
Video Upload Options
In the human cornea, regeneration of the epithelium is regulated by the stem cell reservoir of the limbus, which is the marginal region of the cornea representing the anatomical and functional border between the corneal and conjunctival epithelium. In support of this concept, extensive limbal damage, e.g., by chemical or thermal injury, inflammation, or surgery, may induce limbal stem cell deficiency (LSCD) leading to vascularization and opacification of the cornea and eventually vision loss. These acquired forms of limbal stem cell deficiency may occur uni- or bilaterally, which is important for the choice of treatment. Moreover, a variety of inherited diseases, such as congenital aniridia or dyskeratosis congenita, are characterized by LSCD typically occurring bilaterally. Several techniques of autologous and allogenic stem cell transplantation have been established. The limbus can be restored by transplantation of whole limbal grafts, small limbal biopsies or by ex vivo-expanded limbal cells.
1. Introduction
2. Localization of Limbal Epithelial Stem Cells
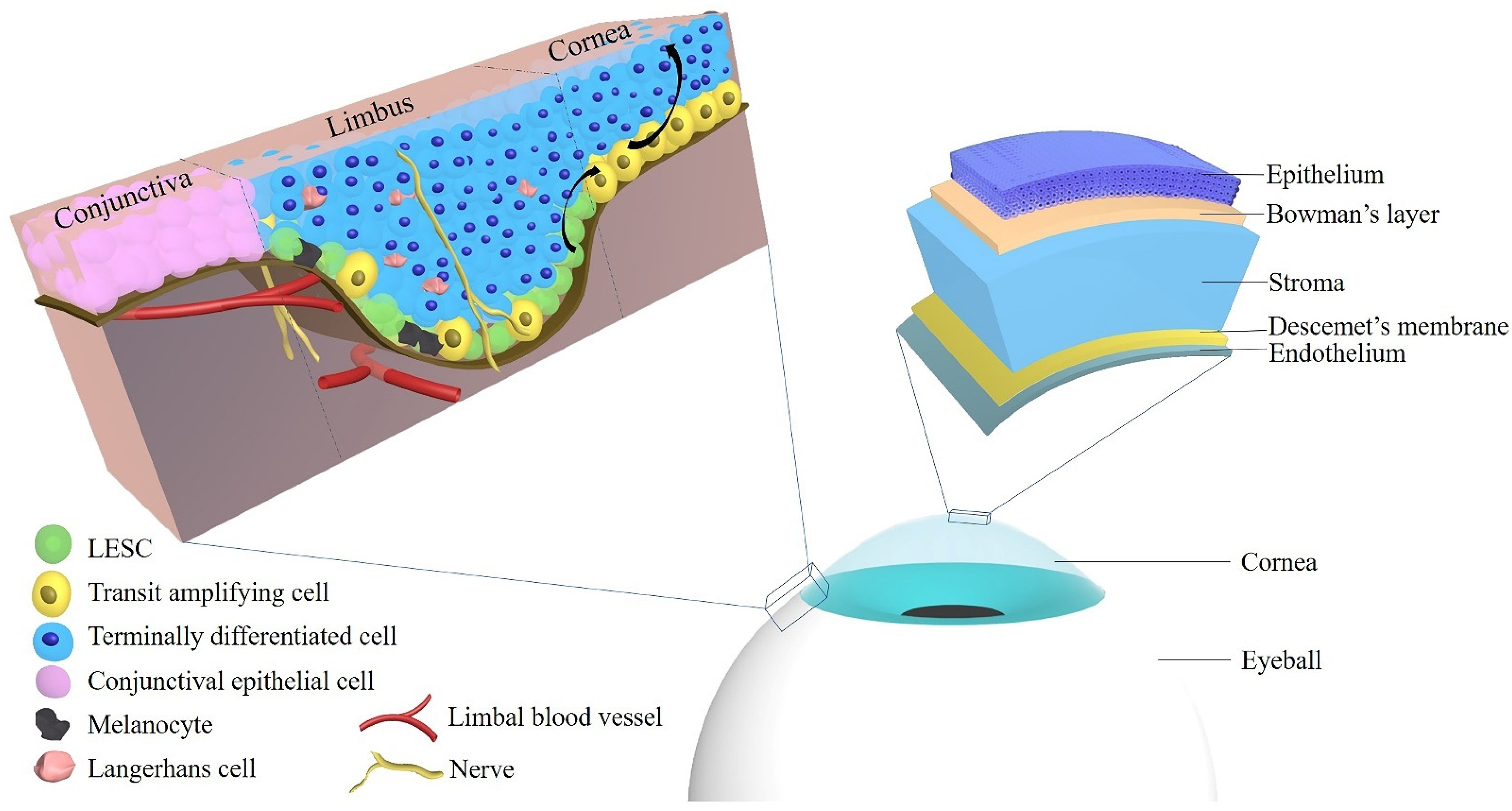
3. Limbal Epithelial Stem Cell Markers
3.1. p63
3.2. ABCG2
3.3. Growth Factor Receptors
3.4. Integrins
3.5. Keratins
| Keratins | Species | Stage | Location | References |
|---|---|---|---|---|
| K3 | Rabbit | 21-day embryos | The peridermal layer of the cornea | [98] |
| 23-day embryos | The suprabasal layer of the cornea | |||
| 7 to 12 days after birth | The basal layer of the cornea | |||
| Adult | The basal layer of the corneal epithelium and the suprabasal layer of the limbal epithelium | |||
| K4 | Mouse | 16-, 18-, and 20-day embryos 2 and 4 days after birth |
The superficial layer of the cornea | [94] |
| Adult | Not observed | |||
| K5/K12 | Mouse | 7 days after birth | The basal and apical cells of the central and peripheral cornea and limbus | [95] |
| K12 | Rabbit | 17-day embryos | The peridermal layer of the cornea | [98] |
| 23-day embryos | The basal layer of the cornea | |||
| Adult | The basal layer of the corneal epithelium and the suprabasal layer of the limbal epithelium | |||
| K12 | Chick | 12-day embryos | Both peridermal and basal ectodermal layers of the cornea | [98] |
| 14- to 21-day embryos | All the epithelial strata of the central cornea |
|||
| 21-day embryos | The suprabasal layer of the limbal epithelium | |||
| K12 | Mouse | 15-day embryos | The superficial layer of the corneal epithelium | [94] |
| 18-day embryos | The suprabasal layer of the cornea | |||
| 0 h after wounding | The peripheral corneal epithelium | [97] | ||
| 24 h after wounding | Occasionally in superficial cells of the central and peripheral cornea | |||
| K14 | Mouse | 15-day embryos | Corneal epithelial cells | [94] |
| 7 days after birth | The basal layers over the entire mouse ocular surface with higher expression at the limbal region compared with the central cornea | [95] | ||
| 49 days after birth | The localization decreases in the central corneal epithelium but remains strong at the limbus. | |||
| 0 h after wounding | Restricted to the limbus | [97] | ||
| 24 h after wounding | The layer of epithelial cells that covered the defect | |||
| 1 day after wounding | Corneal epithelial cells at and behind the leading edge | [95] | ||
| 7 days after wounding | The corneal center | |||
| 28 days after wounding | Cells adjacent to an erosion at the corneal center and around goblet cell clusters at the limbus |
|||
| K15 | Mouse | 7 days after birth | Limbal cells and well spread apical cells of the corneal periphery and center | [95] |
| 49 days after birth | The localization decreases in the central corneal epithelium but remains strong at the limbus. | |||
| 1 day after wounding | Extending toward the leading edge | |||
| 7 days after wounding | The corneal center | |||
| 28 days after wounding | Cells adjacent to an erosion at the corneal center and around goblet cell clusters at the limbus |
|||
| K17 | Human | Within 24 h (for ex vivo) to 48 h after death | Clusters of limbal basal cells in normal corneas and significantly decreased in diabetic limbal basal epithelium | [99] |
| K18 | Mouse | 14 days after birth | The limbus | [95] |
| 7 days after wounding, | The center of the clusters | |||
| 28 days after wounding | Compound niches | |||
| K8/K19 | Mouse | 7 days after birth | Basal and suprabasal cells throughout the corneal epithelium and limbus | [95] |
| 14 days after birth | Fewer cells on the central cornea and more restricted toward the peripheral and limbal region | |||
| 1 day after wounding | K19 + clusters are migrating away from the limbus | |||
| 7 days after wounding | K19 is localized to cells at the edges of large clusters | |||
| 28 days after wounding | Near the limbal region and on the peripheral cornea | [95][96] |
References
- Haeckel, E. Natürliche Schöpfungsgeschichte: Gemeinverständliche Wissenschaftliche Vorträge Über die Entwickelungslehre im Allgemeinen und Diejenige von Darwin, Goethe und Lamarck und Besonderen; Biodiversity Heritage Libary: Berlin, Germany, 1868.
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68.
- Lavker, R.M.; Sun, T.-T. Epidermal stem cells: Properties, markers, and location. Proc. Natl. Acad. Sci. USA 2000, 97, 13473–13475.
- Fuchs, E.; Segre, J.A. Stem Cells: A New Lease on Life. Cell 2000, 100, 143–155.
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147.
- Montagnani, S.; Rueger, M.A.; Hosoda, T.; Nurzynska, D. Adult Stem Cells in Tissue Maintenance and Regeneration. Stem Cells Int. 2016, 2016, 1–2.
- Henningson, C.T.; Stanislaus, M.A.; Gewirtz, A.M. Embryonic and adult stem cell therapy. J. Allergy Clin. Immunol. 2003, 111, S745–S753.
- Nuti, N.; Corallo, C.; Chan, B.M.F.; Ferrari, M.; Gerami-Naini, B. Multipotent Differentiation of Human Dental Pulp Stem Cells: A Literature Review. Stem Cell Rev. Rep. 2016, 12, 511–523.
- Catacchio, I.; Berardi, S.; Reale, A.; De Luisi, A.; Racanelli, V.; Vacca, A.; Ria, R. Evidence for Bone Marrow Adult Stem Cell Plasticity: Properties, Molecular Mechanisms, Negative Aspects, and Clinical Applications of Hematopoietic and Mesenchymal Stem Cells Transdifferentiation. Stem Cells Int. 2013, 2013, 589139.
- So, W.-K.; Cheung, T.H.; Lacorazza, H.D. Molecular Regulation of Cellular Quiescence: A Perspective from Adult Stem Cells and Its Niches. Adv. Struct. Saf. Stud. 2017, 1686, 1–25.
- Bhartiya, D.; Mohammad, S.A.; Guha, A.; Singh, P.; Sharma, D.; Kaushik, A. Evolving Definition of Adult Stem/Progenitor Cells. Stem Cell Rev. Rep. 2019, 15, 456–458.
- Almada, A.E.; Wagers, A.E.A.A.J. Molecular circuitry of stem cell fate in skeletal muscle regeneration, ageing and disease. Nat. Rev. Mol. Cell Biol. 2016, 17, 267–279.
- Davanger, M.; Evensen, A. Role of the Pericorneal Papillary Structure in Renewal of Corneal Epithelium. Nat. Cell Biol. 1971, 229, 560–561.
- Keivyon, K.R.; Tseng, S.C. Limbal Autograft Transplantation for Ocular Surface Disorders. Ophthalmology 1989, 96, 709–723.
- Pellegrini, G.; Golisano, O.; Paterna, P.; Lambiase, A.; Bonini, S.; Rama, P.; De Luca, M. Location and Clonal Analysis of Stem Cells and Their Differentiated Progeny in the Human Ocular Surface. J. Cell Biol. 1999, 145, 769–782.
- Huang, A.J.; Tseng, S.C. Corneal epithelial wound healing in the absence of limbal epithelium. Investig. Ophthalmol. Vis. Sci. 1991, 32, 96–105.
- Amano, S.; Yamagami, S.; Mimura, T.; Uchida, S.; Yokoo, S. Corneal Stromal and Endothelial Cell Precursors. Cornea 2006, 25, S73–S77.
- Yu, W.Y.; Sheridan, C.; Grierson, I.; Mason, S.; Kearns, V.; Lo, A.C.Y.; Wong, D. Progenitors for the Corneal Endothelium and Trabecular Meshwork: A Potential Source for Personalized Stem Cell Therapy in Corneal Endothelial Diseases and Glaucoma. J. Biomed. Biotechnol. 2011, 2011, 412743.
- Du, Y.; Funderburgh, M.L.; Mann, M.M.; SundarRaj, N.; Funderburgh, J.L. Multipotent Stem Cells in Human Corneal Stroma. Stem Cells 2005, 23, 1266–1275.
- Funderburgh, J.L.; Funderburgh, M.L.; Du, Y. Stem Cells in the Limbal Stroma. Ocul. Surf. 2016, 14, 113–120.
- Pinnamaneni, N.; Funderburgh, J.L. Concise Review: Stem Cells in the Corneal Stroma. Stem Cells 2012, 30, 1059–1063.
- Joe, A.W.; Yeung, S.N. Concise Review: Identifying Limbal Stem Cells: Classical Concepts and New Challenges. Stem Cells Transl. Med. 2014, 3, 318–322.
- Liu, X.-N.; Mi, S.-L. Corneal stromal mesenchymal stem cells: Reconstructing a bioactive cornea and repairing the corneal limbus and stromal microenvironment. Int. J. Ophthalmol. 2021, 14, 448–455.
- Massoudi, D.; Malecaze, F.; Galiacy, S.D. Collagens and proteoglycans of the cornea: Importance in transparency and visual disorders. Cell Tissue Res. 2016, 363, 337–349.
- Mukhija, R.; Gupta, N.; Vashist, P.; Tandon, R.; Gupta, S.K. Population-based assessment of visual impairment and pattern of corneal disease: Results from the CORE (Corneal Opacity Rural Epidemiological) study. Br. J. Ophthalmol. 2019, 104, 994–998.
- World Health Organization. World Report on Vision. 2019. World Health Organization Website. Available online: https://www.who.int/docs/default-source/documents/publications/world-vision-report-accessible.pdf?sfvrsn=223f9bf7_2 (accessed on 20 June 2021).
- McMahon, T.T.; Robin, J.B. Corneal trauma: I--Classification and management. J. Am. Optom. Assoc. 1991, 62, 170–178.
- Shaheen, B.S.; Bakir, M.; Jain, S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014, 59, 263–285.
- Matthaei, M.; Hribek, A.; Clahsen, T.; Bachmann, B.; Cursiefen, C.; Jun, A.S. Fuchs Endothelial Corneal Dystrophy: Clinical, Genetic, Pathophysiologic, and Therapeutic Aspects. Annu. Rev. Vis. Sci. 2019, 5, 151–175.
- Haagdorens, M.; Van Acker, S.I.; Van Gerwen, V.; Dhubhghaill, S.N.; Koppen, C.; Tassignon, M.-J.; Zakaria, N. Limbal Stem Cell Deficiency: Current Treatment Options and Emerging Therapies. Stem Cells Int. 2016, 2016, 9798374.
- Mathews, P.M.; Lindsley, K.; Aldave, A.J.; Akpek, E.K. Etiology of Global Corneal Blindness and Current Practices of Corneal Transplantation: A Focused Review. Cornea 2018, 37, 1198–1203.
- Alio, J.L.; Montesel, A.; El Sayyad, F.; Barraquer, R.I.; Arnalich-Montiel, F.; Del Barrio, J.L.A. Corneal graft failure: An update. Br. J. Ophthalmol. 2020, 105.
- Shaharuddin, B.; Ahmad, S.; Meeson, A.; Ali, S. Concise Review: Immunological Properties of Ocular Surface and Importance of Limbal Stem Cells for Transplantation. Stem Cells Transl. Med. 2013, 2, 614–624.
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194.
- Dua, H.S.; Faraj, L.A. Human corneal anatomy redefined: A novel pre-descemet’s layer (dua’s layer). Ophthalmology 2013, 120, 1778–1785.
- Dua, H.S.; Faraj, L.A. Author reply: To pmid 23714320. Ophthalmology 2014, 121, e25-6.
- Parker, J.S.; Birbal, R.S. Are descemet membrane ruptures the root cause of corneal hydrops in keratoconic eyes? Am. J. Ophthalmol. 2019, 205, 147–152.
- Yahia Chérif, H.; Gueudry, J. Efficacy and safety of pre-descemet’s membrane sutures for the management of acute corneal hydrops in keratoconus. Br. J. Ophthalmol. 2015, 99, 773–777.
- Ross, A.R.; Said, D.G.; Gisoldi, R.A.M.C.; Nubile, M.; El-Amin, A.; Gabr, A.F.; El-Moniem, M.A.; Mencucci, R.; Pocobelli, A.; Mastropasqua, L.; et al. Optimizing pre-Descemet’s endothelial keratoplasty (PDEK) technique. J. Cataract Refract. Surg. 2020, 46, 667–674.
- Pereira, N.C.; Forseto, A.D.S.; Maluf, R.C.P.; Dua, H.S. Pre-Descemet’s endothelial keratoplasty: A simple, Descemet’s membrane scoring technique for successful graft preparation. Br. J. Ophthalmol. 2021.
- Wasielica-Poslednik, J.; Lisch, W.; Bell, K.; Weyer, V.; Pfeiffer, N.; Gericke, A. Reproducibility and Daytime-Dependent Changes of Corneal Epithelial Thickness and Whole Corneal Thickness Measured with Fourier Domain Optical Coherence Tomography. Cornea 2016, 35, 342–349.
- Dohlman, C.H. The function of the corneal epithelium in health and disease: The jonas s. Friedenwald memorial lecture. Investig. Ophthalmol. Vis. Sci. 1971, 10, 383–407.
- Thoft, R.A.; Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443.
- Lehrer, M.S.; Sun, T.T.; Lavker, R.M. Strategies of epithelial repair: Modulation of stem cell and transit amplifying cell proliferation. J. Cell Sci. 1998, 111, 2867–2875.
- Auran, J.D.; Koester, C.J.; Kleiman, N.; Rapaport, R.; Bomann, J.S.; Wirotsko, B.M.; Florakis, G.J.; Koniarek, J.P. Scanning Slit Confocal Microscopic Observation of Cell Morphology and Movement within the Normal Human Anterior Cornea. Ophthalmology 1995, 102, 33–41.
- Thoft, R.A. The role of the limbus in ocular surface maintenance and repair. Acta Ophthalmol. 2009, 67, 91–94.
- Schermer, A.; Galvin, S.; Sun, T.-T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J. Cell Biol. 1986, 103, 49–62.
- Chung, E.H.; Bukusoglu, G.; Zieske, J. Localization of corneal epithelial stem cells in the developing rat. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2199–2206.
- Moriyama, H.; Kasashima, Y.; Kuwano, A.; Wada, S. Anatomical location and culture of equine corneal epithelial stem cells. Vet. Ophthalmol. 2013, 17, 106–112.
- De Paiva, C.S.; Chen, Z. Abcg2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells 2005, 23, 63–73.
- Ebato, B.; Friend, J.; Thoft, R.A. Comparison of central and peripheral human corneal epithelium in tissue culture. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1450–1456.
- Ebato, B.; Friend, J.; Thoft, R.A. Comparison of limbal and peripheral human corneal epithelium in tissue culture. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1533–1537.
- Dua, H.S.; Shanmuganathan, V.A.; O Powell-Richards, A.; Tighe, P.; Joseph, A. Limbal epithelial crypts: A novel anatomical structure and a putative limbal stem cell niche. Br. J. Ophthalmol. 2005, 89, 529–532.
- Goldberg, M.F.; Bron, A.J. Limbal palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1982, 80, 155–171.
- Shortt, A.J.; Secker, G.A.; Munro, P.M.; Khaw, P.T.; Tuft, S.J.; Daniels, J.T. Characterization of the Limbal Epithelial Stem Cell Niche: Novel Imaging Techniques Permit In Vivo Observation and Targeted Biopsy of Limbal Epithelial Stem Cells. Stem Cells 2007, 25, 1402–1409.
- Yang, A.; Kaghad, M.; Wang, Y.; Gillett, E.; Fleming, M.; Dotsch, V.; Andrews, N.; Caput, D.; McKeon, F. p63, a p53 Homolog at 3q27–29, Encodes Multiple Products with Transactivating, Death-Inducing, and Dominant-Negative Activities. Mol. Cell 1998, 2, 305–316.
- Barbaro, V.; Testa, A.; Di Iorio, E.; Mavilio, F.; Pellegrini, G.; De Luca, M. C/EBPδ regulates cell cycle and self-renewal of human limbal stem cells. J. Cell Biol. 2007, 177, 1037–1049.
- Pellegrini, G.; Dellambra, E.; Golisano, O.; Martinelli, E.; Fantozzi, I.; Bondanza, S.; Ponzin, D.; McKeon, F.; De Luca, M. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3156–3161.
- Koster, M.I.; Kim, S.; Mills, A.A.; DeMayo, F.; Roop, D.R. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004, 18, 126–131.
- Di Iorio, E.; Barbaro, V. Isoforms of δnp63 and the migration of ocular limbal cells in human corneal regeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 9523.
- Baba, K.; Sasaki, K.; Morita, M.; Tanaka, T.; Teranishi, Y.; Ogasawara, T.; Oie, Y.; Kusumi, I.; Inoie, M.; Hata, K.-I.; et al. Cell jamming, stratification and p63 expression in cultivated human corneal epithelial cell sheets. Sci. Rep. 2020, 10, 9282.
- Mills, A.A.; Zheng, B.; Wang, X.-J.; Vogel, O.H.; Roop, D.R.; Bradley, A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nat. Cell Biol. 1999, 398, 708–713.
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal Stem-Cell Therapy and Long-Term Corneal Regeneration. New Engl. J. Med. 2010, 363, 147–155.
- Chen, Z.; de Paiva, C.S. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells 2004, 22, 355–366.
- Kim, M.; Turnquist, H.; Jackson, J.; Sgagias, M.; Yan, Y.; Gong, M.; Dean, M.; Sharp, J.G.; Cowan, K. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin. Cancer Res. 2002, 8, 22–28.
- Watanabe, K.; Nishida, K. Human limbal epithelium contains side population cells expressing the atp-binding cassette transporter abcg2. FEBS Lett. 2004, 565, 6–10.
- Morita, M.; Fujita, N.; Takahashi, A.; Nam, E.R.; Yui, S.; Chung, C.S.; Kawahara, N.; Lin, H.Y.; Tsuzuki, K.; Nakagawa, T.; et al. Evaluation of ABCG2 and p63 expression in canine cornea and cultivated corneal epithelial cells. Vet. Ophthalmol. 2014, 18, 59–68.
- Kethiri, A.R.; Basu, S.; Shukla, S.; Sangwan, V.S.; Singh, V. Optimizing the role of limbal explant size and source in determining the outcomes of limbal transplantation: An in vitro study. PLoS ONE 2017, 12, e0185623.
- Kubota, M.; Shimmura, S.; Miyashita, H.; Kawashima, M.; Kawakita, T.; Tsubota, K. The Anti-oxidative Role of ABCG2 in Corneal Epithelial Cells. Investig. Opthalmol. Vis. Sci. 2010, 51, 5617–5622.
- Yeh, H.-J.; Yao, C.-L.; Chen, H.-I.; Cheng, H.-C.; Hwang, S.-M. Cryopreservation of Human Limbal Stem Cells Ex Vivo Expanded on Amniotic Membrane. Cornea 2008, 27, 327–333.
- Zieske, J.D.; Wasson, M. Regional variation in distribution of EGF receptor in developing and adult corneal epithelium. J. Cell Sci. 1993, 106, 145–152.
- Liu, Z.; Carvajal, M.; Carraway, C.A.C.; Carraway, K.; Pflugfelder, S.C. Expression of the Receptor Tyrosine Kinases, Epidermal Growth Factor Receptor, ErbB2, and ErbB3, in Human Ocular Surface Epithelia. Cornea 2001, 20, 81–85.
- Li, D.Q.; Tseng, S.C. Differential regulation of keratinocyte growth factor and hepatocyte growth factor/scatter factor by different cytokines in human corneal and limbal fibroblasts. J. Cell. Physiol. 1997, 172, 361–372.
- Cheng, C.C.; Wang, D.Y. The growth-promoting effect of kgf on limbal epithelial cells is mediated by upregulation of deltanp63alpha through the p38 pathway. J. Cell Sci. 2009, 122, 4473–4480.
- Yoshihara, M.; Sasamoto, Y.; Hayashi, R.; Ishikawa, Y.; Tsujikawa, M.; Hayashizaki, Y.; Itoh, M.; Kawaji, H.; Nishida, K. High-resolution promoter map of human limbal epithelial cells cultured with keratinocyte growth factor and rho kinase inhibitor. Sci. Rep. 2017, 7, 2845.
- Li, D.-Q.; Tseng, S.C.G. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J. Cell. Physiol. 1995, 163, 61–79.
- Takada, Y.; Ye, X. The integrins. Genome Biol. 2007, 8, 215.
- Stepp, M.A.; Spurr-Michaud, S.; Gipson, I.K. Integrins in the wounded and unwounded stratified squamous epithelium of the cornea. Investig. Ophthalmol. Vis. Sci. 1993, 34, 1829–1844.
- Tervo, K.; Tervo, T.; Van Setten, G.-B.; Virtanen, I. Integrins in Human Corneal Epithelium. Cornea 1991, 10, 461–465.
- Pajoohesh-Ganji, A.; Pal-Ghosh, S.; Simmens, S.J.; Stepp, M.A. Integrins in Slow-Cycling Corneal Epithelial Cells at the Limbus in the Mouse. Stem Cells 2006, 24, 1075–1086.
- Hayashi, R.; Yamato, M.; Saito, T.; Oshima, T.; Okano, T.; Tano, Y.; Nishida, K. Enrichment of corneal epithelial stem/progenitor cells using cell surface markers, integrin α6 and CD71. Biochem. Biophys. Res. Commun. 2008, 367, 256–263.
- Reinshagen, H.; Auw-Haedrich, C.; Sorg, R.V.; Boehringer, D.; Eberwein, P.; Schwartzkopff, J.; Sundmacher, R.; Reinhard, T. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol. 2011, 89, 741–748.
- Matic, M.; Petrov, I.N.; Chen, S.; Wang, C.; Wolosin, J.M.; Dimitrijevich, S.D. Stem cells of the corneal epithelium lack connexins and metabolite transfer capacity. Differentiation 1997, 61, 251–260.
- Ordonez, P.; Di Girolamo, N. Limbal Epithelial Stem Cells: Role of the Niche Microenvironment. Stem Cells 2012, 30, 100–107.
- Li, A.; Simmons, P.J.; Kaur, P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc. Natl. Acad. Sci. USA 1998, 95, 3902–3907.
- Maseruka, H.; Ridgway, A.; Tullo, A.; Bonshek, R. Developmental changes in patterns of expression of tenascin-C variants in the human cornea. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4101–4107.
- Fuchs, E. Keratins and the skin. Annu. Rev. Cell Dev. Biol. 1995, 11, 123–153.
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733.
- Kao, W.W.-Y. Keratin expression by corneal and limbal stem cells during development. Exp. Eye Res. 2020, 200, 108206.
- Figueira, E.C.; Di Girolamo, N.; Coroneo, M.T.; Wakefield, D. The Phenotype of Limbal Epithelial Stem Cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 144–156.
- Romano, R.-A.; Ortt, K.; Birkaya, B.; Smalley, K.; Sinha, S. An Active Role of the ΔN Isoform of p63 in Regulating Basal Keratin Genes K5 and K14 and Directing Epidermal Cell Fate. PLoS ONE 2009, 4, e5623.
- Chen, B.; Mi, S.; Wright, B.; Connon, C.J. Investigation of K14/K5 as a Stem Cell Marker in the Limbal Region of the Bovine Cornea. PLoS ONE 2010, 5, e13192.
- Irvine, A.D.; Corden, L.D. Mutations in cornea-specific keratin k3 or k12 genes cause meesmann’s corneal dystrophy. Nat. Genet. 1997, 16, 184–187.
- Kurpakus, M.A.; Maniaci, M.T.; Esco, M. Expression of keratins K12, K4 and K14 during development of ocular surface epithelium. Curr. Eye Res. 1994, 13, 805–814.
- Pajoohesh-Ganji, A.; Pal-Ghosh, S.; Tadvalkar, G.; Stepp, M.A. K14 + Compound niches are present on the mouse cornea early after birth and expand after debridement wounds. Dev. Dyn. 2015, 245, 132–143.
- Pajoohesh-Ganji, A.; Pal-Ghosh, S.; Tadvalkar, G.; Stepp, M.A. Corneal Goblet Cells and Their Niche: Implications for Corneal Stem Cell Deficiency. Stem Cells 2012, 30, 2032–2043.
- Park, M.; Richardson, A.; Pandzic, E.; Lobo, E.P.; Whan, R.; Watson, S.L.; Lyons, G.; Wakefield, D.; Di Girolamo, N. Visualizing the Contribution of Keratin-14+ Limbal Epithelial Precursors in Corneal Wound Healing. Stem Cell Rep. 2019, 12, 14–28.
- Chaloin-Dufau, C.; Dhouailly, D.; Sun, T.-T. Appearance of the keratin pair K3/K12 during embryonic and adult corneal epithelial differentiation in the chick and in the rabbit. Cell Differ. Dev. 1990, 32, 97–108.
- Saghizadeh, M.; Soleymani, S.; Harounian, A.; Bhakta, B.; Troyanovsky, S.M.; Brunken, W.J.; Pellegrini, G.; Ljubimov, A.V. Alterations of epithelial stem cell marker patterns in human diabetic corneas and effects of c-met gene therapy. Mol. Vis. 2011, 17, 2177–2190.




