Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aref Zayed | -- | 4838 | 2022-04-08 11:06:10 | | | |
| 2 | Conner Chen | -4 word(s) | 4834 | 2022-04-11 04:57:54 | | | | |
| 3 | Conner Chen | Meta information modification | 4834 | 2022-04-11 04:59:26 | | | | |
| 4 | Conner Chen | Meta information modification | 4834 | 2022-04-11 05:14:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zayed, A. Determination of Steroids by High Performance Liquid Chromatography-Fluorescence. Encyclopedia. Available online: https://encyclopedia.pub/entry/21509 (accessed on 08 February 2026).
Zayed A. Determination of Steroids by High Performance Liquid Chromatography-Fluorescence. Encyclopedia. Available at: https://encyclopedia.pub/entry/21509. Accessed February 08, 2026.
Zayed, Aref. "Determination of Steroids by High Performance Liquid Chromatography-Fluorescence" Encyclopedia, https://encyclopedia.pub/entry/21509 (accessed February 08, 2026).
Zayed, A. (2022, April 08). Determination of Steroids by High Performance Liquid Chromatography-Fluorescence. In Encyclopedia. https://encyclopedia.pub/entry/21509
Zayed, Aref. "Determination of Steroids by High Performance Liquid Chromatography-Fluorescence." Encyclopedia. Web. 08 April, 2022.
Copy Citation
Steroids are compounds widely available in nature and synthesized for therapeutic and medical purposes. Although several analytical techniques are available for the quantification of steroids in clinical samples, their analysis is challenging due to their low levels and complex matrices. The efficiency and quick separation of the high performance liquid chromatography (HPLC) combined with the sensitivity, selectivity, simplicity, and cost-efficiency of fluorescence, make HPLC coupled to fluorescence detection (HPLC-FLD) an ideal tool for routine measurement and detection of steroids for clinical and medical applications.
high performance liquid chromatography
clinical
steroids
fluorescence
quantification
1. Clinical Applications of HPLC-FLD Techniques
Quantification of steroids plays an important role in the diagnosis and treatment of endocrine disorders. Diseases, such as cancer, metabolic syndromes, and neurodegenerative diseases, are associated with abnormalities in the endocrine system. Steroids also play an important role in biochemical processes, such as aging, reproduction, and metabolic pathways [1]. Furthermore, disturbances in the endocrine system due to EDC from environment and food are becoming a major clinical concern. HPLC-FLD has been employed in many research studies, both in humans and animal models, to quantify the various types of steroids and investigate their role in diseases and clinical conditions.
2. Detection of Glucocorticosteroids
Glucocorticosteroids are widely recognized as markers for adrenal activity. Cortisol can reflect the short-term changes in the activity of the hypothalamic–pituitary–adrenocortical axis (HPA axis), making it a valuable surrogate marker for stress and glucose metabolism [2].
Many methods have been developed to detect unconjugated cortisol levels in various biological samples. However, the use of certain samples, such as urine, may not be recommended as these samples may contain many interfering substances thus complicating steroids extraction procedure [3]. In addition, the concentrations of corticosteroids in urine are low compared with those found in plasma, which makes the latter a better medium for measurement. Steroids have been measured by HPLC-FLD methods for various clinical purposes, such as investigating disease courses, pathogenesis, and metabolic processes [4].
The detection of corticosteroids in biological samples can be done directly depending on fluorescence signal enhancement upon the treatment of samples with the deproteinizing agent (ethanol) and sulfuric acid (Figure 1). A study to quantify corticosteroids in serum samples was performed using C18 analytical column after treatment with ethanol and sulfuric acid [5]. Cortisol, testosterone and corticosterone emitted fluorescence, with LOD for cortisol = 0.3 pg/dL (S/N = 3). In another study, the same group measured cortisol in urine samples producing a LOD = 0.26 pg/dL (S/N = 3) [6]. Sudo et al. used post-column derivatization with sulfuric acid to measure cortisol and corticosterone in rat urine (LOD was 0.5 pmol for corticosterone at (S/N = 5)) [3]. They analyzed other corticoids, such as prednisolone, 6α-methylprednisolone, dexamethasone, and betamethasone, but with lower sensitivity. Moreover, ethyl acetate was used for extraction after sulfuric acid hydrolysis to prevent the acid from entering the HPLC system. It was noticed that the fluorescence intensities were dependent on the reactor temperature and sulfuric acid [3][7].

Figure 1. Fluorescence emission of steroids after treatment with sulfuric acid.
Since cortisol levels in hair might be used as a biomarker of chronic stress, a study developed a HPLC-FLD method to measure cortisol distribution in human hair samples. The method has achieved equal precision to mass spectrometry. Samples were prepared by pulverization and incubation in 0.1 M HCl, and extraction with ethyl acetate followed by derivatization with sulfuric acid. A detection limit of 1 pg/mg was achieved [2].
Derivatization with fluorophore-containing reagents was employed in many studies to detect and quantify corticosteroids in serum and urine samples. The derivatization process depends on functionalization of specific groups in corticosteroids structure (Figure 2). The primary alcohol functionality can be esterified through its reaction with either 2-(4-carboxyphenyl)-5,6-dimethylbenzimidazole (CDB) (Figure 2), 9-anthroyl nitrile (9-AN) (Figure 3), or 1-anthroyl nitrile (1-AN) (Figure 3). These derivatization processes proved to be selective as neither secondary alcohol, nor tertiary alcohol can react with fluorophore-containing reagents (Figure 3).
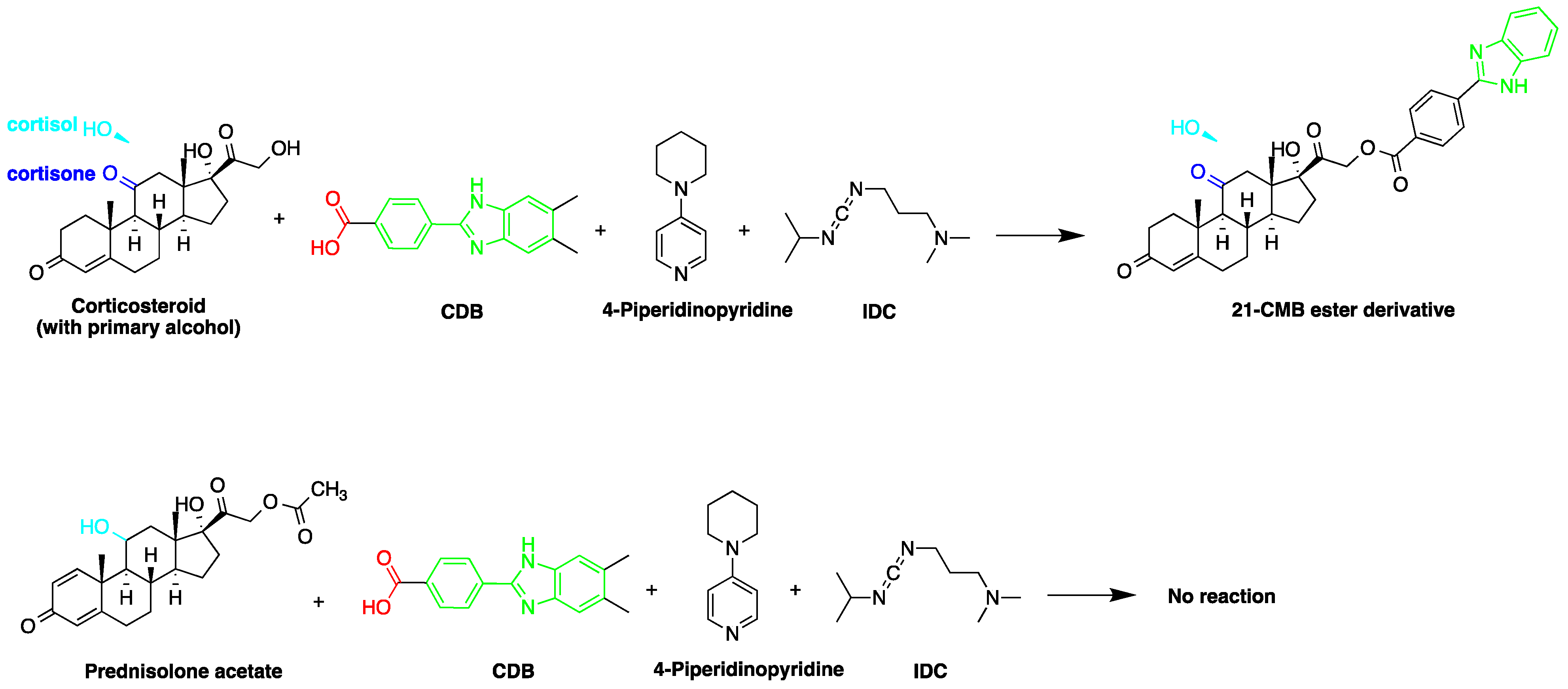
Figure 2. Esterification of primary alcohol functionality of cortisol and cortisone by 2-(4-carboxyphenyl)-5,6-dimethylbenzimidazole (CDB) in the presence of 4-piperidinopyridine and 1-isopropyl-3-(3-dimethylaminopropyl) carbodiimide (IDC) perchlorate. Prednisolone secondary alcohol shows no reaction.
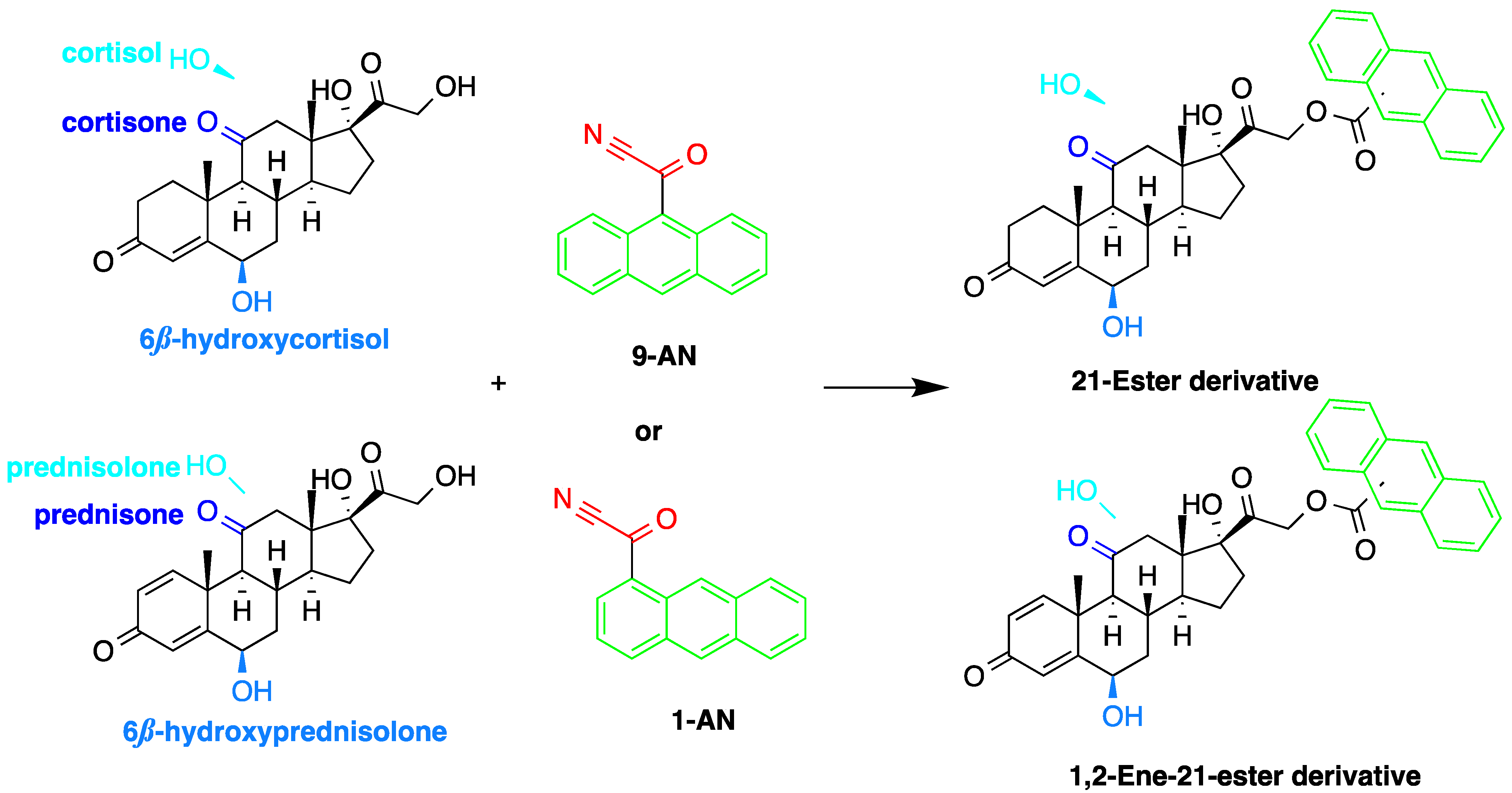
Figure 3. Esterification of primary alcohol functionality of cortisol and cortisone by 9-anthroyl nitrile (9-AN) or 1-anthroyl nitrile (1-AN).
The derivatization through esterification of corticosteroid primary alcohol functionality with CDB was studied by Katayama et al. [8]. The results showed that the detector response to secondary alcohols was less than one fiftieth that of the primary alcohol. However, certain secondary alcohols in steroids, such as prednisolone acetate and testosterone, and tertiary alcohols, showed no reaction. Later, Katayama et al. used human plasma to detect eight corticosteroids by derivatization with CDB to their esters in acetonitrile. The esters were separated on a reversed-phase column (Zorbax ODS) with water:methanol (25:75, v/v) containing 5 mmol/L tetramethylammonium hydrogen sulfate as a mobile phase. The LOD ranged between 0.06 and 0.3 pg per 100 µL of plasma (S/N = 3) [9].
The use of 9-AN as a derivatizing agent for corticosteroids was reported in several studies. Neufled et al. used 9-AN to derivatize the primary alcohol in C21 corticosteroids. The reaction was carried out at 45 °C for 2 h and the fluorescent derivatives were separated on silica stationary phase with a mixture of 2-propanol and hexane as a mobile phase in the gradient mode. The low temperature in derivatization prevented the thermal degradation of corticosteroids and avoided reaction with secondary hydroxyl groups [10][11].
Measuring endogenous glucocorticoids and their metabolites is important as disturbances in the enzyme responsible for their metabolism can cause hypertension. One study described an HPLC-FLD method using 9-AN derivatization for the determination of cortisol, cortisone, and their tetrahydro- and allo-tetrahydro-metabolites in plasma and urine [12]. Following extraction with dichloromethane and SPE, 9-AN was used to derivatize the steroids in the samples to their fluorescent products. LOD (S/N = 3:1) achieved with this method was 3.0 ng/mL for all analytes. Similarly, Shibata et al. employed 9-AN for fluorescent derivatization and developed a method to investigate cortisone levels in plasma and urine samples of renal transplant patients who received prednisolone [13]. Cortisol, cortisone, prednisolone, prednisone, 6β-hydroxycortisol, and 6β-hydroxyprednisolone were derivatized to their fluorescent esters after extraction with ethyl acetate (Figure 4). The 6β-hydroxycortisone was used as an internal standard. LODs achieved for cortisol, cortisone, prednisolone and prednisone in plasma or urine were 0.1 ng/mL, while those for 6β-hydroxycortisol and 6β-hydroxyprednisolone in plasma or urine were 0.5 ng/mL.
Similar to 9-AN, fluorescent derivatization can be achieved using 1-AN. A study described the use of 1-AN to derivatize 18-oxygenated corticosteroids: 18-hydroxycortisol, 18-hydroxycortisone and 18-oxocortisol in human urine into their fluorescent 21-anthroyl esters [14] (Figure 4). A mixture of ether and dichloromethane was used to extract the steroids, and the anthroyl derivatives were enriched by SPE using a CN cartridge column. The LOD was 0.1 pmol (S/N = 5).

Figure 4. Fluorescent derivatization of 18-oxygenated corticosteroids by or 1-anthroyl nitrile (1-AN).
Fluorescent derivatization of corticosteroids can also be carried out using dansyl hydrazine targeting the carbonyl groups (Figure 5). In a study to determine corticosteroids in human plasma and urine samples were derivatized by dansyl hydrazine and quantified by HPLC-FLD, following extraction with methylene chloride. The linearity range of the method was between 0.5 and 60 ng of cortisol, proving it to be suitable for the routine analysis of cortisol in plasma and urine [15].

Figure 5. Fluorescent derivatization of carbonyl functionality of cortisol by dansyl hydrazine.
The detection of corticosteroids with ketolic groups in urine is key part in diagnostic procedure of Cushing’s syndrome. Ketol-containing corticosteroids, such as 17-hydroxycorticosteroid (breakdown product of cortisol), are usually excreted in urine as tetrahydro form making their detection using UV absorption at 240 nm not possible. To detect these steroids in urine, derivatization with amidine-containing compounds was used yielding fluorescent compounds [16] (Figure 6). Using this approach, the best sensitivity was achieved for cortisol.

Figure 6. Fluorescent derivatization of ketolic-containing corticosteroids by benzamidine.
The utilization of ketolic groups was also performed by Yamaguchi et al. to detect and quantify corticosteroids in urine after their conversion into fluorescent quinoxalines (Figure 7) [17]. They detected nineteen 21-hydroxycorticosteroids in human urine samples achieving LOD = 0.14–29.4 pmol/50 µL injection volume (S/N = 3). In another study by the same group, prednisolone and prednisone were quantified in plasma samples following similar derivatization procedure and analyzed by reversed-phase liquid chromatography with isocratic elution. The LOD of prednisolone and prednisone was 3 ng/mL in plasma (S/N = 3) [18].
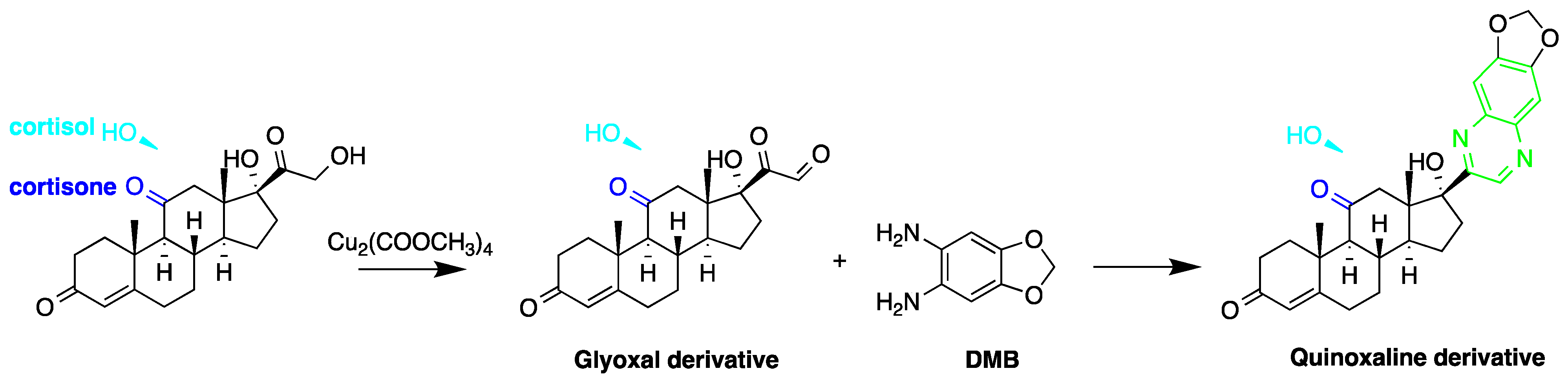
Figure 7. Fluorescent derivatization of ketol-containing corticosteroids to quinoxaline derivative.
Cholesterol quantification is commonly required in the analysis of biosamples. A study described a simple and sensitive method for the determination of cholesterol and phytosterols, such as β-sitosterol in biosamples (e.g., saliva and urine matrices) and food samples (cow and soybean milk) after derivatization with naproxen acyl chloride in toluene (Figure 8). The method employed a C8 column with a mixture of methanol, isopropanol, and water, achieving a LOD of about 25 nM (S/N = 3). The analysis of cholesterol and sitosterol is usually time-consuming, but using the method, a relatively short period of time was required since solvent concentration, evaporation, and replacement steps were not necessary [19].
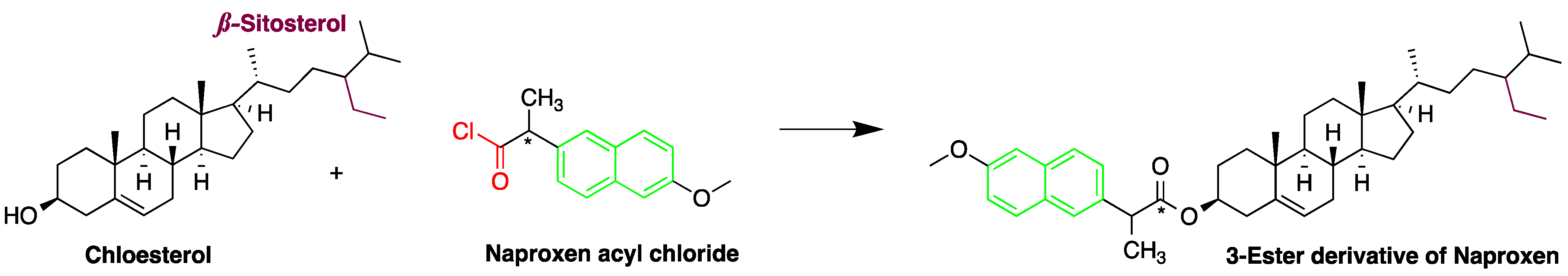
Figure 8. Derivatization of cholesterol by naproxen acyl chloride.
Free 7α-hydroxycholesterol (7-HC) levels in human serum were reported to be a good indicator for bile acid synthesis. An HPLC-FLD method was developed by Saisho et al. to quantify 7-HC levels in dog plasma, with the purpose of studying the effect of cholestyramine on plasma levels of 7-HC. 7-HC was converted to its fluorescence derivative, by two procedures, after being extracted and then purified [20]. The two derivatization reagents used were 1-AN and 7-methoxycoumarin-3-carbonyl azide (MC-CON3). The MC-CON3 derivatization resulted in higher fluorescence intensity compared to 1-AN route. 1-AN produced only a C-3 fluorescent derivative due to the bulky anthracene group and steric hindrance around the C-7 position. In comparison, MC-CON3 yielded a double coumarin derivative at the C-3 and C-7 positions (Figure 9) producing a LOD of 4 pg (S/N = 5).

Figure 9. Derivatization of 7α-hydroxycholesterol (7-HC) by 1-anthroyl nitrile (1-AN) or 7-methoxycoumarin-3-carbonyl azide (MC-CON3).
3. Detection of Steroid Hormones
Detection of steroid hormones and their metabolites are important in diagnosis of metabolic diseases. Several HPLC-FLD methods were described for the detection of steroid hormones. A study developed a method for monitoring progesterone and 17-hydroxyprogesterone in the serum from pregnant women. The quantification employed fluorescent derivatization using 4,4-difluoro-5,7-dimethyl-4-bora-3a,4adiaza-s-indacene-3-propionohydrazide (BODIPYTM FL hydrazide) [15] (Figure 10). The derivatization was carried out in ethanol at room temperature (about 22 °C) for 15 h. This derivatization method was reported to be 50 times faster than that using dansyl hydrazine. The LODs for progesterone, 17-hydroxyprogesterone, dehydroepiandrosterone, androstenedione, testosterone and 17-methyltestosterone were in the range of 550–3700 fmol per 10 µL injection (S/N = 5).

Figure 10. Derivatization of progesterone by 4,4-difluoro-5,7-dimethyl-4-bora-3a,4adiaza-s-indacene-3-propionohydrazide (BOD-IPYTM FL hydrazide).
The derivatization reagent 1-AN was used by Shimada et al. to determine the endogenous steroid pregnenolone in Wistar and Sprague–Dawley rat brain samples [10]. The samples were homogenized in isotonic saline then deproteinized with methanol, before subjected to sequential steps of extraction and derivatization. The samples were derivatized with 1-AN (Figure 11) and the excess reagent was removed by purification, carried out on two successive silica gel columns. The 3β-hydroxy-16-methylpregna-5,16-dien-20-one was used as an internal standard.
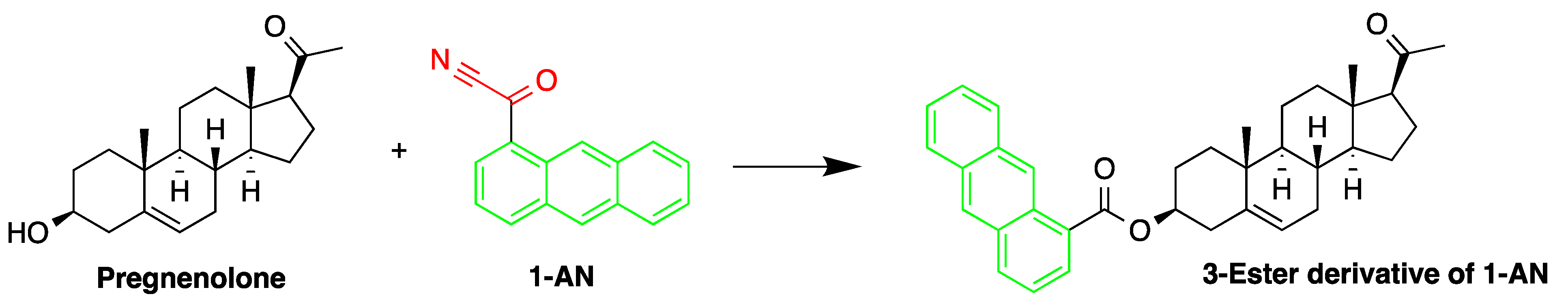
Figure 11. Derivatization of pregnenolone by 1-anthroyl nitrile (1-AN).
Several steroids in animal tissue were detected in another study using ultraviolet, fluorometric, and electrochemical detectors [21]. The study demonstrated the selectivity of FLD in determination of estradiol by exploiting its fluorescence emission, although it was eluted with the same fraction containing other steroids and their respective metabolites. Extraction with methanol allowed the separation of acidic corticosteroids (diethylstilbestrol, estradiol, zeranol/zearalenone, and their metabolites) from their neutral anabolic counterparts (testosterone, trenbolone and progesterone).
Urine is the best source for the estimation of estrogen concentrations, since they are primarily excreted renally as glucuronides and sulfates. Previous studies used urine samples for the determination of estrogen levels in their studies, albeit with different extraction methods [22][23]. Mao et al. used chemical hydrolysis in methanol and concentrated hydrochloric acid at 70 °C for 1 h to release the conjugation before SPE sample preparation, whereas Kumar et al. used a fabric phase sorptive extraction (FBSE) procedure, which offered shorter sample preparation times and 400 times higher sorbent loading. This method achieved a lower detection limit and analysis time, proving to be greener and more economical. The LODs for β-estradiol, 17α-ethinylestradiol, and bisphenol A were 20 pg/mL, 36 pg/mL, and 42 pg/mL, respectively.
In another study, Mao et al. used p-nitrobenzoyl chloride at 25 °C as a derivatization reagent, which can easily react with the hydroxyl and phenolic hydroxyl groups of organic chemicals (Figure 12) without the need for a catalyst [22]. Moreover, 4-nonylphenol, bisphenol A (BPA), 17α-ethinylestradiol, and three endogenic estrogens, including 17α-estradiol, 17β-estradiol, and estriol, were determined in urine samples collected from 20 healthy volunteers. Samples were hydrolyzed with HCl and subjected to SPE purification. Separation was performed on a C18 column with gradient elution using acetonitrile and water as a mobile phase. The LODs of the method were 2.7 µg/L for BPA and 17β-estradiol, 2.9 µg/L for 4-nonylphenol, 4.6 µg/L for 17α-estradiol and 17α-ethinylestradiol, and 8.3 µg/L for estriol.
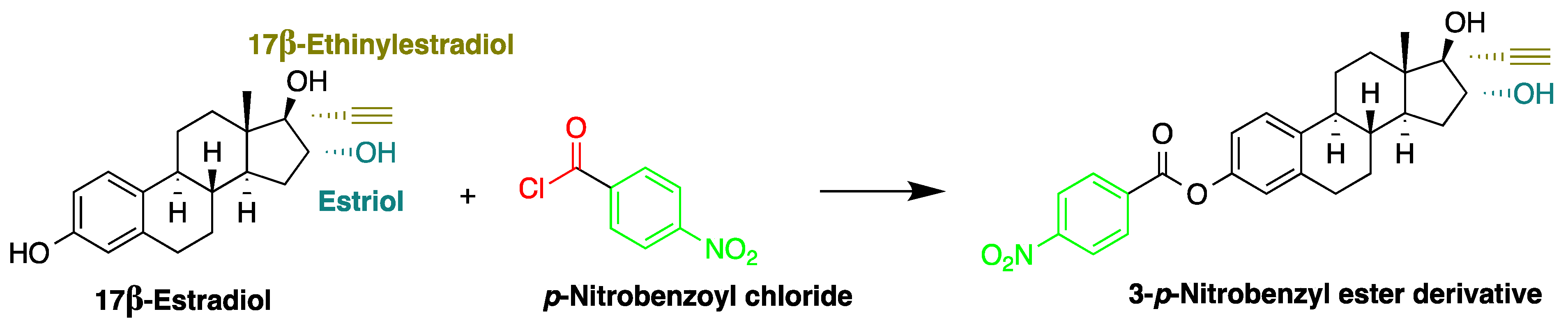
Figure 12. Derivatization of 17β-estradiol by p-nitrobenzoyl chloride.
A recent study examined the determination of estrone (E1), 17β-estradiol (E2), and estriol (E3) and their conjugated metabolites levels in cow and river buffalo meat [24]. Samples were extracted with methanol, enzymatically deconjugated and purified by C18 SPE before they are analyzed by HPLC-FLD. The effect of temperature on the studied steroid concentrations was also investigated and it was shown that heating processes was not able to significantly affect the level of phenolic estrogens in meat.
Estrogens in human urine samples were determined by HPLC-FLD following vortex-assisted dispersive liquid–liquid microextraction (VA-DLLME). Fine droplets of nonanoic acid floating on the top of sample solution were used to extract the estrogens using vortex-mix to assist the dispersion of the extraction solvent into the aqueous sample. Derivatization was not required in this work as the high extraction efficiency of DLLME improved the sensitivity and shortened the analysis time. LOD values were 0.01 ng/ml for E3, 0.01 ng/mL for βE2, and 0.06 ng/mL for E1, respectively [25].
The abuse of anabolic steroids has gained worldwide concerns, as frequent high doses can irreversibly affect the endocrine system, mineral metabolism, and may result in hepatic carcinomas [26]. Therefore, several committees have prohibited or imposed strict rules on their use for illegal purposes, such as increasing performance activity for athletes and stimulating meat production in cattle and poultry. Amin et al. used micellar chromatography with the detergent solution as the mobile phase to detect testosterone and bolasterone in human urine samples [26]. The method made use of fluorescence by means of energy transfer from the aromatic carbonyls in the anabolic steroids to the terbium ion in micellar media. No sample preparation was required and urine samples were injected directly onto the HPLC column. Excitation of terbium by means of energy transfer from steroids resulted in 183-fold fluorescence enhancement. The detection limits were 10 ng/200 µL injection volume for testosterone and 2 ng/200 µL for bolasterone.
4. Detection of Endocrine Disruptive Chemicals (EDCs)
The analysis and detection of steroid blood levels is vital for the investigation of food safety and effect on health [22][27]. Many studies have reported the presence of synthetic steroids in our daily food, both from plant and animal sources [28][29][30][31]. BPA, an industrial chemical, was found in biological fluids because of its ability to leach into food or liquids or through dental sealants into patient’s saliva [24][22][28][32][33]. One of the reasons behind their existence is that farmers increase their profit by using endocrine disruptive chemicals (EDCs) to support the feed conversion and growth rate in animals. Substances with hormonal actions are prohibited in the European community for use in animals intended for meat production due to their possible toxic effects on public health [27].
When bound to human estrogen receptors, they can stimulate the transcriptional activity of various estrogen receptor subtypes. The increase in levels of estrogen may be linked to the possibility of cancer occurrence among meat consumers [30].
In addition, studies have proved the ability of BPA to cross the placenta and blood brain barrier, in turn affecting the endocrine organs in animals and humans. This includes increasing prostate size, decreasing the number of produced sperms, and causing early puberty in females, in addition to effects on sexual differentiation [32][34]. Therefore, several HPLC-FLD methods were developed and used for quantification of EDC’s.
A sensitive HPLC method was developed for quantification of BPA and eight compounds of alkylphenol [35]. The alkylphenols assessed were; 4-sec-butylphenol, 2-tert-butylphenol, 3-tert-butylphenol, 4-tert-butylphenol, 4-n-pentylphenol, 4-tert-pentylphenol, 4-n-hexylphenol, and 4-n-heptylphenol. Derivatization was done using 2-(4-carboxyphenyl)-5,6-dimethylbenzimidazole (CDB) at 40 °C for 60 min. A C18 stationary phase was used for separation. The reported detection limits were in the range of 0.1–10.0 pg/mL. The method was applied to determination of bisphenol A in mother and infant rat serum samples.
The fluorescent reagent 4-(4,5-diphenyl-1H-imidazol-2-yl) benzoyl chloride (DIB-Cl) (Figure 13) was used by Sun et al. to detect EDC’s. To the evaporated sample residue, DIB-Cl suspension in acetonitrile and triethylamine in acetonitrile were added and reacted at room temperature [33].
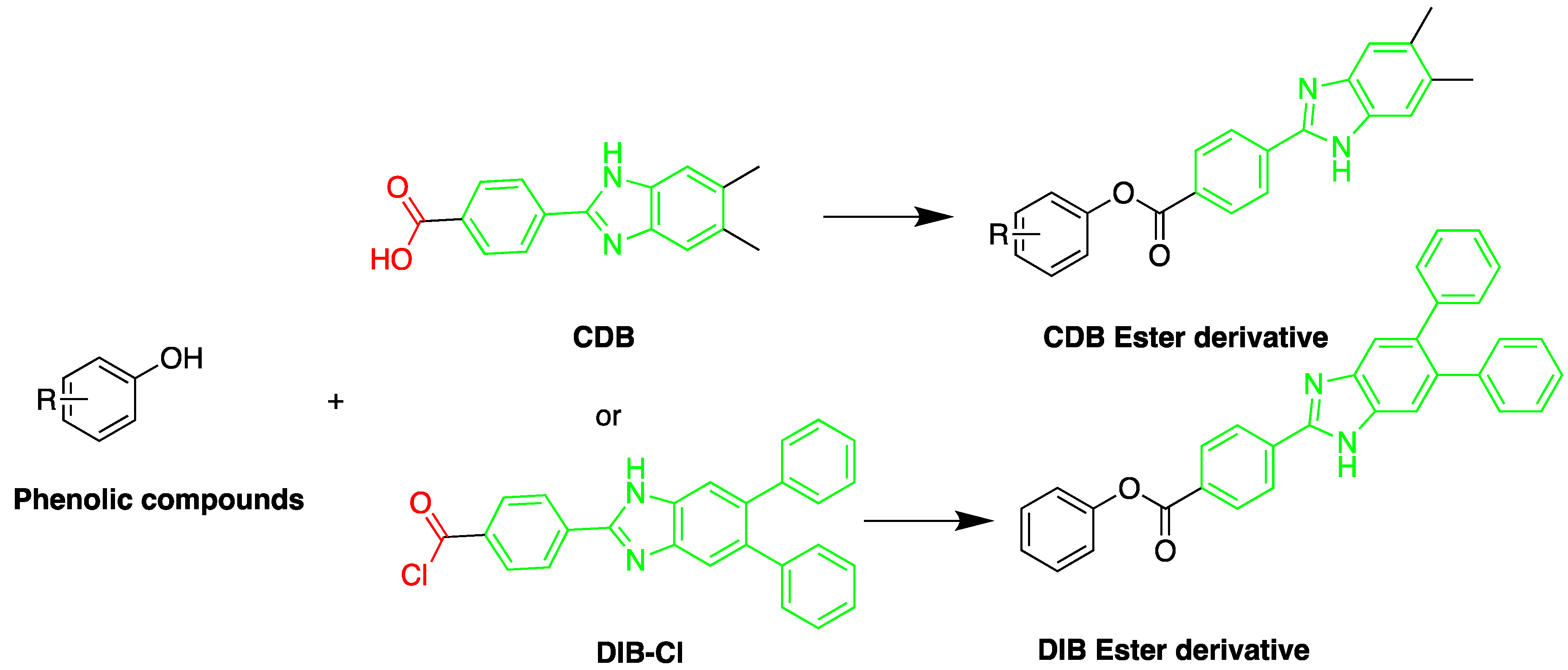
Figure 13. Derivatization of alkyl phenols by 2-(4-carboxyphenyl)-5,6-dimethylbenzimidazole (CDB) or 4-(4,5-diphenyl-1H-imidazol-2-yl) benzoyl chloride (DIB-Cl).
The study determined BPA in rat brain samples using HPLC-FLD coupled with a microdialysis [33]. A microdialysis probe was inserted into the hypothalamus of rat brains and artificial cerebrospinal fluid was used for perfusion. After the administration of a single intravenous or oral dose of BPA, concentrations were monitored in brain and plasma for 8 h. The obtained data proved that BPA could penetrate the blood brain barrier. The LOD of BPA was 0.3 ppb in 60 µL brain microdialysate at (S/N = 3).
Kuroda et al. used a similar method for determination of BPA in human blood serum and ascitic fluid samples. Samples were extracted by LLE using chloroform [32]. The LOD of BPA for both samples was 0.04 ppb at (S/N = 3). Human breast milk was also used to investigate the presence of BPA employing DIB-Cl as a fluorescent derivatizing agent [28]. Two steps of LLE were applied, and two C18 columns were used to separate DIB-BPA from the endogenous material in breast milk. The detection limit in 23 samples of healthy lactating women was 0.11 ng/mL at (S/N = 3).
The chromatographic conditions, sample preparation and detection limits of the HPLC-FLD methods that were employed in the steroid studies are summarized and presented in Table 1.
Table 1. HPLC-FLD method conditions used for steroid detection.
| Detected Steroids | Sample Type | Column Chemistry | Mobile Phase (v/v) | Derivatization Agent | Excitation (λex) and Emission Wavelengths (λem) (nm) | Extraction Method | LOD | References |
|---|---|---|---|---|---|---|---|---|
| PL and PN |
Human plasma | C18 | MeOH:ACN:1.0 M ammonium acetate (38:25:45) | DMB | 350, 390 | LLE | 3 ng/mL | Yamaguchi et al. 1991 [18] |
| F and E | Biological samples | Keystone Hypersil | H2O:MeOH:ACN (50:33.3:16.7) | 9-AN | 305–395, 430–470 | SPE | F: 50 pg E: 70 pg |
Haegele et al. 1991 [36] |
| F | Human serum | C18 | 1: 10 mM potassium biphthalate 2: ACN Tetrahydrofuran: 19 mM potassium biphthalate (40:6:54) Both adjusted to pH 1.85 with trifluoroacetic acid |
Sulfuric acid-ethanol | 365, 520 | - | 0.30 pg/dL | Nozaki et al. 1991 [5] |
| F | Human urine | C18 | Gradient of ACN: 36.4 mmol/L phosphate (45:55; pH 1.85 with trifluoroacetic acid) |
Sulfuric acid-ethanol | 365, 520 | SPE | 0.26 pg/dL | Nozaki et al. 1992 [6] |
| 7α-Hydroxycholesterol | Dog plasma | Develosil Ph-5 | Acetonitrile: Water (5:2) |
1-AN | 338, 411 | LLE | 4 pg | Saisho et al. 1998 [20] |
| Corticosteroids | Urine | Silica | 2-Propanol–hexane | 9-AN | 370, 470 | Enzyme hydrolysis, extraction with 0.5 M NaOH | NR | Neufeld et al. 1998 [11] |
| F and E | Human plasma | C18 | ACN: 0.3 mM ortho-phosphoric acid (470:530) | 9-AN | 360, 460 | SPE | 3.0 ng/mL | Glowka et al. 2009 [12] |
| Corticosterone | Rat urine | CN | ACN: H2O (24.5:75.5) | post-column reaction with sulfuric acid |
460, 510 | LLE | 0.5 pmol | Sudo et al. 1990 [3] |
| F | Human hair | C18 | MeOH:H2O 60:40 |
sulfuric acid | 360, 480 | LLE | 1 pg/mg | Gao et al. 2010 [2] |
| EE2, E2, and BPA | Human urine and aqueous samples | C18 | ACN:MeOH:H2O (30:15:55) | - | 280, 310 | FPSE | E2: 20 pg/mL EE2: 36 pg/mL BPA: 42 pg/mL |
Kumar et al. 2014 [23] |
| BPA, NP, E2, EE2, and E3 | Human urine | C18 | Gradient elution of ACN and H2O | p-nitrobenzoyl chloride | E2, E3: 282, 315 BPA, NP, EE2: 228, 316 |
SPE | BPA and E2: 2.7 μg/L NP: 2.9 μg/L E2 and EE2: 4.6 μg/L E3: 8.3 μg/L |
Mao et al. 2004 [22] |
| E, testosterone, methyltestosterone, bolasterone, testosterone acetate, progesterone | Urine | C18 | 0.01 M Tb(NO3)3, 0.1 M sodium dodecyl sulfate (SDS), and 20% acetonitrile |
- | 245, 547 | SPE | Down to 100 pg/mL | Amin et al. 1993 [26] |
| BPA and 8 alkylphenols (4-sec-Butylphenol, 2-tert-Butylphenol, 3-tert-butylphenol, 4-tert-butylphenol, 4-n-Pentylphenol, 4-tert-pentylphenol, 4-n-hexylphenol, and 4-n-heptylphenol) |
Rat plasma and blood | C18 | MeOH:Water (10:90) | 2-(4-carboxyphenyl)-5,6- dimethylbenzimidazole |
Derivatized: 336, 440 Native: 275, 315 |
LLE | BPA: 0.1 pg/mL Alkylphenol: 0.7–10 pg/mL |
Katayama et al. 2001 [35] |
| progesterone and 17-hydroxyprogesterone dehydroepiandrosterone, androstenedione, testosterone and 17-methyltestosterone |
Serum from pregnant and non-pregnant women | Wakosil 5C4 | Acetonitrile:Water (7:3) | BODIPY FL hydrazide | 495, 516 | LLE | 550–3700 fmol per 10 μL | Katayama et al. 1998 [37] |
| aldosterone, corticosterone, F, E, dexamethasone, fluocinolone acetonide, triamcinolone and triamcinolone acetonide |
Human plasma | C18 | Water:MeOH (25:75) containing 5 mmol/L tetramethylammonium hydrogen sulphate | CDB | 334, 418 | LLE | 0.06–0.3 pg per 100 μL | Katayama et al. 1992 [9] |
| BPA | Breast Milk | C18 | 1: ACN:H2O:MeOH (72:13:15) 2: ACN:0.1 M acetate buffer (pH 5.5):MeOH (55:12:33) |
DIB-Cl | 350, 475 | SPE then LLE | 0.11 ng/mL | Sun et al. 2004 [28] |
| BPA | Human blood serum and ascitic fluid samples | C18 | 1: ACN: H2O:MeOH (72:13:15) 2: ACN:0.1 M Acetate buffer (pH 5.5):MeOH (55:12:33) |
DIB-Cl | 350, 475 | LLE | 0.04 ppb | Kuroda et al. 2003 [32] |
| BPA | Rat brain rat plasma |
C18 | 1: ACN: H2O:MeOH:Tetrahydrofuran (55:10:35:2.5) 2: ACN:0.1 M Acetate buffer (pH 3.0):MeOH (35:10:55) |
DIB-Cl | 350, 475 | LLE | 0.3 ppb in 60 μL rat brain 4.6 ppb in 50 μL rat plasma |
Sun et al. 2002 [33] |
| F, E, PL, PN, 6β-OHF, 6β-OHP and 6β-OHE |
Human plasma and urine | Cosmosil 5SL | diethylene dioxide: ethyl acetate:chloroform:n-hexane:pyridine (500:100:100:1400:21) | 9-AN | 360, 460 | LLE | F, E, PL and PN: 0.1 ng/mL 6β-OHF and 6β-OHP: 0.5 ng/mL |
Shibata et al. 1997 [13] |
| Corticosterone | Rat serum | C18 | 60% MeOH: 40% 5 mM triethylamine, pH 3.3 |
- | 375, 485 | LLE | 0.1 ng | Mason et al. 1992 [7] |
| 18-Oxygenated corticosteroids, 18-hydroxycortisol, 18- hydroxycortisone and 18-oxocortisol |
Human urine | μBondasphere phenyl | A: 10 mM ammonium acetate: MeOH (50:50) B: ACN |
1-AN | 370, 470 | LLE and SPE | 0.1 pmol | Kurosawa et al. 1995 [14] |
| Cholesterol and sitosterol | Saliva and urine biosamples, Cow milk, and Soybean milk | C8 | MeOH:isopropanol:H2O (90:5:5) |
naproxen acyl chloride | 231, 352 | LLE | 25 nM per 10 μL injected volume | Lin et al. 2007 [19] |
| Pregnenolone | Rat brain | C18 | MeOH:H2O (9:1) | 1-AN | 370, 470 | SPE | NR | Shimada et al. 1996 [38] |
| C21 steroids; corticoids | Steroid standards | C18 | MeOH:H2O:cyclodextrin | 1-AN | 360, 460 | NR | NR | Shimada et al. 1991 [39] |
| Triamcinolone | Human plasma | C18 | ACN and 0.3 mM ortho-phosphoric acid |
9-AN | 360, 460 | SPE | 1 ng/mL | Glowka et al. 2006 [40] |
| Butane acid-(5-androsten-17-one-3beta-ol)-diester (A1998) | Rat plasma | C18 | 25 mM acetate buffer (pH 3.7):ACN Alfaxalone: (45:55) Pregnanolone: (40:60) |
Dansyl hydrazine | 332, 516 | LLE | 10 ng/mL | Visser et al. 2000 [41] |
| Alfaxalone and pregnanolone | Rat plasma | C18 | Gradient mixture of ACN and H2O |
Dansyl Hydrazine | 350, 520 | - | 0.025 μg/mL | Peng et al. 2007 [42] |
| EED | Oral contraceptive tablets | STAR RP-18e | ACN:H2O (47:53) | - | EED: 285, 310 | - | EED: 0.0538 μg/ml | Sarafinovska et al. 2006 [43] |
| EED and drospirenone | Oral contraceptive tablets | STAR RP-18e RP | ACN:H2O (47:53) | - | 285, 310 | - | EED: 0.00065 μg/mL DROSP: 0.0774 μg/mL |
Sarafinovska et al. 2009 [44] |
| EED | Coated tablets | LiChroCART 100RP | ACN:H2O (50:50) | - | 280, 310 | - | EED: 0.02 μg/mL | Silva et al. 2013 [45] |
| Sodium E1 sulphate, sodium equilin sulphate, E1 and equilin |
Raw materials and Pharmaceuticals |
5 ODS2 | TEA phosphate buffer (pH 4.0; 0.05 M):ACN 1— (70:30, v:v) 2—for unconjugated estrogens: (66:34) |
Postcolumn on line photochemical derivatization | 280, 410 or 312 | - | 0.01–1.38 pmol | Gatti et al. 1998 [46] |
| E1, 17β-Estradiol, E3, BPA, NP, OP |
Fish, chicken, aquaculture pond water sample | C18 | Gradient program of 1: H2O: 5% ACN 2: H2O: ACN |
BCEC-Cl | 279, 380 | DLLME | 0.02–0.07 µg/L | Wu et al. 2015 [47] |
| E1, E2, and E3 |
Cow and river Buffalo | C18 | 1: ACN:H2O:Formic Acid (40:60:0.4) 2: ACN:H2O:Formic Acid (90:10:0.4) |
- | 280, 310 | LLE and SPE | 5–10 ng/kg | Shahbazi et al. 2016 [48] |
| α- and β-Trenbolone | Bovine muscle and liver | C18 | MeOH:H2O (60:40) | - | 364, 460 | LLE then SPE | bovine muscle: 0.2 ng/g liver: 1.0 ng/g |
Yoshioka et al. 2000 [49] |
| NP, 4-nonylphenol mono-(NP1EO), diethoxylates (NP2EO), BPA, TBP, and OP |
Fish and shellfish | Inertsil PH | Gradient program of A: H2O B: MeOH |
- | 275, 300 | LLE | NP NP1EO and NP2EO : 2 ng/g BPA, BP and OP: 1 ng/g |
Tsuda et al. 2000 [24] |
| Nonylphenol and its ethoxylates | Fish tissue | Hypersil APS | Hexane:ethanol (98:2) | - | 230, 300 | Pressurized fluid extraction | 4–15 ng/mL | Datta et al. 2002 [50] |
| E2 and EE2 | Poultry litter | C18 | Gradient program of A: H2O B: ACN |
- | 280, 312 | LLE | E2: 4.0 μg/kg EE2: 2.6 μg/kg |
Lu et al. 2014 [51] |
| E2 and EE2 | Waste Water | C18 | Gradient program of A: H2O B: ACN |
- | 282, 306 | SPE | 2.5 ng/L | Liz et al. 2017 [52] |
| E3, E2, EE2, HEX, mestranol |
Water, sediment | Poroshell 120 EC | H2O:ACN 50:50 | - | 275, 310 | SPE | Water: 6–24 ng/L Sediment: 0.1–0.9 ng/g |
Perez et al. 2015 [53] |
| E2, 17α-EE2, and E1 | Water | C18 | Gradient program of A: ACN B: H2O acidified at pH 3.6 with glacial acetic acid |
- | 230, 302 | SPE | 10 to 1100 ng/L |
Patrolecco et al. 2013 [54] |
| OP, NP, BPA, diethylstilbestrol, E1, EE2, E2, and E3 |
Wastewater samples |
C18 | Gradient program of A: 5% ACN B: ACN |
EASC | 262, 430 | SPE | 0.3–0.7 ng/L | Zhang et al. 2012 [55] |
| E2 and EE2 |
Tap, surface and waste water | C18 | Water:acetonitrile mixture (50:50) | - | 280, 310 | DLLME | E2: 2.0 ng/L EE2: 6.5 ng/L |
Lima et al. 2013 [56] |
| BPA, 17β-estradiol, and 17α-ethynyl estradiol | Drinking water | LiChro-sorbs RP18 | 10 mM H3PO4:55% MeOH (45:55) | - | 280, 310 | PAC | BPA: 201 ng/L E2: 313 ng/L EE2: 284.5 ng/L |
Yoon et al. 2003 [57] |
| 17α- and 17β-Trenbolone | River water | C18 | Gradient program of MeOH:H2O |
- | 359, 458 | SPE | 4 ng/L | Durhan et al. 2005 [58] |
| NP, OP, NP polyethoxylates |
municipal wastewater treatment plants | C18 | Gradient program of H2O:ACN |
- | 229, 310 | LLE | OP: 2 ng/L NP: 11 ng/L NPE: 52 ng/L |
Snyder et al. 1999 [59] |
| E2, E3, BPA, and 17β-ethinylestradiol |
environmental waters |
C18 | ACN:0.02 mol/L phosphate solution (45:55) | - | 227, 315 | Synthesized in-tube SPME device | 0.006–0.10 ng/mL | Wen et al. 2006 [60] |
| E2, E3, EE2, 3-methyl ether EE2, NP, OP, POE(1-2) nonyl phenol and BPA |
River water | C18 | Gradient program of Milli-Q H2O and ACN |
- | 230, 290 | on-line SPE | 20–50 ng/L | Ying et al. 2002 [61] |
| Endocrine disruptors; BPA and EE2 | Environmental water samples | C18 | MeOH:0.025 mol/L Na2HPO4 buffer (70:30) | - | 220, 315 | A poly(acrylamide-vinylpyridine) monolithic capillary column |
BPA: 0.064 ng/mL 17α-ethinylestradiol: 0.12 ng/mL |
Fan et al. 2005 [62] |
| Brassinosteroids | Plant: (Vicia faba L.) |
C18 | ACN: H2O (90:10) | 9-Phenanthreneboronic acid | 305, 375 | LLE | 50 pg | Gamoh et al. 1989 [63] |
| Brassinolide | Arabidopsis thaliana, Daucus carota and Brassica campestris L. leaves’ samples |
C18 | Gradient program of A: H2O and ACN B: ACN and 0.1% Formic Acid |
- | 305, 375 | UA-DLLME | 8.0 ng/L | Lv et al. 2014 [64] |
| Brassinolide and castasterone | Pollen of orange (Citrus sinensis Osbeck) | C18 | ACN:H2O (80:20) | Dansylaminophenylboronic acid |
345, 515 | LLE | NR | Motegi et al. 1994 [65] |
| Norgestrel, norethindrone, EE2, gestodene, and norethisterone acetate | Meat samples | Hypersil GOLD | Gradient program of (A) H2O containing 5% ACN (B)ACN |
Fmoc-Cl | 250, 395 | MSPE | 1.4 × 103–8.7 × 103 | Qianyu Li et al. 2018 [66] |
| F and E | Human urine samples | C18 | ACN:0.3 mM orthophosphoric acid (470: 530) | 9-AN | 360, 460 | LLE | LLOQ: F: 27.6 nmol/L E: 27.7 nmol/L |
Kosicka et al. 2018 [67] |
| FFA | Edible oils and foodstuff | C8 | Gradient system: A: H2O B: ACN/DMF (1:1) C: ACN (100%) |
BCETS | 279, 380 | Supercritical CO2 and organic solvent extraction | 0.22–1.06 ng/mL | Li et al. 2011 [68] |
| OP, NP, TBP, BPA, E1, E2, E3 | Milk samples | C18 | Gradient program of A:5% ACN in H2O B: ACN |
BQEIC | 302, 401 | LLE | 10.5–13.8 ng/L | Liu et al. 2018 [69] |
| E3, 2-OHE2, 17β-E2, 17α-E2, EE2, HEX | Dairy products | C18 | Gradient Flow A: 1 mM formic acid in ACN B: 1 mM formic acid at pH 3.50 |
- | 280, 310/320 | HF-LPME | 0.23–14.8 μg/kg | Bárbara Socas-Rodríguez et al. 2014 [70] |
| Zearalenone | Edible oil | C18 | Gradient program of A: H2O, B: ACN |
274, 456 | SPE | 10 μg/kg | Drzymala et al. 2015 [71] | |
| EE2, E1, E2, E3, and progesterone | Drinking water and wastewater samples | C18 | Gradient program of A: H2O/CH3CN 90/10 v/v B: CH3CN |
200, 315 | SPE | Drinking water: 1–3.8 ng/L Sewage water: 3.8–7.5 ng/L |
Kozłowska-Tylingo et al. 2015 [72] | |
| E3, E2, E1 | Human urine | C18 | Gradient program of A: H2O, B: ACN |
280, 310 | VA-DLLME-FOA | E3: 0.01 ng/mL β-E2: 0.01 ng/mL E1: 0.06 ng/mL |
Wang et al. 2015 [25] | |
| 17-α-E2, 17-β-E2 benzoate and quinestrol |
Environmental water samples | Zorbax Eclipse SB-C18 | Gradient program of A: ACN, B: H2O |
265, 311 | IF-IHLME | 17-α-Estradiol: 0.04 ng/mL E2 and Quinestrol: 0.05 ng/mL |
Zhang et al. 2017 [73] | |
| E2 and EE2 | Tap water samples | Pursuit 5 C18 column | ACN:H2O (50:50), with 200 μL of H3PO4 | 230, 306 | Nanoparticles of graphene oxide/γ-Fe2O3 as a sorbent for SPE |
E2: 2.7 ng/L EE2: 0.8 ng/L |
Fernanda Nunes Ferreira et al. 2020 [74] | |
| 17-E2 and E3 | Water samples | C18 | H2O:MeOH:ACN (50:30:20) |
280, 310 | ultrasonication assisted DLLME | DLLME-HPLC/FLD: 7.16–69.22 ng/L | Zhang et al. 2020 [75] | |
| E2, 1,3,5(10)-Estratriene-3,17β-diol | Fish and prawn tissue samples | ODS C18 | H2O:MeOH (30:70) | 280,310 | MISPE | 0.023 mg/L | Jiang et al. 2009 [76] | |
| BPA, EE2, 4-t- OP, 4-OP, and 4-NP |
River water | C18 | Gradient program of A: ACN B: H2O |
277, 307 | Disposable pipette extraction (DPX) | BPA, EE2, 4-OP and 4-NP: 0.30 μg/L 4-t-OP: 0.60 μg/L |
Gabriela Corazza et al. 2017 [77] | |
| E1 and EE2 | Digested sludge | C18-PFP | A: H2O B: ACN E1: (50:50) EE2 (55:45) |
280, 310 | ultrasonic liquid extraction | E1: 0.305 μg/g EE2: 0.052 μg/g |
Vitória L. Louros et al. 2019 [78] | |
| E2 | Milk sample | XDB-C18 | MeOH:H2O (70:30) |
280, 310 | SPE | 0.7 ng/mL | Yanan Yuan et al. 2019 [79] | |
| Nine BPs | milk samples | C18 | Gradient program of 0.1% formic acid: ACN |
230, 305 | ultrasonically with acetonitrile and cleaned using the QuEChERS technique. |
1.0–3.1 µg/kg | Xiong et al. 2017 [80] | |
| EE | river water samples | 5C18 MS-II | ACN: 5.0 mM Tris-HNO3 buffer, pH 7.4 (60:40) | 310, 400 | C18 SPE disk | 7.4 ng/L | Ali et al. 2020 [81] | |
| E3, 17β-estradiol glucuronide, 17β-E2, 17α-E2, 17β-E2-3-methyl ether |
wastewater | UPLC C18 | Gradient program of water with 0.1% of ammonia: ACN |
280, 310 | Molecularly Imprinted SPE | 1.4 to 2.5 ng/mL | Rayco Guedes-Alonso et al. 2015 [82] |
Abbreviation: solid phase extraction (SPE), liquid–liquid extraction (LLE), dispersive-liquid–liquid extraction (DLLME), ultrasonic-assisted dispersive liquid–liquid microextraction (UA-DLLME), vortex-assisted dispersive liquid–liquid microextraction method based on floating organic acid droplet (VA-DLLME-FOA), ionic liquid foam floatation coupled with an ionic liquid-based homogeneous liquid–liquid microextraction (IF-IHLME), magnetic solid phase extraction (MSPE), fabric phase sorptive extraction (FPSE), molecularly imprinted solid-phase extraction (MISPE), hollow fiber liquid-phase microextraction (HF-LPME), 4-octylphenol (OP), 4-tert-octylphenol (4-t-OP), 4-nonylphenol (NP), 4-tert-butylphenol (TBP), bisphenol A (BPA), estrone (E1), 17β-estradiol (17β-E2), 17α-estradiol (17α-E2), estriol (E3), free fatty acids (FFA), 17α-ethinylestradiol (EE2), ethinylestradiol (EED), cortisol (F), cortisone (E), 4,40-(1,2-diethylethylene)diphenol (HEX), prednisolone (PL), prednisone (PN), 6β-hydroxycortisol (6β-OHF), 6β-hydroxyprednisolone (6β-OHP) and 6β-hydroxycortisone (6β-OHE), room temperature (RT).
References
- Norman, A.W.; Litwack, G. Hormones; Elsevier: Amsterdam, The Netherlands, 1997; ISBN 9780125214414.
- Gao, W.; Xie, Q.; Jin, J.; Qiao, T.; Wang, H.; Chen, L.; Deng Huihua, H.; Lu, Z. HPLC-FLU detection of cortisol distribution in human hair. Clin. Biochem. 2010, 43, 677–682.
- Sudo, A. Analysis of corticosterone in rat urine by high-performance liquid chromatography and fluorimetry using post-column reaction with sulphuric acid. J. Chromatogr. B Biomed. Sci. Appl. 1990, 528, 453–458.
- Nozaki, O. Steroid analysis for medical diagnosis. J. Chromatogr. A 2001, 935, 267–278.
- Nozaki, O.; Ohata, T.; Ohba, Y.; Moriyama, H.; Kato, Y. Determination of serum cortisol by reversed-phase liquid chromatography using precolumn sulphuric acid-ethanol fluorescence derivatization and column switching. J. Chromatogr. B Biomed. Sci. Appl. 1991, 570, 1–11.
- Nozaki, O.; Ohata, T.; Ohba, Y.; Moriyama, H.; Kato, Y. Determination of urinary free cortisol by high performance liquid chromatography with sulphuric acid–ethanol derivatization and column switching. Biomed. Chromatogr. 1992, 6, 109–114.
- Mason, S.R.; Ward, L.C.; Reilly, P.E.B. Fluorimetric detection of serum corticosterone using high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1992, 581, 267–271.
- Katayama, M.; Masuda, Y.; Taniguchi, H. Determination of alcohols by high-performance liquid chromatography after pre-column derivatization with 2-(4-carboxyphenyl)-5,6-dimethylbenzimidazole. J. Chromatogr. A 1991, 585, 219–224.
- Katayama, M.; Masuda, Y.; Taniguchi, H. Determination of corticosteroids in plasma by high-performance liquid chromatography after pre-column derivatization with 2-(4-carboxyphenyl)-5,6-dimethylbenzimidazole. J. Chromatogr. B Biomed. Sci. Appl. 1993, 612, 33–39.
- Goto, J.; Goto, N.; Shamsa, F.; Saito, M.; Komatsu, S.; Suzaki, K.; Nambara, T. New sensitive derivatization of hydroxysteroids for high-performance liquid chromatography with fluorescence detection. Anal. Chim. Acta 1983, 147, 397–400.
- Neufeld, E.; Chayen, R.; Stern, N. Fluorescence derivatisation of urinary corticosteroids for high-performance liquid chromatographic analysis. J. Chromatogr. B Biomed. Appl. 1998, 718, 273–277.
- Główka, F.K.; Kosicka, K.; Karaźniewicz-Łada, M. HPLC method for determination of fluorescence derivatives of cortisol, cortisone and their tetrahydro- and allo-tetrahydro-metabolites in biological fluids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 283–289.
- Shibata, N.; Hayakawa, T.; Takada, K.; Hoshino, N.; Minouchi, T.; Yamaji, A. Simultaneous determination of glucocorticoids in plasma or urine by high-performance liquid chromatography with precolumn fluorimetric derivatization by 9-anthroyl nitrile. J. Chromatogr. B Biomed. Appl. 1998, 706, 191–199.
- Kurosawa, S.; Koike, T.; Yoshimura, T.; Kurosawa, T.; Tohma, M.; Chiba, H.; Kobayashi, K. Simultaneous determination of 18-oxygenated corticosteroids by high-performance liquid chromatography with fluorescence detection. J. Liq. Chromatogr. 1995, 18, 2383–2396.
- Kawasaki, T.; Maeda, M.; Tsuji, A. Determination of plasma and urinary cortisol by high-performance liquid chromatography using fluorescence derivatization with dansyl hydrazine. J. Chromatogr. B Biomed. Sci. Appl. 1979, 163, 143–150.
- Seki, T.; Yamaguchi, Y. New fluorimetric determination of 17-hydroxycorticosteroids after high-performance liquid chromatography using post-column derivatization with benzamidine. J. Chromatogr. B Biomed. Sci. Appl. 1984, 305, 188–193.
- YAMAGUCHI, M.; YOSHITAKE, T.; ISHIDA, J.; NAKAMURA, M. Determination of 21-hydroxycorticosteroids in human urine by high-performance liquid chromatography with fluorescence detection. Chem. Pharm. Bull. 1989, 37, 3022–3025.
- Yamaguchi, M.; Ishida, J.; Yoshitake, T.; Nakamura, M. Determination of prednisolone and prednisone in plasma by liquid chromatography with fluorescence detection. Anal. Chim. Acta 1991, 242, 113–116.
- Lin, Y.T.; Wu, S.S.; Wu, H.L. Highly sensitive analysis of cholesterol and sitosterol in foods and human biosamples by liquid chromatography with fluorescence detection. J. Chromatogr. A 2007, 1156, 280–287.
- Saisho, Y.; Shimada, C.; Umeda, T. Determination of 7α-hydroxycholesterol in dog plasma by high- performance liquid chromatography with fluorescence detection. Anal. Biochem. 1998, 265, 361–367.
- Laganà, A.; Marino, A. General and selective isolation procedure for high-performance liquid chromatographic determination of anabolic steroids in tissues. J. Chromatogr. A 1991, 588, 89–98.
- Mao, L.; Sun, C.; Zhang, H.; Li, Y.; Wu, D. Determination of environmental estrogens in human urine by high performance liquid chromatography after fluorescent derivatization with p-nitrobenzoyl chloride. Anal. Chim. Acta 2004, 522, 241–246.
- Kumar, R.; Gaurav; Heena; Malik, A.K.; Kabir, A.; Furton, K.G. Efficient analysis of selected estrogens using fabric phase sorptive extraction and high performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2014, 1359, 16–25.
- Tsuda, T.; Suga, K.; Kaneda, E.; Ohsuga, M. Determination of 4-nonylphenol, nonylphenol monoethoxylate, nonylphenol diethoxylate and other alkylphenols in fish and shellfish by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 2000, 746, 305–309.
- Wang, P.; Qiu, X.; Yang, Y. Determination of estrogens in human urine by vortex-assisted dispersive liquid-liquid microextraction based on floating organic acid droplet combined with high-performance liquid chromatography-fluorescence detection. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 640–646.
- Amin, M.; Harrington, K.; Von Wandruszka, R. Determination of Steroids in Urine by Micellar HPLC with Detection by Sensitized Terbium Fluorescence. Anal. Chem. 1993, 65, 2346–2351.
- Impens, S.; De Wasch, K.; Cornelis, M.; De Brabander, H.F. Analysis on residues of estrogens, gestagens and androgens in kidney fat and meat with gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2002, 970, 235–247.
- Sun, Y.; Irie, M.; Kishikawa, N.; Wada, M.; Kuroda, N.; Nakashima, K. Determination of bisphenol A in human breast milk by HPLC with column-switching and fluorescence detection. Biomed. Chromatogr. 2004, 18, 501–507.
- Kawamura, Y.; Koyano, Y.; Takeda, Y.; Yamada, T. Migration of Bisphenol a from Polycarbonate Products. J. Food Hyg. Soc. Japan 1998.
- Cho, E.; Chen, W.Y.; Hunter, D.J.; Stampfer, M.J.; Colditz, G.A.; Hankinson, S.E.; Willett, W.C. Red meat intake and risk of breast cancer among premenopausal women. Arch. Intern. Med. 2006, 166, 2253–2259.
- Noda, M.; Komatsu, H.; Sano, H. HPLC analysis of dental resin composites components. J. Biomed. Mater. Res. 1999, 47, 374–378.
- Kuroda, N.; Kinoshita, Y.; Sun, Y.; Wada, M.; Kishikawa, N.; Nakashima, K.; Makino, T.; Nakazawa, H. Measurement of bisphenol A levels in human blood serum and ascitic fluid by HPLC using a fluorescent labeling reagent. J. Pharm. Biomed. Anal. 2003, 30, 1743–1749.
- Sun, Y.; Nakashima, M.N.; Takahashi, M.; Kuroda, N.; Nakashima, K. Determination of bisphenol A in rat brain by microdialysis and column switching high-performance liquid chromatrography with fluorescence detection. Biomed. Chromatogr. 2002, 16, 319–326.
- Vom Saal, F.S.; Cooke, P.S.; Buchanan, D.L.; Palanza, P.; Thayer, K.A.; Nagel, S.C.; Parmigiani, S.; Welshons, W.V. A Physiologically Based Approach To the Study of Bisphenol a and Other Estrogenic Chemicals On the Size of Reproductive Organs, Daily Sperm Production, and Behavior. Toxicol. Ind. Health 1998, 14, 239–260.
- Katayama, M.; Sasaki, T.; Matsuda, Y.; Kaneko, S.; Iwamoto, T.; Tanaka, M. Sensitive determination of bisphenol A and alkylphenols by high performance liquid chromatography with pre-column derivatization with 2-(4-carboxyphenyl)-5,6-dimethylbenzimidazole. Biomed. Chromatogr. 2001, 15, 403–407.
- Haegele, A.D.; Wade, S.E. Ultrasensitive differential measurement of cortisol and cortisone in biological samples using fluorescent ester derivatives in normal phase hplc. J. Liq. Chromatogr. 1991, 14, 1133–1148.
- Katayama, M.; Nakane, R.; Matsuda, Y.; Kaneko, S.; Hara, I.; Sato, H. Determination of progesterone and 17-hydroxyprogesterone by high performance liquid chromatography after pre-column derivatization with 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a- diaza-s-indacene-3-propionohydrazide. Analyst 1998, 123, 2339–2342.
- Shimada, K.; Nakagi, T. Studies on neurosteroids. IV. Quantitative determination of pregnenolone in rat brains using high-performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 1996, 19, 2593–2602.
- Shimada, K.; Nonaka, M. Utility of cyclodextrin in mobile phase for high-performance liquid chromatographic separation of C21 steroids. J. Liq. Chromatogr. 1991, 14, 2109–2117.
- Główka, F.K.; Karaźniewicz, M.; Lipnicka, E. RP-HPLC method with fluorescence detection for determination of small quantities of triamcinolone in plasma in presence of endogenous steroids after derivatization with 9-anthroyl nitrile; pharmacokinetic studies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 839, 54–61.
- Visser, S.A.G.; Smulders, C.J.G.M.; Gladdines, W.W.F.T.; Irth, H.; Van Der Graaf, P.H.; Danhof, M. High-performance liquid chromatography of the neuroactive steroids alphaxalone and pregnanolone in plasma using dansyl hydrazine as fluorescent label: Application to a pharmacokinetic-pharmacodynamic study in rats. J. Chromatogr. B Biomed. Sci. Appl. 2000, 745, 357–363.
- Peng, X.D.; Xu, D.H.; Jin, J.; Mei, X.T.; Lv, J.Y.; Xu, S.B. Determination of a new active steroid by high performance liquid chromatography with laser-induced fluorescence detection following the pre-column derivatization. Int. J. Pharm. 2007, 337, 25–30.
- Arsova-Sarafinovska, Z.; Ugrinova, L.; Starkoska, K.; Djordjev, D.; Dimitrovska, A. Determination of ethinylestradiol and levonorgestrel in oral contraceptives with HPLC methods with UV detection and UV/fluorescence detection. Maced. Pharm. Bull. 2006, 52, 9–16.
- Arsova-sarafinovska, Z.; Polozhani, A.; Dimitrovska, A. Determination of ethinylestradiol and drospirenone in oral contraceptives with HPLC method with UV and fluorescence detection Determination of ethinylestradiol and drospirenone in oral contraceptives with HPLC method with UV and fluorescence detection. Arch. Public Health 2009, 1, 67.
- Silva, V.B.; Galdos, A.A.G.; Mothe, C.M.A.; Pallastrelli, M.B.; Prado, M.S.A.; Singh, A.K.; Kedor-Hackmann, E.R.M.; Santoro, M.I.R.M. Simultaneous determination of ethinyl estradiol and drospirenone in oral contraceptive by high performance liquid chromatography. Brazilian J. Pharm. Sci. 2013, 49, 521–528.
- Gatti, R.; Gotti, R.; Gioia, M.G.; Cavrini, V. HPLC analysis of pharmaceutical estrogens in raw materials and dosage forms. J. Pharm. Biomed. Anal. 1998, 17, 337–347.
- Wu, H.; Li, G.; Liu, S.; Hu, N.; Geng, D.; Chen, G.; Sun, Z.; Zhao, X.; Xia, L.; You, J. Monitoring the contents of six steroidal and phenolic endocrine disrupting chemicals in chicken, fish and aquaculture pond water samples using pre-column derivatization and dispersive liquid-liquid microextraction with the aid of experimental design metho. Food Chem. 2016, 192, 98–106.
- Shahbazi, Y.; Malekinejad, H.; Tajik, H. Determination of naturally occurring estrogenic hormones in cow’s and river buffalo’s meat by HPLC-FLD method. J. Food Drug Anal. 2016, 24, 457–463.
- Yoshioka, N.; Akiyama, Y.; Takeda, N. Determination of α- and β-trenbolone in bovine muscle and liver by liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 2000, 739, 363–367.
- Datta, S.; Loyo-Rosales, J.E.; Rice, C.P. A simple method for the determination of trace levels of alkylphenolic compounds in fish tissue using pressurized fluid extraction, solid phase cleanup, and high-performance liquid chromatography fluorescence detection. J. Agric. Food Chem. 2002, 50, 1350–1354.
- Lu, J.; Kong, D.; Zhao, L.; Zhou, Q. Analysis of oestrogenic hormones in chicken litter by HPLC with fluorescence detection. Int. J. Environ. Anal. Chem. 2014, 94, 783–790.
- De Liz, M.V.; Do Amaral, B.; Stets, S.; Nagata, N.; Peralta-Zamora, P. Sensitive estrogens determination in wastewater samples by HPLC and fluorescence detection. J. Braz. Chem. Soc. 2017, 28, 1453–1460.
- Pérez, R.L.; Escandar, G.M. Multivariate calibration-assisted high-performance liquid chromatography with dual UV and fluorimetric detection for the analysis of natural and synthetic sex hormones in environmental waters and sediments. Environ. Pollut. 2016, 209, 114–122.
- Patrolecco, L.; Ademollo, N.; Grenni, P.; Tolomei, A.; Barra Caracciolo, A.; Capri, S. Simultaneous determination of human pharmaceuticals in water samples by solid phase extraction and HPLC with UV-fluorescence detection. Microchem. J. 2013, 107, 165–171.
- Zhang, S.; You, J.; Sun, Z.; Song, C.; Ning, S.; Zhao, C.; Suo, Y. A sensitive method for extraction and determination of endocrine-disrupting compounds from wastewater using 10-ethyl-acridone-2-sulfonyl chloride as pre-column labeling reagent by high-performance liquid chromatography with fluorescence detection. Microchem. J. 2012, 103, 90–96.
- Lima, D.L.D.; Silva, C.P.; Otero, M.; Esteves, V.I. Low cost methodology for estrogens monitoring in water samples using dispersive liquid-liquid microextraction and HPLC with fluorescence detection. Talanta 2013, 115, 980–985.
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Esparza, M. HPLC-fluorescence detection and adsorption of bisphenol A, 17β-estradiol, and 17α-ethynyl estradiol on powdered activated carbon. Water Res. 2003, 37, 3530–3537.
- Durhan, E.J.; Lambright, C.S.; Makynen, E.A.; Lazorchak, J.; Hartig, P.C.; Wilson, V.S.; Gray, L.E.; Ankley, G.T. Identification of metabolites of trenbolone acetate in androgenic runoff from a beef feedlot. Environ. Health Perspect. 2006, 114, 65–68.
- Snyder, S.A.; Keith, T.L.; Verbrugge, D.A.; Snyder, E.M.; Gross, T.S.; Kannan, K.; Giesy, J.P. Analytical methods for detection of selected estrogenic compounds in aqueous mixtures. Environ. Sci. Technol. 1999, 33, 2814–2820.
- Wen, Y.; Zhou, B.S.; Xu, Y.; Jin, S.W.; Feng, Y.Q. Analysis of estrogens in environmental waters using polymer monolith in-polyether ether ketone tube solid-phase microextraction combined with high-performance liquid chromatography. J. Chromatogr. A 2006, 1133, 21–28.
- Ying, G.G.; Kookana, R.S.; Chen, Z. On-line solid-phase extraction and fluorescence detection of selected endocrine disrupting chemicals in water by high-performance liquid chromatography. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2002, 37, 225–234.
- Fan, Y.; Zhang, M.; Da, S.L.; Feng, Y.Q. Determination of endocrine disruptors in environmental waters using poly(acrylamide-vinylpyridine) monolithic capillary for in-tube solid-phase microextraction coupled to high-performance liquid chromatography with fluorescence detection. Analyst 2005, 130, 1065–1069.
- Gamoh, K.; Omote, K.; Okamoto, N.; Takatsuto, S. High-performance liquid chromatography of brassinosteroids in plants with derivatization using 9-phenanthreneboronic acid. J. Chromatogr. A 1989, 469, 424–428.
- Lv, T.; Zhao, X.E.; Zhu, S.; Ji, Z.; Chen, G.; Sun, Z.; Song, C.; You, J.; Suo, Y. Development of an Efficient HPLC Fluorescence Detection Method for Brassinolide by Ultrasonic-Assisted Dispersive Liquid–Liquid Microextraction Coupled with Derivatization. Chromatographia 2014, 77, 1653–1660.
- Motegi, C.; Takatsuto, S.; Gamoh, K. Identification of brassinolide and castasterone in the pollen of orange (Citrus sinensis Osbeck) by high-performance liquid chromatography. J. Chromatogr. A 1994, 658, 27–30.
- Li, N.; Wu, D.; Liu, J.; Hu, N.; Shi, X.; Dai, C.; Sun, Z.; Suo, Y.; Li, G.; Wu, Y. Magnetic covalent organic frameworks based on magnetic solid phase extraction for determination of six steroidal and phenolic endocrine disrupting chemicals in food samples. Microchem. J. 2018, 143, 350–358.
- Kosicka, K.; Siemiątkowska, A.; Szpera-Goździewicz, A.; Krzyścin, M.; Bręborowicz, G.; Główka, F. High-performance liquid chromatography methods for the analysis of endogenous cortisol and cortisone in human urine: Comparison of mass spectrometry and fluorescence detection. Ann. Clin. Biochem. 2019, 56, 82–89.
- Li, G.; You, J.; Suo, Y.; Song, C.; Sun, Z.; Xia, L.; Zhao, X.; Shi, J. A developed pre-column derivatization method for the determination of free fatty acids in edible oils by reversed-phase HPLC with fluorescence detection and its application to Lycium barbarum seed oil. Food Chem. 2011, 125, 1365–1372.
- Liu, J.; You, J.; Zhang, S.; Song, C.; Ji, Z.; Zhuang, J.; Yu, Y. New fluorescent labeling reagent Benzimidazoquinazoline-12(6H) -one-5-ethylimidazole ester and its application in the analysis of endocrine disrupting compounds in milk by high performance liquid chromatography with fluorescence detection. Microchem. J. 2018, 138, 309–315.
- Socas-Rodríguez, B.; Asensio-Ramos, M.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Analysis of oestrogenic compounds in dairy products by hollow-fibre liquid-phase microextraction coupled to liquid chromatography. Food Chem. 2014, 149, 319–325.
- Drzymala, S.S.; Weiz, S.; Heinze, J.; Marten, S.; Prinz, C.; Zimathies, A.; Garbe, L.A.; Koch, M. Automated solid-phase extraction coupled online with HPLC-FLD for the quantification of zearalenone in edible oil. Anal. Bioanal. Chem. 2015, 407, 3489–3497.
- Kozłowska-Tylingo, K.; Konieczka, P.; Gustaw, E.; Wasik, A.; Namieśnik, J. Comparison of High Performance Liquid Chromatography Methods with Different Detectors for Determination of Steroid Hormones in Aqueous Matrices. Anal. Lett. 2014, 47, 1449–1464.
- Zhang, R.; Cheng, X.; Guo, J.; Zhang, H.; Hao, X. Comparison of Two Ionic Liquid-Based Pretreatment Methods for Three Steroids’ Separation and Determination in Water Samples by HPLC. Chromatographia 2017, 80, 237–246.
- Ferreira, F.N.; Benevides, A.P.; Cesar, D.V.; Luna, A.S.; de Gois, J.S. Magnetic solid-phase extraction and pre-concentration of 17β-estradiol and 17α-ethinylestradiol in tap water using maghemite-graphene oxide nanoparticles and determination via HPLC with a fluorescence detector. Microchem. J. 2020, 157, 104947.
- Zhang, G.; Yang, Y.; Lu, Y.; Chen, Y.; Li, W.; Wang, S. Effect of heavy metal ions on steroid estrogen removal and transport in SAT using DLLME as a detection method of steroid estrogen. Water (Switzerland) 2020, 12, 589.
- Jiang, T.; Zhao, L.; Chu, B.; Feng, Q.; Yan, W.; Lin, J.M. Molecularly imprinted solid-phase extraction for the selective determination of 17β-estradiol in fishery samples with high performance liquid chromatography. Talanta 2009, 78, 442–447.
- Corazza, G.; Merib, J.; Magosso, H.A.; Bittencourt, O.R.; Carasek, E. A hybrid material as a sorbent phase for the disposable pipette extraction technique enhances efficiency in the determination of phenolic endocrine-disrupting compounds. J. Chromatogr. A 2017, 1513, 42–50.
- Louros, V.L.; Lima, D.L.D.; Leitão, J.H.; Esteves, V.I.; Nadais, H.G. Determination of estrone and 17α-ethinylestradiol in digested sludge by ultrasonic liquid extraction and high-performance liquid chromatography with fluorescence detection. J. Sep. Sci. 2019, 42, 1585–1592.
- Yuan, Y.; Wang, M.; Jia, N.; Zhai, C.; Han, Y.; Yan, H. Graphene/multi-walled carbon nanotubes as an adsorbent for pipette-tip solid-phase extraction for the determination of 17β-estradiol in milk products. J. Chromatogr. A 2019, 1600, 73–79.
- Xiong, L.; Yan, P.; Chu, M.; Gao, Y.Q.; Li, W.H.; Yang, X.L. A rapid and simple HPLC–FLD screening method with QuEChERS as the sample treatment for the simultaneous monitoring of nine bisphenols in milk. Food Chem. 2018, 244, 371–377.
- Ali, M.F.B.; Uejo, Y.; Kishikawa, N.; Ohyama, K.; Kuroda, N. A selective and highly sensitive high performance liquid chromatography with fluorescence derivatization approach based on Sonogashira coupling reaction for determination of ethinyl estradiol in river water samples. J. Chromatogr. A 2020, 1628, 461440.
- Guedes-Alonso, R.; Santana-Viera, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Molecularly imprinted solid-phase extraction coupled with ultra high performance liquid chromatography and fluorescence detection for the determination of estrogens and their metabolites in wastewater. J. Sep. Sci. 2015, 38, 3961–3968.
More
Information
Subjects:
Chemistry, Analytical; Others; Endocrinology & Metabolism
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.5K
Revisions:
4 times
(View History)
Update Date:
11 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




