H.; Song, C.; Guo, Z.; Shi, H. Associations of low handgrip strength with cancer mortality: A multicentre observational study. J. Cachex-Sarcopenia Muscle 2020, 11, 1476–1486.
- Mehmet, H.; Robinson, S.R.; Yang, A.W.H. Assessment of Gait Speed in Older Adults. J. Geriatr. Phys. Ther. 2020, 43, 42–52.
- Verweij, N.M.; Schiphorst, A.H.W.; Pronk, A.; Bos, F.V.D.; Hamaker, M.E. Physical performance measures for predicting outcome in cancer patients: A systematic review. Acta Oncol. 2016, 55, 1386–1391.
- Schmidt, K.; Vogt, L.; Thiel, C.; Jäger, E.; Banzer, W. Validity of the Six-Minute Walk Test in Cancer Patients. Int. J. Sport Med. 2013, 34, 631–636.
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148.
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. N. Engl. J. Med. 1995, 332, 556–562.
- Reuben, D.B.; Siu, A.L. An Objective Measure of Physical Function of Elderly Outpatients: The Physical Performance Test. J. Am. Geriatr. Soc. 1990, 38, 1105–1112.
- Terret, C.; Albrand, G.; Moncenix, G.; Droz, J.P. Karnofsky Performance Scale (KPS) or Physical Performance Test (PPT)? That is the question. Crit. Rev. Oncol. Hematol. 2011, 77, 142–147.
- Blackwood, J.; Karczewski, H.; Huang, M.H.; Pfalzer, L. Katz activities of daily living disability in older cancer survivors by age, stage, and cancer type. J. Cancer Surviv. 2020, 14, 769–778.
- Reeve, B.B.; Potosky, A.L.; Smith, A.W.; Han, P.; Hays, R.D.; Davis, W.W.; Arora, N.K.; Haffer, S.C.; Clauser, S.B. Impact of Cancer on Health-Related Quality of Life of Older Americans. J. Natl. Cancer Inst. 2009, 101, 860–868.
- Van Abbema, D.; van Vuuren, A.; Berkmortel, F.V.D.; Akker, M.V.D.; Deckx, L.; Buntinx, F.; van Kampen, R.; Lambooij, E.; de Boer, M.; de Vos-Geelen, J.; et al. Functional status decline in older patients with breast and colorectal cancer after cancer treatment: A prospective cohort study. J. Geriatr. Oncol. 2017, 8, 176–184.
- La Carpia, D.; Liperoti, R.; Guglielmo, M.; Di Capua, B.; Devizzi, L.F.; Matteucci, P.; Farina, L.; Fusco, D.; Colloca, G.; Di Pede, P.; et al. Cognitive decline in older long-term survivors from Non-Hodgkin Lymphoma: A multicenter cross-sectional study. J. Geriatr. Oncol. 2020, 11, 790–795.
- Granger, C.L.; McDonald, C.F.; Irving, L.; Clark, R.A.; Gough, K.; Murnane, A.; Mileshkin, L.; Krishnasamy, M.; Denehy, L. Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer 2014, 83, 292–299.
- DeCoster, L.; Kenis, C.; Schallier, D.; Vansteenkiste, J.; Nackaerts, K.; Vanacker, L.; Vandewalle, N.; Flamaing, J.; Lobelle, J.P.; Milisen, K.; et al. Geriatric Assessment and Functional Decline in Older Patients with Lung Cancer. Lung 2017, 195, 619–626.
- Ramos, J.; Dalleck, L.C.; Tjonna, A.E.; Beetham, K.; Coombes, J.S. The Impact of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training on Vascular Function: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 679–692.
- Palma, S.; Hasenoehrl, T.; Jordakieva, G.; Ramazanova, D.; Crevenna, R. High-intensity interval training in the prehabilitation of cancer patients—A systematic review and meta-analysis. Supportive Care Cancer 2021, 29, 1781–1794.
- Hoppe, S.; Rainfray, M.; Fonck, M.; Hoppenreys, L.; Blanc, J.-F.; Ceccaldi, J.; Mertens, C.; Blanc-Bisson, C.; Imbert, Y.; Cany, L.; et al. Functional Decline in Older Patients with Cancer Receiving First-Line Chemotherapy. J. Clin. Oncol. 2013, 31, 3877–3882.
- Hurria, A.; Soto-Perez-De-Celis, E.; Ms, J.B.A.; Cohen, H.J.; Arsenyan, A.; Ballman, K.; Le-Rademacher, J.; Jatoi, A.; Filo, J.; Mandelblatt, J.; et al. Functional Decline and Resilience in Older Women Receiving Adjuvant Chemotherapy for Breast Cancer. J. Am. Geriatr. Soc. 2019, 67, 920–927.
- Kenis, C.; Decoster, L.; Bastin, J.; Bode, H.; Van Puyvelde, K.; De Grève, J.; Conings, G.; Fagard, K.; Flamaing, J.; Milisen, K.; et al. Functional decline in older patients with cancer receiving chemotherapy: A multicenter prospective study. J. Geriatr. Oncol. 2017, 8, 196–205.
- Alibhai, S.M.; Breunis, H.; Timilshina, N.; Johnston, C.; Tomlinson, G.; Tannock, I.; Krahn, M.; Fleshner, N.E.; Warde, P.; Canning, S.D.; et al. Impact of Androgen-Deprivation Therapy on Physical Function and Quality of Life in Men with Nonmetastatic Prostate Cancer. J. Clin. Oncol. 2010, 28, 5038–5045.
- Ursem, C.; Diaz-Ramirez, L.G.; Boscardin, J.; Lee, S. Changes in functional status associated with radiation for prostate cancer in older veterans. J. Geriatr. Oncol. 2020, 12, 808–812.
- Amemiya, T.; Oda, K.; Ando, M.; Kawamura, T.; Kitagawa, Y.; Okawa, Y.; Yasui, A.; Ike, H.; Shimada, H.; Kuroiwa, K.; et al. Activities of Daily Living and Quality of Life of Elderly Patients after Elective Surgery for Gastric and Colorectal Cancers. Ann. Surg. 2007, 246, 222–228.
- Van Egmond, M.A.; van der Schaaf, M.; Klinkenbijl, J.H.; Twisk, J.W.; Engelbert, R.H.; Henegouwen, M.I.V.B. The pre- and postoperative course of functional status in patients undergoing esophageal cancer surgery. Eur. J. Surg. Oncol. 2020, 46, 173–179.
- Tang, V.; Zhao, S.; Boscardin, J.; Sudore, R.; Covinsky, K.; Walter, L.C.; Esserman, L.; Mukhtar, R.; Finlayson, E. Functional Status and Survival after Breast Cancer Surgery in Nursing Home Residents. JAMA Surg. 2018, 153, 1090–1096.
- Finlayson, E.; Zhao, S.; Boscardin, W.J.; Fries, B.E.; Landefeld, C.S.; Dudley, R.A. Functional Status after Colon Cancer Surgery in Elderly Nursing Home Residents. J. Am. Geriatr. Soc. 2012, 60, 967–973.
- Oelsner, E.C.; Balte, P.P.; Bhatt, S.P.; Cassano, P.A.; Couper, D.; Folsom, A.R.; Freedman, N.D.; Jacobs, D.R.; Kalhan, R.; Mathew, A.R.; et al. Lung function decline in former smokers and low-intensity current smokers: A secondary data analysis of the NHLBI Pooled Cohorts Study. Lancet Respir. Med. 2020, 8, 34–44.
- Arday, D.R.; Milton, M.H.; Husten, C.G.; Haffer, S.C.; Wheeless, S.C.; Jones, S.M.; Johnson, R.E. Smoking and functional status among Medicare managed care enrollees. Am. J. Prev. Med. 2003, 24, 234–241.
- Cancer Prevention & Early Detection Facts & Figures 2019–2020; American Cancer Society: Atlanta, GA, USA, 2019.
- Habraken, J.M.; van der Wal, W.M.; ter Riet, G.; Weersink, E.J.M.; Toben, F.; Bindels, P.J.E. Health-related quality of life and functional status in end-stage COPD: A longitudinal study. Eur. Respir. J. 2010, 37, 280–288.
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and Cancer. Oncology 2010, 15, 556–565.
- Bell, J.A.; Sabia, S.; Singh-Manoux, A.; Hamer, M.; Kivimaki, M. Healthy obesity and risk of accelerated functional decline and disability. Int. J. Obes. 2017, 41, 866–872.
- Van Cleave, J.H.; Egleston, B.L.; McCorkle, R. Factors Affecting Recovery of Functional Status in Older Adults after Cancer Surgery. J. Am. Geriatr. Soc. 2011, 59, 34–43.
- Nightingale, G.; Battisti, N.M.L.; Loh, K.P.; Puts, M.; Kenis, C.; Goldberg, A.; Haase, K.R.; Krok-Schoen, J.; Liposits, G.; Sattar, S.; et al. Perspectives on functional status in older adults with cancer: An interprofessional report from the International Society of Geriatric Oncology (SIOG) nursing and allied health interest group and young SIOG. J. Geriatr. Oncol. 2020, 12, 658–665.
- Edbrooke, L.; Granger, C.L.; Denehy, L. Physical activity for people with lung cancer. Aust. J. Gen. Pract. 2020, 49, 175–181.
- Amidei, C.; Kushner, D.S. Clinical implications of motor deficits related to brain tumors. Neuro-Oncol. Pract. 2015, 2, 179–184.
- Santos, D.Z.; Leite, I.C.G.; Guerra, M.R. Functional status of patients with metastatic spinal cord compression. Support. Care Cancer 2018, 26, 3225–3231.
- Barsevick, A.M.; Dudley, W.N.; Beck, S.L. Cancer-related Fatigue, Depressive Symptoms, and Functional Status: A mediation model. Nurs. Res. 2006, 55, 366–372.
- Littlewood, T.; Mandelli, F. The effects of anemia in hematologic malignancies: More than a symptom. Semin. Oncol. 2002, 29, 40–44.
- Van den Beuken-van Everdingen, M.H.J.; Hochstenbach, L.M.J.; Joosten, E.A.J.; Tjan-Heijnen, V.C.G.; Janssen, D.J.A. Update on Prevalence of Pain in Patients with Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090.e9.
- Bower, J.E. Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609.
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2020.
- Ward, P.R.; Wong, M.D.; Moore, R.; Naeim, A. Fall-related injuries in elderly cancer patients treated with neurotoxic chemotherapy: A retrospective cohort study. J. Geriatr. Oncol. 2014, 5, 57–64.
- Winters-Stone, K.M.; Torgrimson, B.; Horak, F.; Eisner, A.; Nail, L.; Leo, M.C.; Chui, S.; Luoh, S.-W. Identifying Factors Associated with Falls in Postmenopausal Breast Cancer Survivors: A Multi-Disciplinary Approach. Arch. Phys. Med. Rehabil. 2011, 92, 646–652.
- Brawer, M.K. Hormonal therapy for prostate cancer. Rev. Urol. 2006, 8 (Suppl. S2), S35–S47.
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Prim. 2019, 5, 13.
- Kleinpell, R.M.; Fletcher, K.; Jennings, B.M. Chapter 11: Reducing Functional Decline in Hospitalized Elderly. In Patient Safety and Quality: An Evidence-Based Handbook for Nurses; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008.




