Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lu, L.; Tibpromma, S.; Karunarathna, S.; , .; Lumyong, S.; Xu, J.; Hyde, K.D. Fungi on Coffee. Encyclopedia. Available online: https://encyclopedia.pub/entry/21503 (accessed on 07 February 2026).
Lu L, Tibpromma S, Karunarathna S, , Lumyong S, Xu J, et al. Fungi on Coffee. Encyclopedia. Available at: https://encyclopedia.pub/entry/21503. Accessed February 07, 2026.
Lu, Li, Saowaluck Tibpromma, Samantha Karunarathna, , Saisamorn Lumyong, Jianchu Xu, Kevin D. Hyde. "Fungi on Coffee" Encyclopedia, https://encyclopedia.pub/entry/21503 (accessed February 07, 2026).
Lu, L., Tibpromma, S., Karunarathna, S., , ., Lumyong, S., Xu, J., & Hyde, K.D. (2022, April 08). Fungi on Coffee. In Encyclopedia. https://encyclopedia.pub/entry/21503
Lu, Li, et al. "Fungi on Coffee." Encyclopedia. Web. 08 April, 2022.
Copy Citation
Coffee is grown in more than 80 countries as a cash crop and consumed worldwide as a beverage and food additive. It is susceptible to fungal infection during growth, processing and storage. Fungal infections, in particular, can seriously affect the quality of coffee and threaten human health.
endophytes
fungal diseases
fungal toxins
pathogens
postharvest diseases
1. Introduction
Coffee has gained in popularity in modern times and is the second-best-selling beverage in the world [1]. As an important economic crop, it is central to the livelihoods of millions of people worldwide [2]; accordingly, more than 80 countries grow coffee and some countries use coffee as a major cash crop [3]. World coffee production for 2020/2021 is forecast to be 5.5 million kg higher than the previous year, reaching a record 176.1 million kg [4]. Brazil is the largest exporter of coffee, and its exports account for one-third of the global total [5]. The Agricultural Trade Office in Sao Paulo (ATO) forecasts the Brazilian coffee production for 2020/2021 at a record of 67.9 million kg, an increase of 15% over 2019 output. Finland is the largest per capita consumer of coffee, while China consumes the most coffee by volume [6]. Coffea arabica and C. canephora (robusta) are the two most-grown coffee species in the world [7], accounting for 60% and 40% of global production, respectively [8].
Throughout the tropics, coffee growers face many problems in agricultural production [9]. As a climate-sensitive plant, implications of climate change have altered coffee production, from decreasing crop yield and quality to increasing fungal diseases and invasive pests [10]. Coffee worldwide suffers from a range of pests and diseases, and fungal infections are also a major problem [11]. Coffee roots, stems, leaves and beans are often damaged by pests and pathogens [12].
Fungi on coffee occur in different life modes: endophytes, pathogens and saprobes [13][14][15]. The largest number of fungi have been recorded from C. arabica and C. canephora (Table S1, supplementary could be found in https://www.mdpi.com/2076-0817/11/4/411#supplementary, the following supplementary is same). Endophytes usually live inside the host without causing injury or obvious symptoms, and this association can provide a better living environment for both the host and fungus [16]. There are also reports on their ability to aid in the defense of host plants [17][18]. Huang et al. [19] screened potential antagonistic endophytes that prevent and control post-harvest diseases. Coffee easily can be infected by pathogenic fungi when growing, during post-harvest handling and storage, and during processing [20]. One of the most virulent diseases is ‘coffee rust’ caused by Hemileia vastatrix, which wiped out coffee 150 years ago and continues to cause problems in coffee plantations worldwide [21][22]. Fungal diseases in coffee can be divided into two types: diseases in pre-harvest and diseases in post-harvest [23][24]. Many post-harvest coffee pathogens are infected shortly before harvest, are generally not found at harvest, and feature low activity; moreover, poor storage conditions during post-harvest favour their development [23]. Fungal invasions before harvest are mainly induced by the interaction between the plant host and other organisms (such as insects), while fungal infections after harvest are controlled by nutrient availability, temperature, humidity and biological factors (insects) [25]. Another pathway is that endophytic fungi in coffee beans change their life modes to saprobic/pathogenic after the beans are harvested, becoming postharvest pathogens [19][26]. Most postharvest fungi produce toxins as secondary metabolites viz. ochratoxin-A, which is a mycotoxin mainly produced as a result of secondary metabolism of many species of Aspergillus and Penicillium and is the most common mycotoxin present in agricultural commodities [27]. Toxin-producing fungi can be isolated from coffee beans both pre-harvest and post-harvest, while the risk of fungal growth and mycotoxin production after harvest is higher in high temperature areas [28][29]. These toxins can cause host infections and reduce coffee bean quality [30] and can be carcinogenic, hepatotoxic, hematotoxic, nephrotoxic and neurotoxic for humans [31][32]. Silva et al. [33] isolated ochratoxin-A from damaged coffee beans, and ochratoxin-A was shown to cause coffee quality and yield losses. Studies have shown that the main toxigenic fungal genera comprise Aspergillus, Penicillium and Fusarium, which are natural coffee contaminants [34], and they can infect hosts in both farms and warehouses [35].
2. Records of Coffee Fungi
A total of 966 records of coffee fungi (Supplementary Table S1) were found in the literatures, belonging to 113 genera of which frequently found families and genera are shown in Figure 1a and Supplementary Table S2.
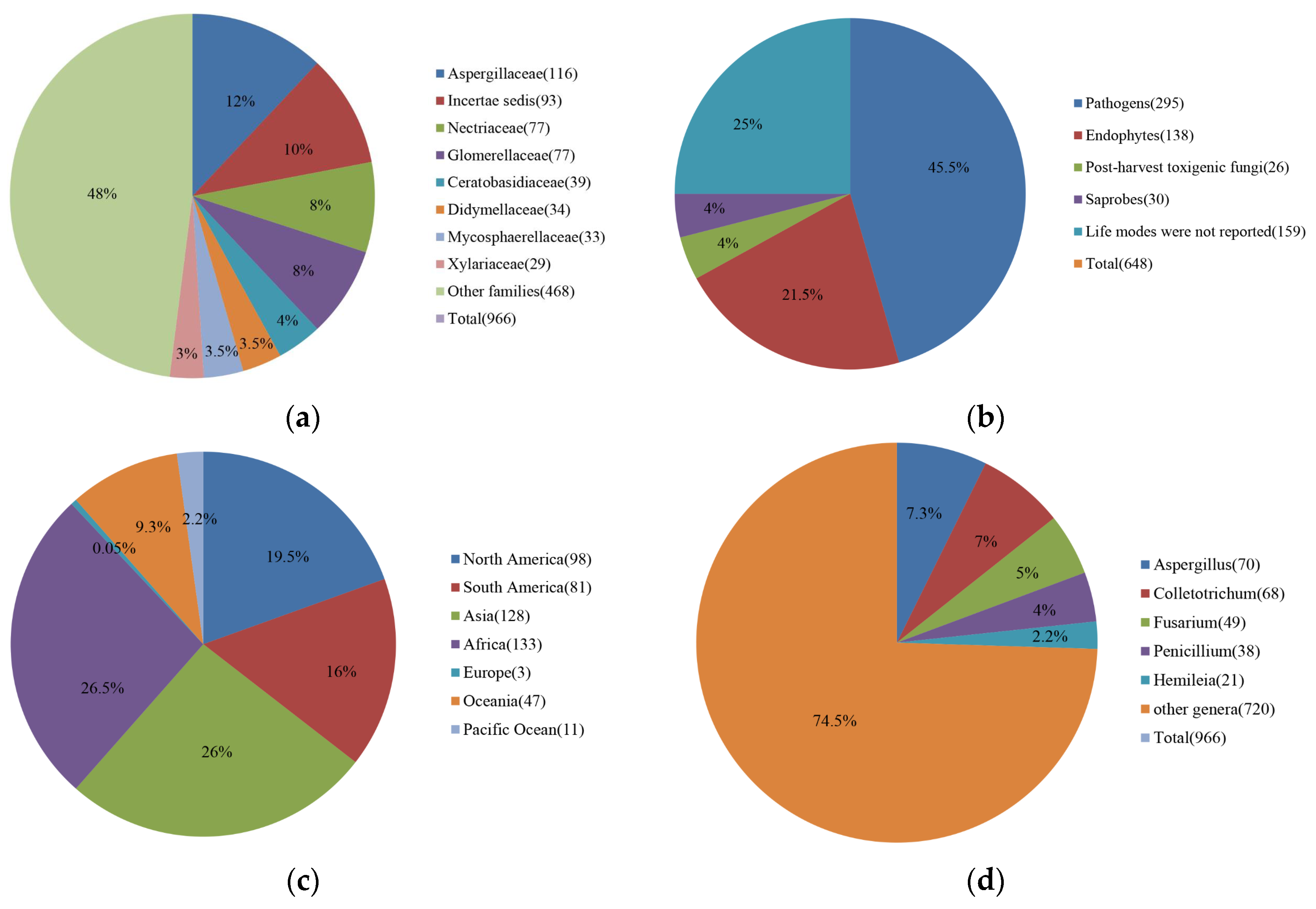
Figure 1. (a) Percentages of different fungal families reported on coffee; (b) Percentages of different life modes of fungi reported on coffee; (c) Percentages of coffee pathogens reported in different countries; (d) Percentages of most frequently reported fungal pathogenic and toxigenic fungal genera on coffee.
Out of 648 fungal species, 295 are pathogenic, 138 are endophytes, and 30 are saprobes, while the life modes of 159 species have not been confirmed and another 26 species are post-harvest disease causing agents that can produce mycotoxins in dried and green coffee beans (Figure 1a,b). It was not confirmed in reported publications whether most reported pathogens caused pre-harvest or post-harvest infections. Of the 295 species of pathogens, 212 species are true pathogens (TP), three species are post-harvest pathogens (PP) and four species are both true pathogens (TP) and post-harvest pathogens (PP), while the other 76 species are listed as unknown (UNK) as their disease symptoms have not been confirmed on coffee (Supplementary Table S4).
3. Endophyte Role in Coffee Plants
Endophytic fungi that can inhibit nematodes, coffee berry borers and pathogenic fungi have the potential to be used as biocontrol agents to control pest and pathogen infections of coffee [36][37][38]. Goates et al. [39] showed that some endophytic fungi reduce fungal diseases by producing volatile organic compounds that can kill or inhibit phytopathogens. Monteiro et al. [40] demonstrated that volatile organic compounds produced by the endophyte Muscodor coffeanum isolated from C. arabica produce fungicidal activity against Aspergillus ochraceus. Three records of Muscodor species belonging to induratiaceae [41] were found in coffee according to researchers' data. De Almeida et al. [42] isolated Aspergillus sp., A. westerdijkiae, A. niger, A. tamari, and A. fumigatus, Lichtheimia ramosa and Rhizopus oryzae from coffee beans to test their abilities to inhibit the growth of Aspergillus species and ochratoxin-A production, and A. niger showed the best inhibitory ability of both growth and ochratoxin-A production. According to researchers' results, Aspergillaceae is the most frequently found family in coffee. Furthermore, Eida et al. [43] isolated Penicillium crustosum, Penicillium verruculosum, Trichoderma harzianum, and Hypocrea lixii from coffee residue compost that can aid in the degradation of lignocellulose waste.
4. Pathogen Effect on Coffee and Coffee Disease
Among 648 species, fungal pathogens are the most common (295 species) as shown in Figure 1b. According to Supplementary Tables S1 and S3: Sixty-eight of Colletotrichum belonging to 35 species have been reported. Coffee production is often affected by Coffee Berry Disease, which is the main factor limiting the production of C. arabica in Kenya and other countries in East Africa, especially in high-altitude areas. It is potentially responsible for 50 to 80% of total crop losses [44]. Forty-nine Fusarium belonging to 23 species have been reported (Supplementary Tables S1 and S3). Fusarium species are one of the most important phytopathogenic and toxin-producing fungi [45]. Coffee Wilt Disease is a devastating disease in East and Central Africa [46]. Coffee Wilt Disease is a vascular disease, and due to its high transmission potential, Coffee Wilt Disease poses a threat to all coffee-producing regions. This disease can kill its host at all ages in a short time [47]. Moreover, after the infected trees and their roots are removed, the infested soil may remain infectious for several years [48]. Twenty-one Hemileia have been reported belonging to two species, and Hemileia vastatrix is an important phytopathogenic fungus that causes Coffee Leaf Rust. Coffee Leaf Rust, one of the major diseases of Arabica coffee is a major threat to coffee production worldwide and it has been reported to cause serious economic losses in more than 50 coffee-growing countries [49]. Brown Eye Spot or Cercospora Blotch caused by Cercospora coffeicola, has been reported on coffee. Besides, Andrade et al. [50] showed that isolates producing brown eye spot and black spot can also cause prompt alterations in the antioxidant metabolism of coffee leaves. Armillaria root rot caused by Armillaria sp., has been reported on coffee, and this disease leads to coffee plant rot and the eventual death of the plant. Since this fungus invades deeper into roots, symptoms are difficult to detect, thus it can last for several years before symptoms appear on the surface. This disease spreads to other plants with the transfer of soil [51]. Major coffee diseases that have a huge impact on coffee plantations worldwide are discussed in detail below [14].
4.1. Detection and Identification of Diseases
Detection and identification of fungal diseases in crops can be done through direct and indirect methods. Direct detection of fungal diseases includes molecular and serological methods, while indirect methods identify the plant diseases through various parameters such as morphological change, temperature change, transpiration rate change, and volatile organic compounds released by infected plants [52]. Among different methods, fungal morphology is a commonly used method to identify coffee pathogens [11], while pathogenicity tests [53] and Polymerase Chain Reaction (PCR) [54] are also used. Generally, three main methods are used for the identification of coffee fungal pathogens.
Fungal morphological characteristics—Different fungal pathogens cause different symptoms on the host surface [14]. Firstly, the disease symptoms on the host are observed and recorded, and then the pure cultures grown on potato dextrose agar (PDA) are obtained according to the isolation method of Senanayake et al. [55]. Finally, colony size, colour of the conidial masses and zonation, size, and shape of conidia harvested from the cultures are recorded under the microscope [44].
Pathogenicity test (Koch’s postulates)—Spore suspensions of pathogenic fungi are obtained by pure cultures grown on PDA for 7 to 10 days, prepare healthy/disease-free hosts, then carry out the pathogenicity test [44]. After inoculation, the changes are recorded from 1 to 15 days of growth, compare the morphological characteristics of the lesions in the host with original disease lesions.
Polymerase Chain Reaction—Pure cultures of pathogenic fungi grown on PDA for 7 to 10 days are used to scrape mycelium from the culture surface. Then, the genomic DNA is extracted using a Genomic DNA Extraction Kit or CTAB. Finally, PCR amplification is done for the specific genes of interest [44].
4.2. Coffee Leaf Rust
-
Hosts: C. arabica (arabica coffee) and C. canephora (robusta coffee), the two most important commercial coffee species [56].
-
Symptoms and signs: Infection occurs on the leaves of coffee. The first observable symptoms are small, and light-yellow spots on the upper surface of leaves. As the diameter of these points gradually increases, a large number of orange urediniospores (=uredospores) appear under the leaf surface. The fungus forms spores through stomata instead of penetrating the epidermis like most rust-causing species, so it does not form many typical rust pustules. Powdery lesions under leaves appear orange-yellow to red-orange with a high degree of variance. Although disease spots can develop anywhere on the leaf, they tend to concentrate around the edges, where dew and raindrops gather. The center of the spot eventually dries out and turns brown, while edges of the lesions continue to expand and produce new spores. At the beginning of the season, the disease usually first appears on the lower branches, and infection progresses slowly up the tree. Infected leaves fall prematurely, leaving long branches without leaves [14][56][57][58][59].
-
Pathogen biology: Hemileia vastatrix mainly exists in the form of dikaryotic, and nutrient-absorbing mycelium between cells in leaves of its coffee host. Short pedicels are clustered throughout stomata and below leaves, with dual-nucleated spores. Towards the end of the season, sometimes under cool, dry conditions, spores are produced from polyspores on older attached leaves. After nuclear division and meiosis, these sporozoites germinate to produce basidia, each of which forms four haploid sporozoites [14][56][57][58][59].
-
Disease cycle: Urediniosporic life cycle as its most important source of inoculum, can cause infection and develop into lesions, producing more urediniospores. Spore adhesion to the host surface, germination of urediniospores, formation of an adhesion layer on stomata, penetration, and intercellular and intracellular colonization are various steps of the disease cycle. The disease cycle of Coffee Leaf Rust is discussed in Talhinhas et al. [22].
-
Disease management:
-
Cultural practices: Agroforestry practices of tree-crop mixing, timely pruning, handling and de-suckering, regular change of crop cycle [49].
-
Biological practices: Pichia membranifaciens is a yeast strain isolated from soil that can reduce the Hemileia vastatrix spore viability [59].
-
Resistant varieties: Such as HDT (Hıbrido de Timor), Catimor and Sarchimor populations [22].
4.3. Coffee Berry Disease
-
Pathogen: Colletotrichum kahawae is a particularly devastating pathogen that affects developing berries, leading to berry rot and shedding before bean formation. Colletotrichum kahawae has not been reported outside Africa or in low altitudes. Coffee Berry Disease was first detected and identified by McDonald in Kenya in 1922 [14].
-
Symptoms and signs: Characteristic symptoms are progressive anthracnose of young and expanding coffee berries. Symptoms present as small water-soaked lesions that rapidly become dark and sunken. These lesions expand, causing rot of the entire berry under humid conditions, and pink spore masses become visible on the lesion surface. Berries are often shed from branches at an early stage of the disease. Lesions may also occur on young berry stalks, causing them to shed before lesions are evident on berries. Pale, corky lesions (scab lesions) also appear on young and mature berries that are resistant to infection. They may completely heal or remain dormant until berries ripen. This disease also affects ripening berries, causing a ‘brown blight’ phase as typical dark, sunken anthracnose lesions that envelop the red berries. Colletotrichum kahawae may also infect flowers under wet conditions, causing brown lesions on petals [14][58][60].
-
Pathogen biology: The fungus settles in the mature bark of coffee buds and infects flowers, mature fruits, and leaves. Under high humidity and high temperature, conidia germinate and form germ tubes and appendages when contacted with susceptible tissues [61].
-
Disease cycle: The Coffee Berry Disease cycle begins each year at the first rain event and is subsequently maintained by rain-splash dispersal and secondary inoculation of conidia onto healthy berries in the rainy season. The disease cycle of Coffee Berry Disease is discussed in De Silva et al. [62].
-
Disease management:
-
Cultural practices: Shading with fruit trees and irrigation to induce early flowering to decrease the severity and all berries should be removed at the end of the planting season to prevent them from becoming a source of inoculation for new crops [60].
-
Biological control: Many components in the microbiota (fungi and bacteria) on coffee trees show very high antagonistic levels and have a strong antagonistic effect on Colletotrichum kahawae. However, these agents have not been developed into commercial biocontrol agents [60].
4.4. Coffee Wilt Disease
-
Symptoms and signs: First, leaves turn yellow before withering and developing brown necrotic lesions. Finally, leaves curl, dry, and fall. This process can start from any part of the plant, but eventually, symptoms spread to the rest of the plant. Symptoms first present on the coffee stem, where fungi colonize, and the host response blocks vascular bundles, resulting in blue-black stains [14][47][58][63].
-
Pathogen biology: Conidia and ascospores are spread by wind, rain and through human activities (harvesting, pruning). Pathogens penetrate wounds, so any agent causing wounds aids the spread of the fungus. Krantz and Mogk in 1973 noted that most dying and dead trees had been wounded during weeding. Insects may also spread the disease from one tree to another tree [64].
-
Disease cycle: Incubation period from first symptoms to death of tree varies, although most affected trees die 2–3 months after initial symptoms were observed. It usually quickly kills infected mature trees within just 6 months after the first external symptoms appear, resulting in a decline of total yield. Coffee quality may also be affected by premature berry ripening. The disease cycle of Coffee Wilt Disease is discussed in Alemu et al. [65].
-
Disease management:
-
Cultural practices: Frequent inspection, along with burning infected material and spraying soil surfaces with 2.5% copper (II) sulphate. Replanting should not be done until 6 months after uprooting infected trees to allow the viability of soil inoculum to decline. It is recommended to grow cover crops such as Desmodium sp. and haricot bean, which are very efficient in suppressing weeds (so reducing the need for slashing) and as legumes, promote the growth of coffee trees [63].
-
Chemical control: Ridomil Gold (metalxyl 8% + Mancozeb 64%) 68% Wp 2.5 kg/ha, when disease on set, used at 7, 14, 21, 28 days. Pencase 80% WP (Mancozeb) at the rate of 2.5 kg/ha, when disease on set, used at 7, 14, 21 days [63].
-
Biological control: The strain of Bacillus subtilis (AUBB20) is the most antagonistic to this disease. Tricoderma viride and Tricoderma harzianum have shown good potential in inhibiting the mycelial growth of Fusarium xylarioides, but no effective methods of biological control are currently available [63].
4.5. Brown Eye Spot or Cercospora Blotch
-
Symptoms and signs: on the leaves, small, round to irregular spots, and brown to light brown lesions first appear. The number and size of lesions then increase before eventually the entire leaf is affected. The edge of the lesion may appear dark purple or black, and it may be encircled by a yellow halo. Severely infected leaves turn yellow and fall off; lesions on green berries are initially brown, sunken, longitudinal, irregular or oval with a gray center. Infection can occur at any stage of berry growth; on the red cherries, first, large, sunken, and blackened areas cover with silvery fungal spores. Penetration into the seeds may cause the pulp to stick to parchment paper during processing, and damage the product. Cercospora coffeicola reduces productivity and lowers the beverage quality of coffee [14][57][67].
-
Pathogen biology: Wind, splashing water and human activities cause spores (conidia) to be deposited on leaves and petioles, beginning the disease cycle. Conidia germinate at moderate to warm (20–28 °C) temperatures [68].
-
Disease cycle: In warm and humid periods, new infections and sporulation occur every 7 to 10 days. Pathogen easily spreads in fields via wind, rain, and irrigation water. It survives as a pathogen in weeds and infested crop fragments, where it is capable of re-infecting grown plants. The disease cycle of Cercospora Blotch is discussed in Souza et al. [69].
-
Disease management:
-
Biological control: No biological control measures have been developed [68].
-
Cultural practices: Elimination of crop debris, weed hosts and provide 35–65% shade. In order to maintain adequate plant nutrition, nitrogen fertilizers are used. Plant only high-quality seeds, and destroy infected crops in time after the final harvest and before replanting. Select a reasonable planting density (10 ft × 10 ft for robusta while 8 ft × 8 ft for arabica). Avoid planting coffee transplants too deep in soils [68].
-
Chemical control: Fungicide sprays are necessary for disease control in wet conditions, but proper fungicides, rates, and fungicide rotations such as Chlorothalonil and Chlorothalonil Mixtures, Strobilurins and Strobilurin Mixtures should be followed [68].
References
- Besttoppers. Available online: https://besttoppers.com/top-10-widely-consumed-drinks (accessed on 16 February 2022).
- Zhang, S.J.; De Bruyn, F.; Pothakos, V.; Contreras, G.F.; Cai, Z.; Moccand, C.; Weckx, S.; De Vuyst, L. Influence of various processing parameters on the microbial community dynamics, metabolomic profiles, and cup quality during wet coffee processing. Front. Microbiol. 2019, 10, 2621.
- Treanor, N.B.; Saunders, J. Tackling (Illegal) Deforestation in Coffee Supply Chains: What Impact Can Demand-Side Regulations Have? Forest Policy Trade and Finance Initiative Report; Forest Trends: Washington, DC, USA, 2021.
- International Coffee Organization. Available online: http://www.ico.org/coffee_prices.asp (accessed on 16 February 2022).
- Veloso, T.G.R.; Silva, M.D.C.S.D.; Cardoso, W.S.; Guarçoni, R.C.; Kasuya, M.C.M.; Pereira, L.L. Effects of environmental factors on microbiota of fruits and soil of Coffea arabica in Brazil. Sci. Rep. 2020, 10, 1–11.
- Coffee Consumption by Country 2022. Available online: https://worldpopulationreview.com/country-rankings/coffee-consumption-by-country (accessed on 16 February 2022).
- Vega, F.E.; Ziska, L.H.; Simpkins, A.; Infante, F.; Davis, A.P.; Rivera, J.A.; Barnaby, J.Y.; Wolf, J. Early growth phase and caffeine content response to recent and projected increases in atmospheric carbon dioxide in coffee (Coffea arabica and C. canephora). Sci. Rep. 2020, 10, 1–11.
- Leitão, A.L. Occurrence of ochratoxin A in coffee: Threads and solutions—A mini-review. Beverages 2019, 5, 36.
- Harvey, C.A.; Rakotobe, Z.L.; Rao, N.S.; Dave, R.; Razafimahatratra, H.; Rabarijohn, R.H.; Rajaofara, H.; MacKinnon, J.L. Extreme vulnerability of smallholder farmers to agricultural risks and climate change in Madagascar. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130089.
- Pham, Y.; Reardon-Smith, K.; Mushtaq, S.; Cockfield, G. The impact of climate change and variability on coffee production: A systematic review. Clim. Chang. 2019, 156, 609–630.
- Rutherford, M.A.; Phiri, N. Pests and Diseases of Coffee in Eastern Africa: A Technical and Advisory Manual; CAB International: Wallingford, UK, 2006.
- Ribeyre, F.; Avelino, J. Impact of field pests and diseases on coffee quality. In Specialty Coffee: Managing Coffee; International Plant Nutrition Institute; IPNI : Penang, Malaysia, 2012; pp. 151–176.
- Santamaría, J.; Bayman, P. Fungal epiphytes and endophytes of coffee leaves (Coffea arabica). Microb. Ecol. 2005, 50, 1–8.
- Muller, R.A.; Berry, D.; Avelino, J.; Bieysse, D. Coffee diseases. In Coffee: Growing, Processing, Sustainable Production. A Guidebook for Growers, Processors, Traders and Researchers; Germany Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2009; pp. 495–549.
- Botrel, D.A.; Laborde, M.C.F.; De Medeiros, F.H.V.; Resende, M.L.V.; Júnio, P.M.R.; Pascholati, S.F.; Gusmão, L.F.P. Saprobic fungi as biocontrol agents of halo blight (Pseudomonas syringae pv. garcae) in coffee clones. Coffee Sci. 2018, 13, 283–291.
- Torres-Mendoza, D.; Ortega, H.E.; Cubilla-Rios, L. Patents on endophytic fungi related to secondary metabolites and biotransformation applications. J. Fungi 2020, 6, 58.
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 1–14.
- da Silva, S.A.; Pereira, R.G.F.A.; Lira, N.D.A.; da Glória, E.M.; Chalfoun, S.M.; Batista, L.R. Fungi associated to beans infested with coffee berry borer and the risk of ochratoxin A. Food Control 2020, 113, 107204.
- Huang, X.; Ren, J.; Li, P.; Feng, S.; Dong, P.; Ren, M. Potential of microbial endophytes to enhance the resistance to postharvest diseases of fruit and vegetables. J. Sci. Food Agric. 2020, 101, 1744–1757.
- Varga, J.; Kocsubé, S.; Péteri, Z.; Vagvolgyi, C.; Toth, B. Chemical, physical and biological approaches to prevent ochratoxin induced toxicoses in humans and animals. Toxins 2010, 2, 1718–1750.
- McCook, S. Global rust belt: Hemileia vastatrix and the ecological integration of world coffee production since 1850. J. Glob. Hist. 2006, 1, 177–195.
- Talhinhas, P.; Batista, D.; Diniz, I.; Vieira, A.; Silva, D.N.; Loureiro, A.; Tavares, S.; Pereira, A.P.; Azinheira, H.G.; Guerra-Guimarães, L. The coffee leaf rust pathogen Hemileia vastatrix: One and a half centuries around the tropics. Mol. Plant Pathol. 2017, 18, 1039–1051.
- Coates, L.; Johnson, G. Postharvest diseases of fruit and vegetables. In Plant Pathogens and Plant Diseases; Elsevier Science: Amsterdam, The Netherlands, 1997; pp. 533–548.
- Roberto, S.R.; Youssef, K.; Hashim, A.F.; Ippolito, A. Nanomaterials as alternative control means against postharvest diseases in fruit crops. Nanomaterials 2019, 9, 1752.
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing fungi and mechanisms of phytotoxicity. Agriculture 2015, 5, 492–537.
- Strobel, G. The emergence of endophytic microbes and their biological promise. J. Fungi 2018, 4, 57.
- Miller, J. Mycotoxin in Grain, Eagan Press: St. Paul, MA, USA, 1994; 326.
- Alvindia, D.G.; de Guzman, M.F. Survey of Philippine coffee beans for the presence of ochratoxigenic fungi. Mycotoxin Res. 2016, 32, 61–67.
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; El Khoury, A. Mycotoxins: Factors influencing production and control strategies. AIMS Agric. Food 2021, 6, 416–447.
- Proctor, R.H.; McCormick, S.P.; Kim, H.-S.; Cardoza, R.E.; Stanley, A.M.; Lindo, L.; Kelly, A.; Brown, D.W.; Lee, T.; Vaughan, M.M. Evolution of structural diversity of trichothecenes, a family of toxins produced by plant pathogenic and entomopathogenic fungi. PLoS Pathog. 2018, 14, e1006946.
- Khan, S.A.; Venancio, E.J.; Ono, M.A.; Fernandes, E.V.; Hirooka, E.Y.; Shimizu, C.F.; Oba, A.; Flaiban, K.K.M.C.; Itano, E.N. Effects of subcutaneous ochratoxin-A exposure on immune system of broiler chicks. Toxins 2019, 11, 264.
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137.
- Silva, C.F.; Schwan, R.F.; Dias, Ë.S.; Wheals, A.E. Microbial diversity during maturation and natural processing of coffee cherries of Coffea arabica in Brazil. Int. J. Food Microbiol. 2000, 60, 251–260.
- Bokhari, F.M. Mycotoxins and toxigenic fungi in Arabic coffee beans in Saudi Arabia. Adv. Biol. Res. 2007, 1, 56–66.
- Rezende, E.D.F.; Borges, J.G.; Cirillo, M.; Prado, G.; Paiva, L.C.; Batista, L. Ochratoxigenic fungi associated with green coffee beans (Coffea arabica L.) in conventional and organic cultivation in Brazil. Braz. J. Microbiol. 2013, 44, 377–384.
- Vega, F.E.; Posada, F.; Aime, M.C.; Pava-Ripoll, M.; Infante, F.; Rehner, S.A. Entomopathogenic fungal endophytes. Biol. Control 2008, 46, 72–82.
- Aldina, R.F.; Indarti, S.; Wibowo, A. Pathogenicity of Nematofagous Fungus for Control of Pratylenchus Coffeae Nematodes on Coffee Plants; Springer: Cham, Germany, 2017; pp. 243–251.
- Schulz, B.; Boyle, C. What Are Endophytes? Microbial Root Endophytes; Springer: Berlin/Heidelberg, Germany, 2006.
- Goates, B.J.; Mercier, J. Effect of biofumigation with volatiles from Muscodor albus on the viability of Tilletia spp. teliospores. Can. J. Microbiol. 2009, 55, 203–206.
- Monteiro, M.C.P.; Alves, N.M.; De Queiroz, M.V.; Pinho, D.B.; Pereira, O.L.; De Souza, S.M.C.; Cardoso, P.G. Antimicrobial activity of endophytic fungi from coffee plants. Biosci. J. 2017, 33, 381–389.
- Samarakoon, M.C.; Thongbai, B.; Hyde, K.D.; Broenstrup, M.; Beutling, U.; Lambert, C.; Miller, A.N.; Liu, J.K.; Promputtha, I.; Stadler, M. Elucidation of the life cycle of the endophytic genus Muscodor and itEvaluation of the effects of temperature on processed coffee beans in the presence of fungi and ochratoxin As transfer to Induratia in Induratiaceae fam. nov., based on a polyphasic taxonomic approach. Fungal Divers. 2020, 101, 177–210.
- de Almeida, Â.B.; Corrêa, I.P.; Furuie, J.L.; de Farias Pires, T.; do Rocio Dalzoto, P.; Pimentel, I.C. Inhibition of growth and ochratoxin-A production in Aspergillus species by fungi isolated from coffee beans. Braz. J. Microbiol. 2019, 50, 1091–1098.
- Eida, M.F.; Nagaoka, T.; Wasaki, J.; Kouno, K. Evaluation of cellulolytic and hemicellulolytic abilities of fungi isolated from coffee residue and sawdust composts. Microbes Environ. 2011, 26, 220–227.
- Prihastuti, H.; Cai, L.; Chen, H.; McKenzie, E.; Hyde, K. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Div. 2009, 39, 89–109.
- Ma, L.-J.; Van Der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.-J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373.
- Peck, L.; Nowell, R.; Flood, J.; Ryan, M.; Barraclough, T. Historical genomics reveals the evolutionary mechanisms behind multiple outbreaks of the host-specific coffee wilt pathogen Fusarium xylarioides. bioRxiv 2020, 22, 1–24.
- Rutherford, M.A. Current knowledge of coffee wilt disease, a major constraint to coffee production in Africa. Phytopathology 2006, 96, 663–666.
- Musoli, P.C.; Cilas, C.; Pot, D.; Nabaggala, A.; Nakendo, S.; Pande, J.; Charrier, A.; Leroy, T.; Bieysse, D. Inheritance of resistance to coffee wilt disease (Fusarium xylarioides Steyaert) in Robusta coffee (Coffea canephora Pierre) and breeding perspectives. Tree Genet. Genomes 2012, 9, 351–360.
- Gichuru, E.; Alwora, G.; Gimase, J.; Kathurima, C. Coffee leaf rust (Hemileia vastatrix) in Kenya—A review. Agronomy 2021, 11, 2590.
- De Andrade, C.C.L.; Vicentin, R.P.; Costa, J.R.; Perina, F.J.; De Resende, M.L.V.; Alves, E. Alterations in antioxidant metabolism in coffee leaves infected by Cercospora coffeicola. Ciênc. Rural 2016, 46, 1764–1770.
- Otieno, W. Armillaria Root Rot of Tea in Kenya: Characterization of the Pathogen and Approaches to Disease Management; Wageningen University and Research; Wageningen University: Wageningen, The Netherlands, 2002; Available online: https://www.semanticscholar.org/paper/Armillaria-root-rot-of-tea-in-Kenya-%3A-of-the-and-to-Otieno/c0f9753b5d198b0a8eb412d40b560c58304eef02#citing-papers (accessed on 16 February 2022).
- Fang, Y.; Ramasamy, R.P. Current and prospective methods for plant disease detection. Biosensors 2015, 5, 537–561.
- López-Lima, D.; Carrión, G.; Sánchez-Nava, P.; Desgarennes, D.; Villain, L. Fungal diversity and Fusarium oxysporum pathogenicity associated with coffee corky-root disease in Mexico. Rev. Fac. Cienc. Agrar. UNCuyo 2020, 52, 276–292.
- Hariharan, G.; Prasannath, K. Recent advances in molecular diagnostics of fungal plant pathogens: A mini review. Front. Cell. Infect. Microbiol. 2021, 10.
- Senanayake, I.; Rathnayaka, A.; Marasinghe, D.; Calabon, M.; Gentekaki, E.; Lee, H.; Hurdeal, V.; Pem, D.; Dissanayake, L.; Wijesinghe, S. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754.
- Arneson, P. Coffee Rust; Plant Health Instructor; The American Phytopathological Society (APS): St. Paul, MN, USA, 2000.
- Abu Mettleq, A.S.; Abu-Naser, S.S. A rule based system for the diagnosis of coffee diseases. Int. J. Acad. Inf. Syst. Res. (IJAISR) 2019, 3, 1–8.
- Hailu, B.Z. Fungal Disease Dynamics, Genetic Variation and Biodiversity-Yield Relationships: A Study Along a Gradient of Coffee Management in Southwestern Ethiopia. Ph.D. Thesis, Stockholm University, Faculty of Science, Department of Ecology, Environment and Plant Sciences, Stockholm, Sweden, 2020.
- de Resende, M.L.V.; Pozza, E.A.; Reichel, T.; Botelho, D.M.S. Strategies for coffee leaf rust management in organic crop systems. Agronomy 2021, 11, 1865.
- Lemma, D.T.; Abewoy, D. Review on integrated pest management of coffee berry disease and coffee berry borer. Int. J. Plant Breed. Crop Sci. 2021, 8, 1001–1008.
- Bailey, J.A. Colletotrichum: Biology, Pathology and Control; CAB International: Wallingford, UK, 1992.
- De Silva, D.D.; Crous, P.W.; Ades, P.K.; Hyde, K.D.; Taylor, P.W.J. Life styles of Colletotrichum species and implications for plant biosecurity. Fungal Biol. Rev. 2017, 31, 155–168.
- Wassie, A.K. Integrated diseased management on coffee wilt disease caused by Fusarium xylarioides and its distribution in Ethiopian review. Agric. Res. Technol. Open Access J. 2019, 23, 302–308.
- Wrigley, G. Coffee; Longman Scientific & Technical: Harlow, UK; Essex, UK, 1988.
- Alemu, T. A review of coffee wilt disease, Gibberella xylarioides (Fusarium xylarioides) in Africa with special reference to Ethiopia. Ethiop. J. Biol. Sci. 2012, 11, 65–103.
- Nelson, S.C. Cercospora Leaf Spot and Berry Blight of Coffee; Plant Disease; University of Hawaii: Mänoa, Hawaii, 2008; Available online: https://www.ctahr.hawaii.edu/oc/freepubs/pdf/PD-41.pdf (accessed on 16 February 2022).
- Vale, P.A.S.; De Resende, M.L.V.; Botelho, D.M.D.S.; De Andrade, C.C.L.; Alves, E.; Ogoshi, C.; Guimarães, S.D.S.C.; Pfenning, L.H. Epitypification of Cercospora coffeicola and its involvement with two different symptoms on coffee leaves in Brazil. Eur. J. Plant Pathol. 2020, 159, 399–408.
- Schwartz, H.F.; Gent, D.H. Cercospora leaf spot (Cucumber, Melon, Pumpkin, Squash, and Zucchini). In High Plains IPM Guide; University of Wyoming: Laramie, WY, USA; University of Nebraska: Lincoln, NE, USA; Colorado State University: Fort Collins, CO, USA; Montana State University: Bozeman, MT, USA, 2007; Available online: http://wiki.bugwood.org/uploads/CercosporaLeafSpot-Cucurbits.pdf (accessed on 16 February 2022).
- Souza, A.G.C.; Rodrigues, F.; Maffia, L.A.; Mizubuti, E.S.G. Infection process of Cercospora coffeicola on coffee leaf. J. Phytopathol. 2010, 159, 6–11.
More
Information
Subjects:
Mycology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.7K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
08 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




