Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Leonor Matias | -- | 999 | 2022-04-08 13:02:34 | | | |
| 2 | Dean Liu | -1 word(s) | 998 | 2022-04-11 05:40:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Matias, M.L.; Nunes, D.; Pimentel, A.; Reis Machado, A.; Rodrigues, J.; Deuermeier, J.; Fortunato, E.; Martins, R. Fe-TiO2 Photocatalysts on Porous Platforms for Water Purification. Encyclopedia. Available online: https://encyclopedia.pub/entry/21514 (accessed on 11 January 2026).
Matias ML, Nunes D, Pimentel A, Reis Machado A, Rodrigues J, Deuermeier J, et al. Fe-TiO2 Photocatalysts on Porous Platforms for Water Purification. Encyclopedia. Available at: https://encyclopedia.pub/entry/21514. Accessed January 11, 2026.
Matias, Maria Leonor, Daniela Nunes, Ana Pimentel, Ana Reis Machado, Joana Rodrigues, Jonas Deuermeier, Elvira Fortunato, Rodrigo Martins. "Fe-TiO2 Photocatalysts on Porous Platforms for Water Purification" Encyclopedia, https://encyclopedia.pub/entry/21514 (accessed January 11, 2026).
Matias, M.L., Nunes, D., Pimentel, A., Reis Machado, A., Rodrigues, J., Deuermeier, J., Fortunato, E., & Martins, R. (2022, April 08). Fe-TiO2 Photocatalysts on Porous Platforms for Water Purification. In Encyclopedia. https://encyclopedia.pub/entry/21514
Matias, Maria Leonor, et al. "Fe-TiO2 Photocatalysts on Porous Platforms for Water Purification." Encyclopedia. Web. 08 April, 2022.
Copy Citation
Polyethylene glycol-modified titanium dioxide (PEG-modified TiO2) nanopowders were prepared using a fast solvothermal method under microwave irradiation, and without any further calcination processes. These nanopowders were further impregnated on porous polymeric platforms by drop-casting. The effect of adding iron with different molar ratios (1, 2, and 5%) of iron precursor was investigated. The approach developed in this work originated novel functionalized photocatalytic platforms, which were revealed to be promising for the removal of organic dyes from wastewater.
TiO2
photocatalysis
water purification
microwave synthesis
iron doping
1. Brief introduction
As a by-product of the society’s rapid development, environmental contaminants, such as dyes, drugs, and pesticides, are widely being released into effluents and represent, not only a risk to human health, but also to the environment, due to their complex structure and high recalcitrance. Spillage of dyes, widely used in various industries, such as water-soluble Rhodamine B (RhB) and Methylene blue (MB), endangers animals, plants, and human beings [1][2][3]. In this regard, a growing interest in the removal of pollution effluents from wastewater through environmentally friendly routes has spurred intensive research over the last few years [4][5][6].
Among the various techniques for decomposition and detoxification of organic dye effluents, photocatalysis is considered an appealing and inexpensive technology, which has been successfully employed for the treatment of pollutants and remediation of water. One of its advantages is the use of solar energy, which makes the process economically viable for large scale applications, while acquiring additional environmental value [2][3][7][8][9].
Concerning photocatalysts, TiO2 is in the spotlight and the subject of intense research, due to its remarkable properties, which include a strong oxidation potential to decompose organic pollutants, physical and chemical stabilities, low cost and non-toxicity [10][11][12][13][14][15]. TiO2 is widely used in photocatalysis for the degradation of contaminants, in both aqueous and gaseous mediums [16].
TiO2 is a semiconductor that presents a wide band gap (~3.2 eV for anatase, ~3.0 eV for rutile and 3.4 eV for brookite [17], at room temperature) and is usually excited with high-energy UV photons, above the semiconductor’s band gap [18][19]. As a result, pure TiO2 absorbs in the UV region (4–5% of the solar spectrum [18]), which makes its photocatalytic efficiency low under visible light (visible region comprehends ~45% of the solar spectrum [20]). To overcome this issue and enhance the photocatalytic activity of TiO2-based materials, several approaches have been tested to extend their use under sunlight, including doping with metal and non-metal elements, through surface modification and by coupling with other semiconductor materials [12][16][20][21][22][23].
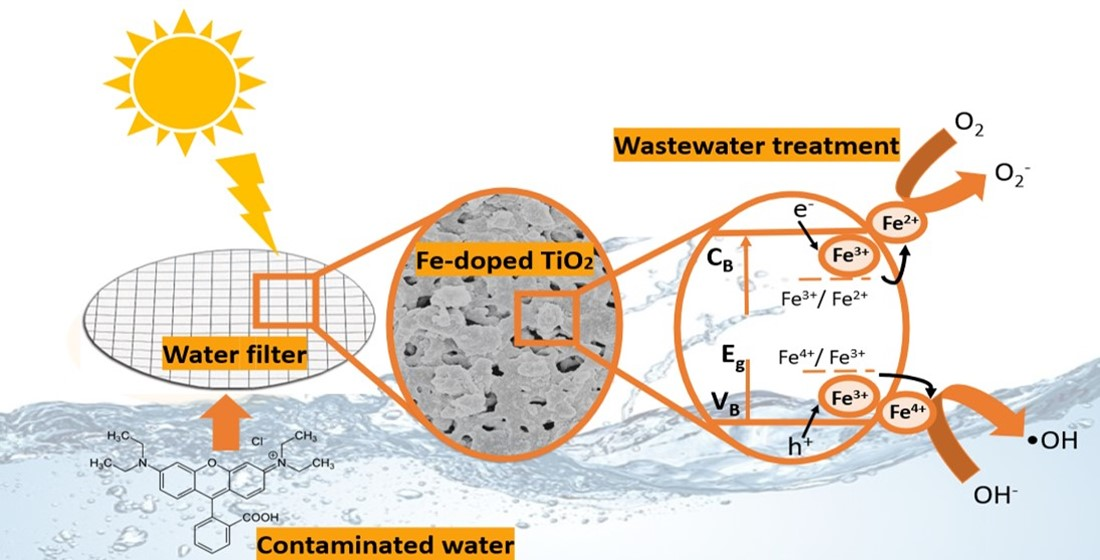
Doping TiO2 with transition metal ions has been reported to reduce the rate of electron-hole recombination, due to the formation of a Schottky barrier with TiO2. Simultaneously, it is responsible for narrowing the TiO2 band gap energy; thus, extending the absorption to the visible spectral range (red-shift), and leading to an improvement in the photocatalytic activity [24][25][26]. Doping could also potentially increase TiO2 crystallinity and enlarge the surface area [27]. Several metal ions have been successfully incorporated into a TiO2 lattice, including nickel [28], copper [29][30], chromium [31], iron [1], vanadium [32], and zinc [33]. Amongst the different metal ions, Fe appears an excellent candidate for doping TiO2, due to its half-filled d-electronic configuration and the similar atomic radius of Fe3+ ion (0.69 Å) and Ti4+ ion (0.75 Å). The titanium positions in the TiO2 lattice can be easily substituted by the cation. Fe3+ is reported to provide trap centers for photogenerated electrons and holes, since the energy level of Fe2+/Fe3+ is located near Ti3+/Ti4+; thus, enhancing the charge separation.
Different substrates have been used for growing Fe-doped TiO2 nanostructures, including glass substrates, polyamide fabric, and Si substrates, but to the best of researchers' knowledge, this has never been reported for porous water filters. The impregnation of these nanostructures on commercial water filters avoids the limitations and costs associated with the recovery of nanometer-sized particles.
In this research, pure TiO2 and 1, 2, and 5 mol% Fe-TiO2 nanostructures were synthesized by a fast surfactant-assisted microwave method (1 h), without any further calcination of the nanostructures, and impregnated using a simple drop-casting technique on polymeric substrates, used as water filters. Enhanced visible light absorption of TiO2-based materials on flexible porous water filter substrates was, thus, produced. The surface modification of TiO2-based materials with PEG was investigated. The materials produced were systematically characterized by XRD, Raman spectroscopy, SEM coupled with EDS, and XPS. Optical characterization was also carried out for the nanostructures, via UV–VIS absorption and room temperature diffuse reflectance measurements, to determine the optical band gap values. Finally, their efficiency as photocatalysts on water filters was investigated for the degradation of the organic model pollutant, RhB, under solar radiation.
2. Conclusions
In summary, pure TiO2 and Fe-TiO2 nanostructures were successfully synthesized by a fast surfactant-assisted microwave irradiation (1 h), without a calcination step, and impregnated by drop-casting on porous polymeric substrates. The approach used in this study enabled the total covering of the porous substrates and the evaluation of their photocatalytic activity in the degradation of RhB under solar radiation. SEM confirmed the formation of fine particles with a sphere-like appearance and films that uniformly coated the substrates. XRD revealed the presence of pure TiO2 anatase in all nanostructures, which was further confirmed by Raman spectroscopy on the impregnated substrates. The platforms impregnated with 5 mol% of Fe-TiO2 nanostructures exhibited an enhanced RhB photodegradation, when compared to pure TiO2 and the pristine substrates. The highest photocatalytic activity for the Fe-TiO2 material under solar radiation reached 85% after 3.5 h, compared to 74% with pure TiO2. The photodegradation rate of RhB dye with the Fe-TiO2 substrate was 1.5-times faster than pure TiO2. The XPS and UV-VIS results support that the presence of Fe ions led to the introduction of new energy levels, as well as defects, such as oxygen vacancies, that played an important role as traps for the photogenerated carriers, leading to a reduction in the recombination rate, followed by a visible enhancement in the photocatalytic activity. In summary, this study demonstrated that the synergy between the micro-porosity of the substrates and the surface-modified Fe-TiO2 nanostructures enhanced the substrates’ photocatalytic properties. Flexible and eco-friendly photocatalytic functionalized substrates were produced with potential for wastewater removal.
Graphical Abstract
References
- Rodríguez, P.A.O.; Pecchi, G.A.; Casuscelli, S.G.; Elías, V.R.; Eimer, G.A. A simple synthesis way to obtain iron-doped TiO2 nanoparticles as photocatalytic surfaces. Chem. Phys. Lett. 2019, 732, 136643.
- Neena, D.; Kondamareddy, K.K.; Bin, H.; Lu, D.; Kumar, P.; Dwivedi, R.K.; Pelenovich, V.O.; Zhao, X.Z.; Gao, W.; Fu, D. Enhanced visible light photodegradation activity of RhB/MB from aqueous solution using nanosized novel Fe-Cd co-modified ZnO. Sci. Rep. 2018, 8, 1–12.
- Rani, S.; Aggarwal, M.; Kumar, M.; Sharma, S.; Kumar, D. Removal of methylene blue and rhodamine B from water by zirconium oxide/graphene. Water Sci. 2016, 30, 51–60.
- Shen, S.; Li, R.; Wang, H.; Fu, J. Carbon Dot–Doped Titanium Dioxide Sheets for the Efficient Photocatalytic Performance of Refractory Pollutants. Front. Chem. 2021, 9, 706343.
- Wu, D.; Zhao, C.; Xu, Y.; Zhang, X.; Yang, L.; Zhang, Y.; Gao, Z.; Song, Y.Y. Modulating Solar Energy Harvesting on TiO2 Nanochannel Membranes by Plasmonic Nanoparticle Assembly for Desalination of Contaminated Seawater. ACS Appl. Nano Mater. 2020, 3, 10895–10904.
- Odling, G.; Robertson, N. Bridging the gap between laboratory and application in photocatalytic water purification. Catal. Sci. Technol. 2019, 9, 533–545.
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986.
- Borges, M.E.; Sierra, M.; Cuevas, E.; García, R.D.; Esparza, P. Photocatalysis with solar energy: Sunlight-responsive photocatalyst based on TiO2 loaded on a natural material for wastewater treatment. Sol. Energy 2016, 135, 527–535.
- Rajeshwar, K.; Osugi, M.E.; Chanmanee, W.; Chenthamarakshan, C.R.; Zanoni, M.V.B.; Kajitvichyanukul, P.; Krishnan-Ayer, R. Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J. Photochem. Photobiol. Photochem. Rev. 2008, 9, 171–192.
- He, F.; Jeon, W.; Choi, W. Photocatalytic air purification mimicking the self-cleaning process of the atmosphere. Nat. Commun. 2021, 12, 1–4.
- Fernández-Catalá, J.; Berenguer-Murcia, Á.; Cazorla-Amorós, D. Photocatalytic oxidation of VOCs in gas phase using capillary microreactors with commercial TiO2 (P25) fillings. Materials 2018, 11, 1149.
- Nunes, D.; Pimentel, A.; Branquinho, R.; Fortunato, E.; Martins, R. Metal oxide-based photocatalytic paper: A green alternative for environmental remediation. Catalysts 2021, 11, 504.
- Nunes, D.; Pimentel, A.; Araujo, A.; Calmeiro, T.R.; Panigrahi, S.; Pinto, J.V.; Barquinha, P.; Gama, M.; Fortunato, E.; Martins, R. Enhanced UV Flexible Photodetectors and Photocatalysts Based on TiO2 Nanoplatforms. Springer Nat. 2018, 61, 1591–1606.
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. Photochem. Rev. 2012, 13, 169–189.
- Xu, C.; Liu, T.; Guo, W.; Sun, Y.; Liang, C.; Cao, K.; Guan, T.; Liang, Z.; Jiang, L. 3D Printing of Powder-Based Inks into Functional Hierarchical Porous TiO2 Materials. Adv. Eng. Mater. 2020, 22, 1901088.
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102.
- Dima, R.S.; Phuthu, L.; Maluta, N.E.; Kirui, J.K.; Maphanga, R.R. Electronic, Structural, and Optical Properties of Mono-Doped and Co-Doped (210) TiO2 Brookite Surfaces for Application in Dye-Sensitized Solar Cells—A First Principles Study. Materials 2021, 14, 3918.
- Heng, Z.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C.; Koo, C.H. Photocatalytic degradation of organic pollutants using green oil palm frond-derived carbon quantum dots/titanium dioxide as multifunctional photocatalysts under visible light radiation. Chin. J. Chem. Eng. 2021, 12, 1–31.
- Bono, N.; Ponti, F.; Punta, C.; Candiani, G. Effect of UV Irradiation and TiO2-Photocatalysis on Airborne Bacteria and Viruses: An Overview. Materials 2021, 14, 1075.
- Reda, S.M.; Khairy, M.; Mousa, M.A. Photocatalytic activity of nitrogen and copper doped TiO2 nanoparticles prepared by microwave-assisted sol-gel process. Arab. J. Chem. 2020, 13, 86–95.
- Zhao, J.; Chen, C.; Ma, W. Photocatalytic Degradation of Organic Pollutants Under Visible Light Irradiation. Top. Catal. 2005, 35, 269–278.
- Landolsi, Z.; Ben Assaker, I.; Nunes, D.; Fortunato, E.; Martins, R.; Chtourou, R.; Ammar, S. Enhanced electrical and photocatalytic properties of porous TiO2 thin films decorated with Fe2O3 nanoparticles. J. Mater. Sci. Mater. Electron. 2020, 31, 20753–20773.
- Nunes, D.; Pimentel, A.; Gonçalves, A.; Pereira, S.; Branquinho, R.; Barquinha, P.; Martins, R. Metal Oxide Nanostructures for Sensor Applications. Semicond. Sci. Technol 2019, 34, 043001.
- Khairy, M.; Zakaria, W. Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egypt. J. Pet. 2014, 23, 419–426.
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The effect of doping elements on photocatalytic activity. Mater. Adv. 2020, 1, 1193–1201.
- Pongwan, P.; Wetchakun, K.; Phanichphant, S.; Wetchakun, N. Enhancement of visible-light photocatalytic activity of Cu-doped TiO2 nanoparticles. Res. Chem. Intermed. 2016, 42, 2815–2830.
- Yu, J.C.; Li, G.; Wang, X.; Hu, X.; Leung, C.W.; Zhang, Z. An ordered cubic Im3m mesoporous Cr-TiO2 visible light photocatalyst. Chem. Commun. 2006, 25, 2717–2719.
- Ganesh, I.; Gupta, A.K.; Kumar, P.P.; Sekhar, P.S.C.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and characterization of Ni-doped TiO2 materials for photocurrent and photocatalytic applications. Sci. World J. 2012, 2012, 1–16.
- Yoong, L.S.; Chong, F.K.; Dutta, B.K. Development of copper-doped TiO2 photocatalyst for hydrogen production under visible light. Energy 2009, 34, 1652–1661.
- Ethiraj, A.S.; Rhen, D.S.; Soldatov, A.V.; Ali, G.A.M.; Bakr, Z.H. Efficient and Recyclable Cu Incorporated TiO2 Nanoparticle Catalyst for Organic Dye Photodegradation. Int. J. Thin Film Sci. Technol. 2021, 10, 169–182.
- Li, X.; Guo, Z.; He, T. The doping mechanism of Cr into TiO2 and its influence on the photocatalytic performance. Phys. Chem. Chem. Phys. 2013, 15, 20037–20045.
- Avansi, W.; Arenal, R.; De Mendonça, V.R.; Ribeiro, C.; Longo, E. Vanadium-doped TiO2 anatase nanostructures: The role of V in solid solution formation and its effect on the optical properties. CrystEngComm 2014, 16, 5021–5027.
- Jiang, K.; Zhang, J.; Luo, R.; Wan, Y.; Liu, Z.; Chen, J. A facile synthesis of Zn-doped TiO2 nanoparticles with highly exposed (001) facets for enhanced photocatalytic performance. RSC Adv. 2021, 11, 7627–7632.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Entry Collection:
Wastewater Treatment
Revisions:
2 times
(View History)
Update Date:
11 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No