
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohamed Aboelhamd | -- | 3239 | 2022-04-07 11:54:28 | | | |
| 2 | Vivi Li | + 55 word(s) | 3294 | 2022-04-08 04:35:45 | | |
Video Upload Options
Injectable hydrogels (IHs) are smart biomaterials and are the most widely investigated and versatile technologies, which can be either implanted or inserted into living bodies with minimal invasion. Their unique features, tunable structure and stimuli-responsive biodegradation properties make these IHs promising in many biomedical applications, including tissue engineering, regenerative medicines, implants, drug/protein/gene delivery, cancer treatment, aesthetic corrections and spinal fusions. Regarding their current prospective and ongoing research, hydrogel formulations have some limitations in their applications, clinical practices and sustainability. Many hydrogel systems (natural/synthetic), such as thermosensitive hydrogels, are free-flowing sols at a low temperature, while upon raising to body temperature (physiological temperature), they are converted to a stable visco-elastic gel phase, such as poly (phosphazene), pluronic and poly (N-isopropyl acrylamide).
1. Introduction
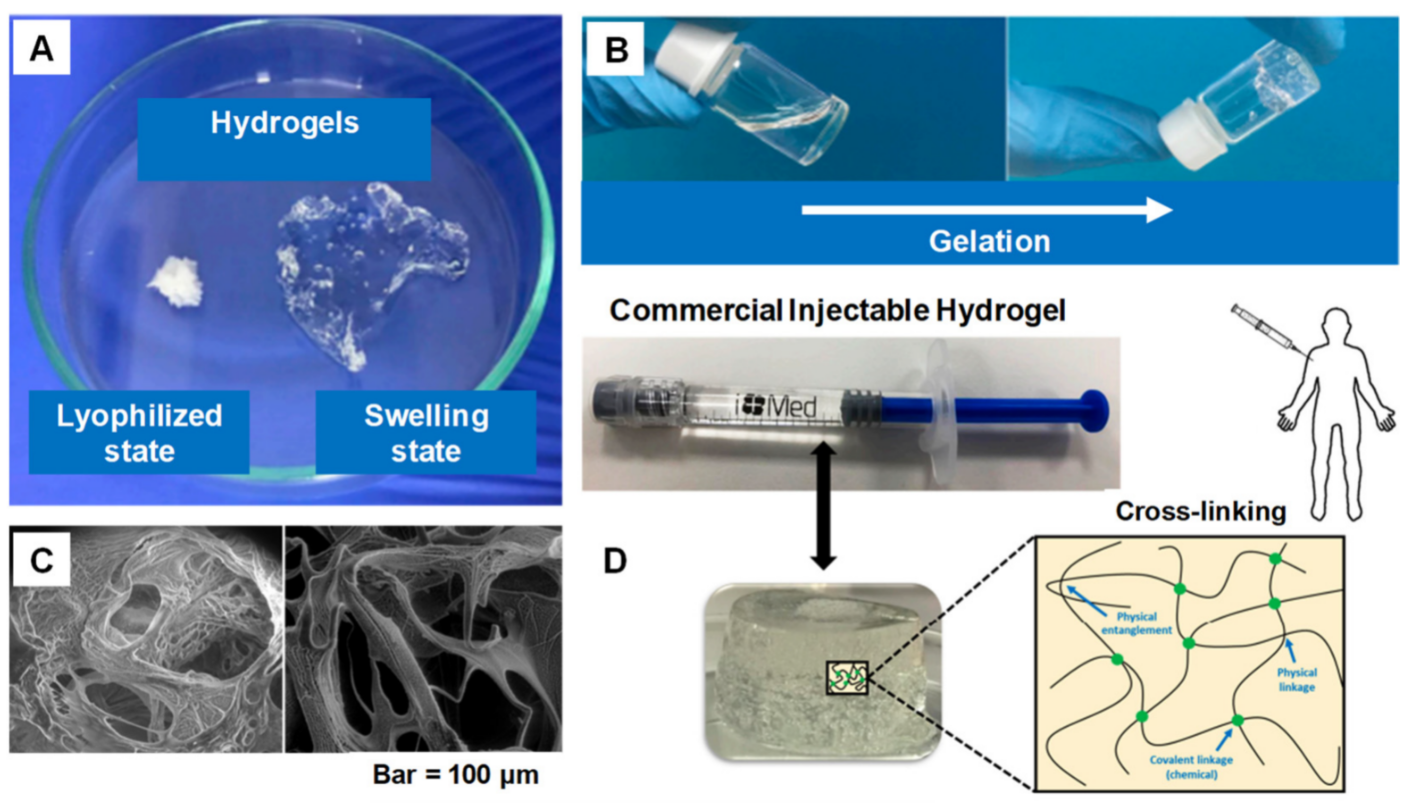
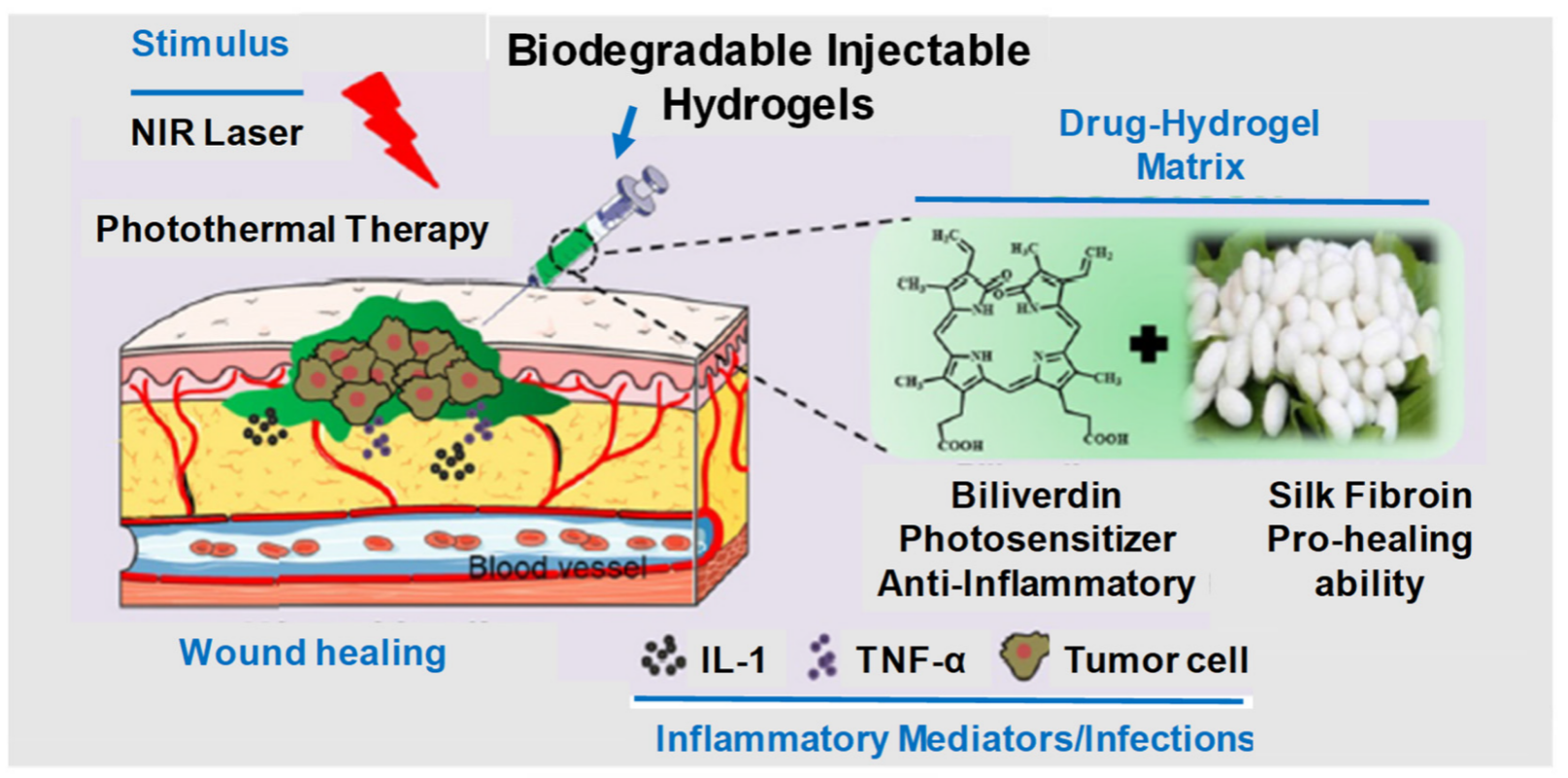
2. Current Trends
2.1. IHs and Its Application in Drug Delivery System and Biomedical Engineering Applications
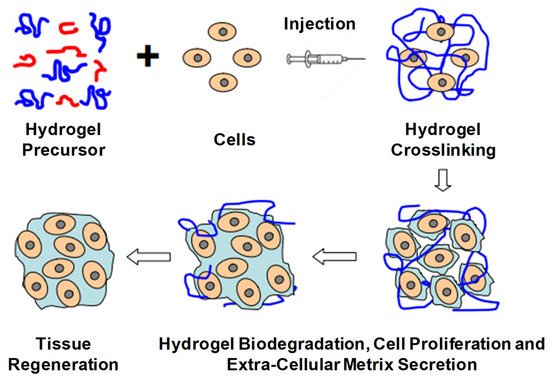
2.2. Drug Delivery
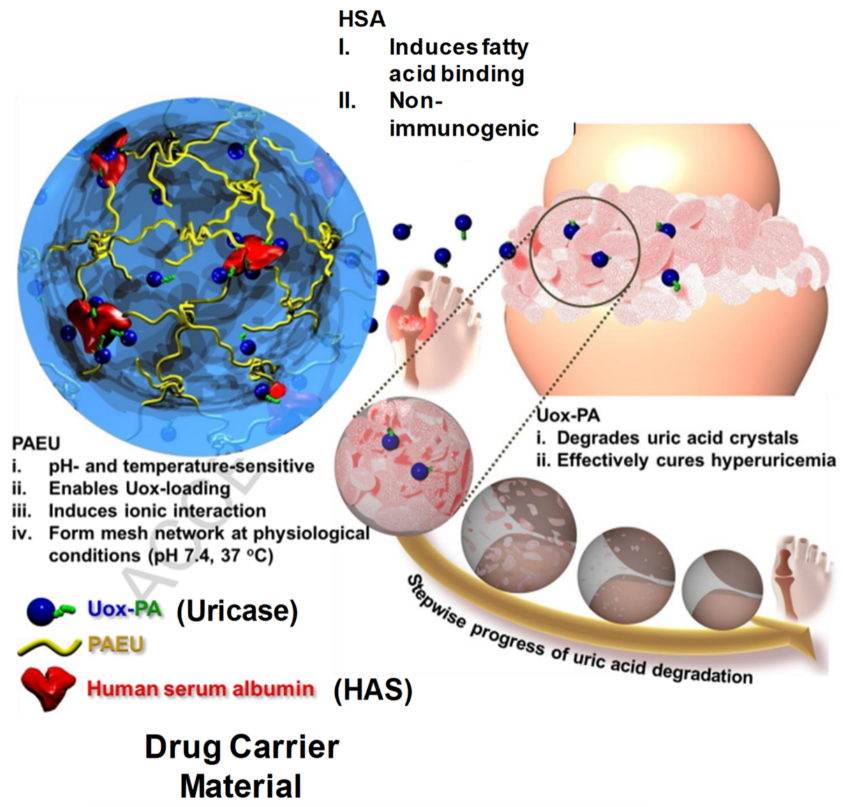
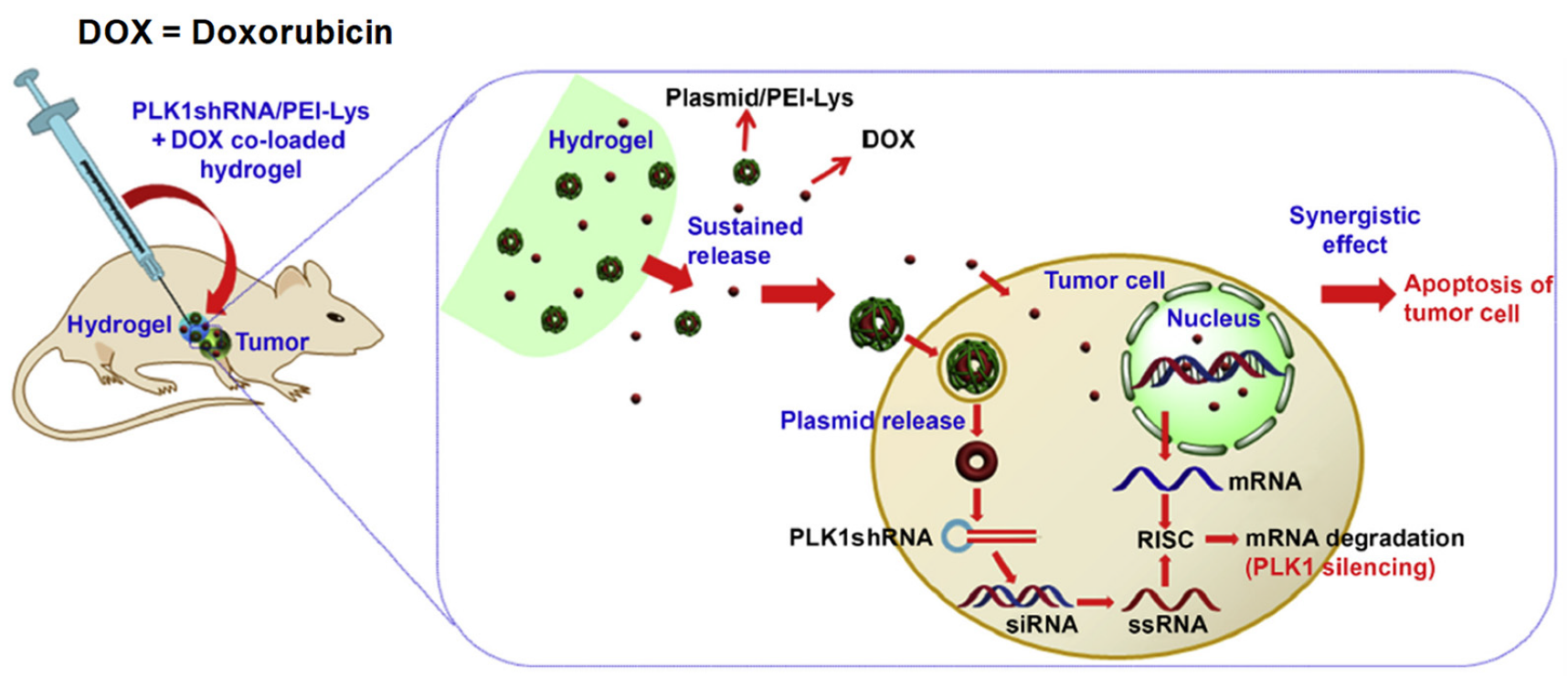
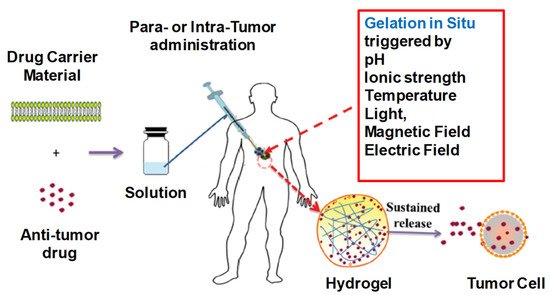
2.3. Therapeutic Applications
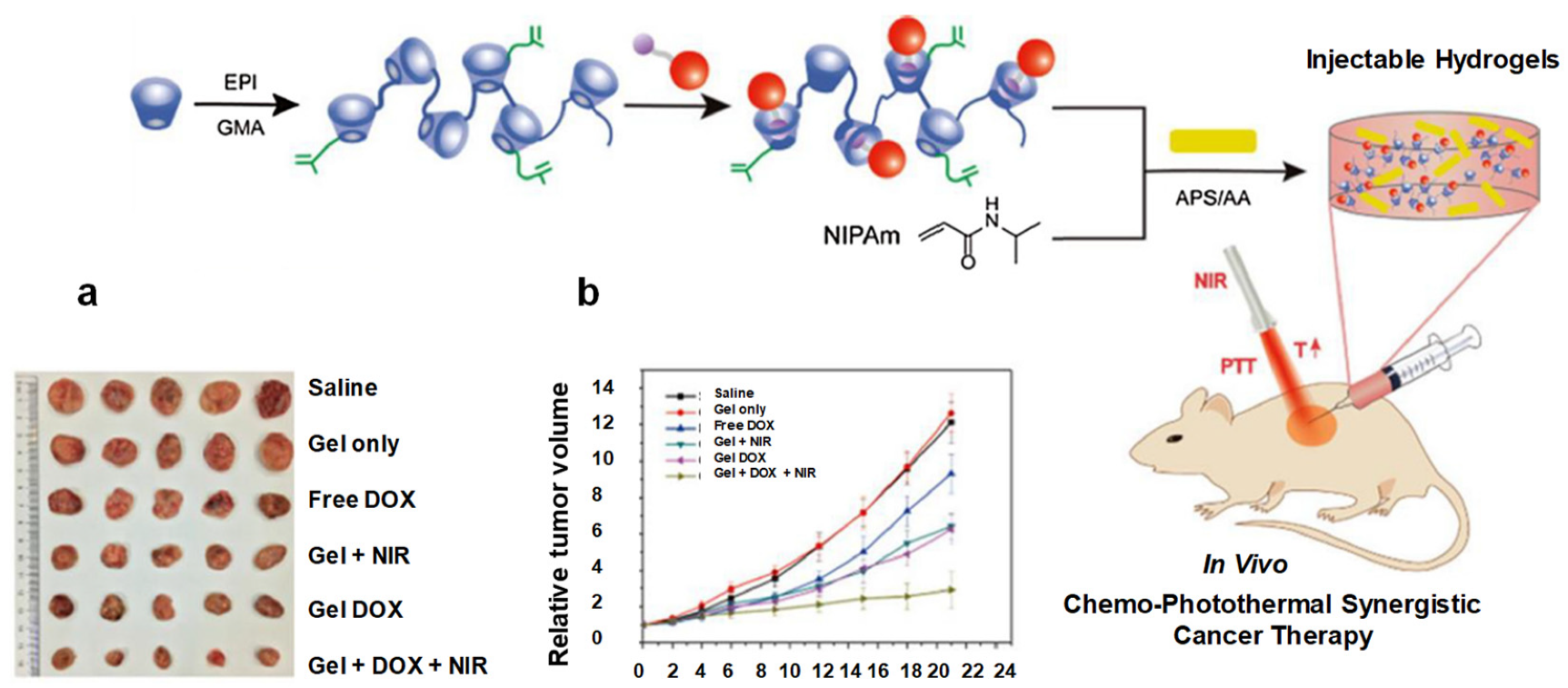
2.3.1. Cancer Therapy
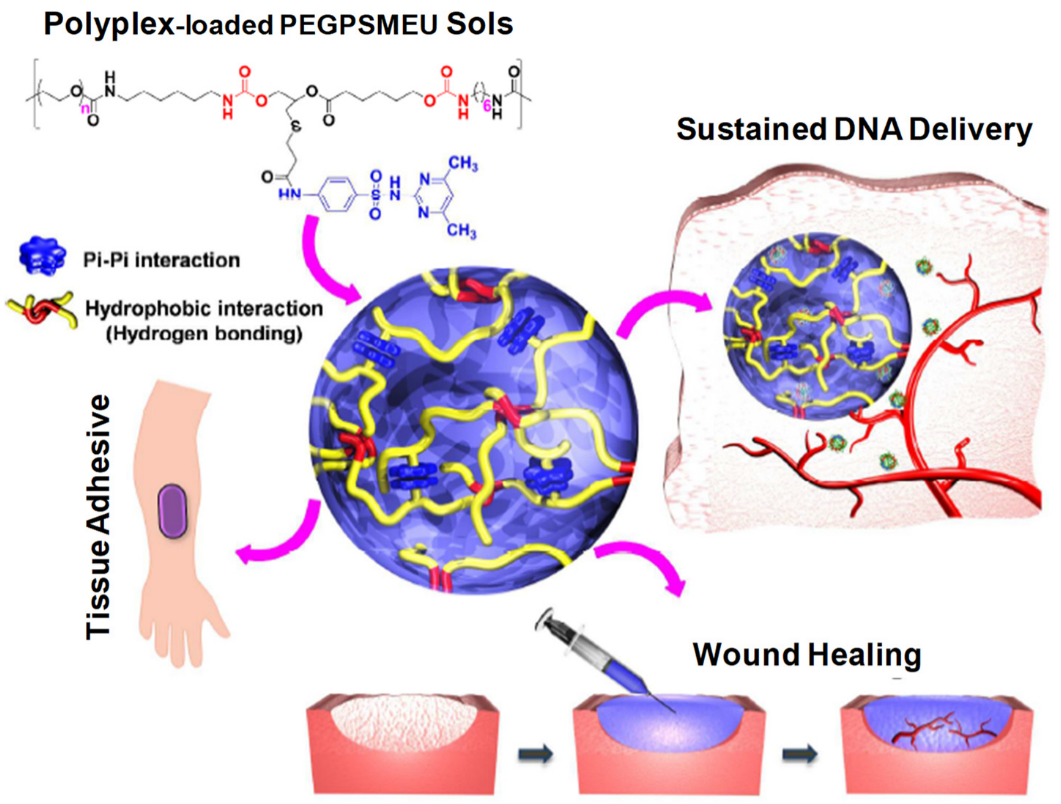
2.3.2. Wound Healings
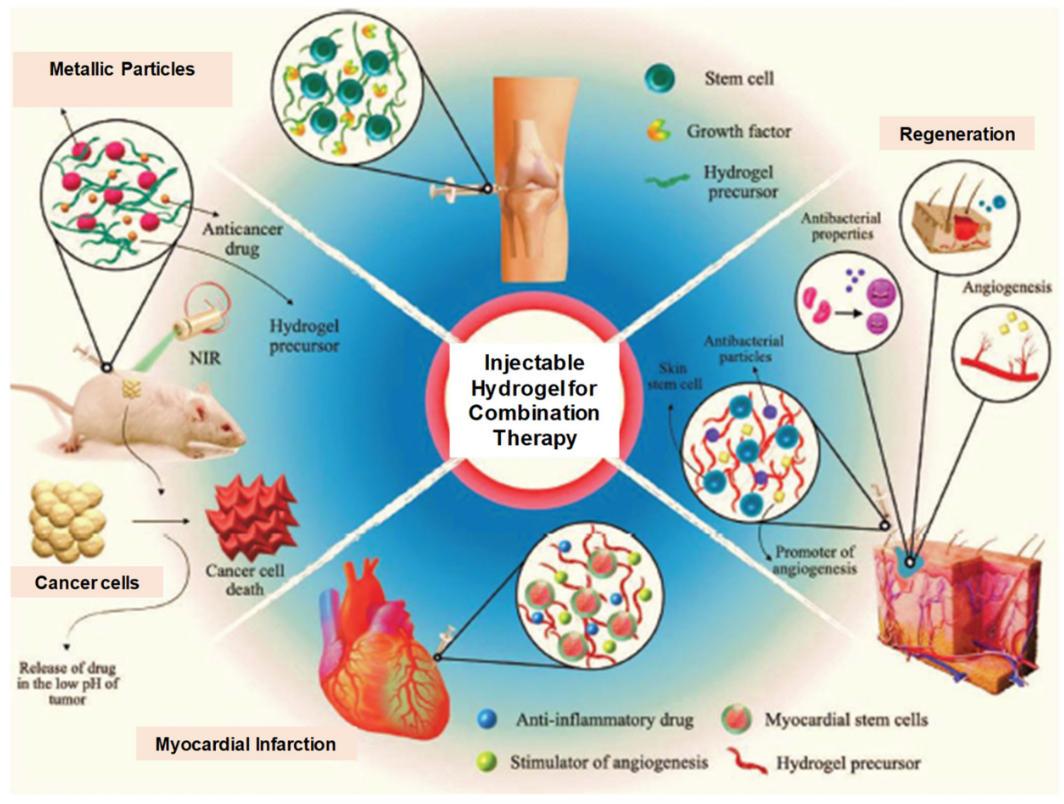
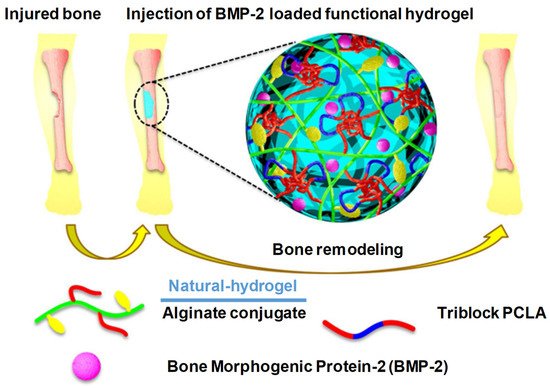
2.3.3. Bone Regeneration
3. Future Prospects
3.1. Limitations and Outcomes/Overcomes
| Chemical/Physical Crosslinking | Types of Hydrogel Material | Hydrogel Synthesis Procedure | Applications and Advantages | Limitations and Disadvantages | Reference |
|---|---|---|---|---|---|
| Hydrophobic interaction | Hydrophilic monomers and hydrophobic co-monomers | Free radical copolymerization of a hydrophilic monomer with a hydrophobic co-monomer | Absence of crosslinking agents and relative ease of production | Poor mechanical characteristics | [36] |
| Ionic interaction | Solution and multivalent ions of opposite charge | Polyelectrolyte ionic interaction through simple ion exchange mechanisms and complex formation | Crosslinking takes place at room temperature and physiological pH Properties can be fine-tuned by cationic and anionic constituents | Limited to ionic polymers and sensitive to impurities | [31] |
| Hydrogen bond | Polymeric functional groups of high electron density with electron-deficient hydrogen atom | Self-assembly through secondary molecular interactions | Increase in polymer concentration can increase the stability of gel | Influx of water can disperse/dissolve the gel within short duration | [32] |
| Bulk polymerization | Monomers and monomer-soluble initiators | The polymerization reaction is initiated with radiation, ultraviolet or chemical catalysts at low rate of conversions | A simple and versatile technique for preparing hydrogels with desired physical properties and forms | Increase in viscosity during high rate of polymerization reaction can generate heat Weak polymer structure | [37] |
| Solution polymerization | Ionic or neutral monomers with the multifunctional crosslinking agent | Reaction initiated thermally with UV irradiation or by redox initiator system | Control of temperature Performed in non-toxic aqueous medium at room temperature High polymerization rate |
To be washed to eliminate reactants, the polymers and other impurities | [38] |
| Suspension polymerization | Hydrophilic monomers, initiators, cross-linkers and suspending agent | The monomers and initiator are dispersed in the organic phase as a homogenous mixture | Directly usable as powders, beads or microspheres Restricted to water insoluble polymer |
Cooling jacket required to dissipate heat Requirement of agitators and dispersant |
[39] |
| Grafting | Viny polymers, initiators and crosslinking agents | Covalent bonding of monomers on free radicals generated on stronger support structures | Improve functional properties of the polymer | Difficulty of characterizing side chains | [40] |
| Irradiation | High energy gamma beams and electron beams as initiators | Irradiation of aqueous polymer solution results in the formation of radicals and macroradicals on the polymer chains | Pure, sterile, residue-free hydrogel Does not require catalyst and other additives Irradiation dose can control swelling capacity |
Irradiation can cause polymer degradation via chain scission and crosslinking events | [41] |
| Step growth polymerization | Bi- or multifunctional monomers and each with attest two sites for bonding | Multifunctional monomers react to form oligomers resulting in long chain polymers | No initiator is required to start the polymerization and termination reactions | Prolonged reaction times required to achieve a high degree of conversion and high molecular weights | [42] |
3.2. Injectable Formulation Challenges
3.2.1. Mechanical Robustness
3.2.2. Loading and Release of Therapeutic Agents
3.2.3. Hydrogel Bioactivity
3.2.4. Immunological Compatibility
3.2.5. Technological Challenges
3.2.6. Scale-Up Strategies and GMP Processes
3.2.7. Regulatory Approvals
References
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 27.
- Chao, Y.; Chen, Q.; Liu, Z. Smart Injectable Hydrogels for Cancer Immunotherapy. Adv. Funct. Mater. 2020, 30, 1902785.
- Wang, S.; Zheng, H.; Zhou, L.; Cheng, F.; Liu, Z.; Zhang, H.; Wang, L.; Zhang, Q. Nanoenzyme-Reinforced Injectable Hydrogel for Healing Diabetic Wounds Infected with Multi-Drug Resistant Bacteria. Nano Lett. 2020, 20, 5149–5158.
- Liu, S.; Guo, R.; Li, C.; Lu, C.; Yang, G.; Wang, F.; Nie, J.; Ma, C.; Gao, M. POSS hybrid hydrogels: A brief review of synthesis, properties and applications. Eur. Polym. J. 2021, 143, 110180.
- Chatterjee, S.; Hui, P.C.L. Review of applications and future prospects of stimuli-responsive hydrogel based on thermo-responsive biopolymers in drug delivery systems. Polymers 2021, 13, 2086.
- Tan, H.; Marra, K.G. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 2010, 3, 1746–1767.
- Li, Y.; Yang, H.Y.; Lee, D.S. Advances in biodegradable and injectable hydrogels for biomedical applications. J. Control. Release 2021, 330, 151–160.
- Nguyen, Q.V.; Huynh, D.P.; Park, J.H.; Lee, D.S. Injectable polymeric hydrogels for the delivery of therapeutic agents: A review. Eur. Polym. J. 2015, 72, 602–619.
- Alonso, J.M.; Del Olmo, J.A.; Gonzalez, R.P.; Saez-martinez, V. Injectable hydrogels: From laboratory to industrialization. Polymers 2021, 13, 650.
- Yao, Q.; Lan, Q.H.; Jiang, X.; Du, C.C.; Zhai, Y.Y.; Shen, X.; Xu, H.L.; Xiao, J.; Kou, L.; Zhao, Y.Z. Bioinspired biliverdin/silk fibroin hydrogel for antiglioma photothermal therapy and wound healing. Theranostics 2020, 10, 11719–11736.
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307.
- Kim, S.H.; Thambi, T.; Giang Phan, V.H.; Lee, D.S. Modularly engineered alginate bioconjugate hydrogel as biocompatible injectable scaffold for in situ biomineralization. Carbohydr. Polym. 2020, 233, 115832.
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent Advances of Injectable Hydrogels for Drug Delivery and. Polym. Test. 2020, 81, 106283.
- Suflet, D.M.; Popescu, I.; Pelin, I.M.; Ichim, D.L.; Daraba, O.M.; Constantin, M.; Fundueanu, G. Dual cross-linked chitosan/pva hydrogels containing silver nanoparticles with antimicrobial properties. Pharmaceutics 2021, 13, 1461.
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158.
- Eslahi, N.; Abdorahim, M.; Simchi, A. Smart Polymeric Hydrogels for Cartilage Tissue Engineering: A Review on the Chemistry and Biological Functions. Biomacromolecules 2016, 17, 3441–3463.
- De France, K.J.; Cranston, E.D.; Hoare, T. Mechanically Reinforced Injectable Hydrogels. ACS Appl. Polym. Mater. 2020, 2, 1016–1030.
- Zhang, X.; Tan, B.; Wu, Y.; Zhang, M.; Liao, J. A review on hydrogels with photothermal effect in wound healing and bone tissue engineering. Polymers 2021, 13, 2100.
- Ilochonwu, B.C.; Urtti, A.; Hennink, W.E.; Vermonden, T. Intravitreal hydrogels for sustained release of therapeutic proteins. J. Control. Release 2020, 326, 419–441.
- Fan, D.Y.; Tian, Y.; Liu, Z.J. Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 2019, 7, 675.
- Giang Phan, V.H.; Duong, H.T.T.; Thambi, T.; Nguyen, T.L.; Turabee, M.H.; Yin, Y.; Kim, S.H.; Kim, J.; Jeong, J.H.; Lee, D.S. Modularly engineered injectable hybrid hydrogels based on protein-polymer network as potent immunologic adjuvant in vivo. Biomaterials 2019, 195, 100–110.
- Nandi, R.; Yucknovsky, A.; Mazo, M.M.; Amdursky, N. Exploring the inner environment of protein hydrogels with fluorescence spectroscopy towards understanding their drug delivery capabilities. J. Mater. Chem. B 2020, 8, 6964–6974.
- Liu, C.; Zhang, Q.; Zhu, S.; Liu, H.; Chen, J. Preparation and applications of peptide-based injectable hydrogels. RSC Adv. 2019, 9, 28299–28311.
- Xiao, Y.; Gu, Y.; Qin, L.; Chen, L.; Chen, X.; Cui, W.; Li, F.; Xiang, N.; He, X. Colloids and Surfaces B: Biointerfaces Injectable thermosensitive hydrogel-based drug delivery system for local cancer therapy. Colloids Surf. B Biointerfaces 2021, 200, 111581.
- Afinjuomo, F.; Abdella, S.; Youssef, S.H. Inulin and Its Application in Drug Delivery. Pharmaceuticals 2021, 14, 855.
- Gil, M.S.; Cho, J.; Thambi, T.; Giang Phan, V.H.; Kwon, I.; Lee, D.S. Bioengineered robust hybrid hydrogels enrich the stability and efficacy of biological drugs. J. Control. Release 2017, 267, 119–132.
- Ma, H.; He, C.; Cheng, Y.; Li, D.; Gong, Y.; Liu, J.; Tian, H.; Chen, X. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials 2014, 35, 8723–8734.
- Mathew, A.P.; Uthaman, S.; Cho, K.H.; Cho, C.S.; Park, I.K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29.
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184.
- Overstreet, D.J.; Dutta, D.; Stabenfeldt, S.E.; Vernon, B.L. Injectable hydrogels. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 881–903.
- Won, J.E.; Wi, T.I.; Lee, C.M.; Lee, J.H.; Kang, T.H.; Lee, J.W.; Shin, B.C.; Lee, Y.J.; Park, Y.M.; Han, H.D. NIR irradiation-controlled drug release utilizing injectable hydrogels containing gold-labeled liposomes for the treatment of melanoma cancer. Acta Biomater. 2021, 136, 508–518.
- Wei, W.; Li, H.; Yin, C.; Tang, F. Research progress in the application of in situ hydrogel system in tumor treatment. Drug Deliv. 2020, 27, 460–468.
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control. Release 2020, 324, 204–217.
- Zhao, L.; Niu, L.; Liang, H.; Tan, H.; Liu, C.; Zhu, F. PH and Glucose Dual-Responsive Injectable Hydrogels with Insulin and Fibroblasts as Bioactive Dressings for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 37563–37574.
- Poustchi, F.; Amani, H.; Ahmadian, Z.; Niknezhad, S.V.; Mehrabi, S.; Santos, H.A.; Shahbazi, M.A. Combination Therapy of Killing Diseases by Injectable Hydrogels: From Concept to Medical Applications. Adv. Healthc. Mater. 2021, 10, e2001571.
- Kong, L.; Wu, Z.; Zhao, H.; Cui, H.; Shen, J.; Chang, J.; Li, H.; He, Y. Bioactive Injectable Hydrogels Containing Desferrioxamine and Bioglass for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 30103–30114.
- Okay, O. Self-healing hydrogels formed via hydrophobic interactions. In Supramolecular Polymer Networks and Gels; Springer: Cham, Switzerland, 2015; Volume 268, ISBN 9783319154046.
- Lin, J.; Zheng, S.Y.; Xiao, R.; Yin, J.; Wu, Z.L.; Zheng, Q.; Qian, J. Constitutive behaviors of tough physical hydrogels with dynamic metal-coordinated bonds. J. Mech. Phys. Solids 2020, 139, 103935.
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236.
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121.
- Qiu, X.; Hu, S. “Smart” materials based on cellulose: A review of the preparations, properties, and applications. Materials 2013, 6, 738–781.
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267.
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357.
- Guvendiren, M.; Lu, H.D.; Burdick, J.A. Shear-thinning hydrogels for biomedical applications. Soft Matter 2012, 8, 260–272.
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481.
- Liang, K.; Bae, K.H.; Kurisawa, M. Recent advances in the design of injectable hydrogels for stem cell-based therapy. J. Mater. Chem. B 2019, 7, 3775–3791.
- Branco, M.C.; Pochan, D.J.; Wagner, N.J.; Schneider, J.P. The effect of protein structure on their controlled release from an injectable peptide hydrogel. Biomaterials 2010, 31, 9527–9534.
- Taha, T.A. Optical properties of PVC/Al2O3 nanocomposite films. Polym. Bull. 2019, 76, 903–918.
- Said, H.M.; Alla, S.G.A.; El-Naggar, A.W.M. Synthesis and characterization of novel gels based on carboxymethyl cellulose/acrylic acid prepared by electron beam irradiation. React. Funct. Polym. 2004, 61, 397–404.
- Zhang, L.; Ren, X.; Zhang, Y.; Zhang, K. Step-Growth Polymerization Method for Ultrahigh Molecular Weight Polymers. ACS Macro Lett. 2019, 8, 948–954.
- Sun, Y.; Su, J.; Liu, G.; Chen, J.; Zhang, X.; Zhang, R.; Jiang, M.; Qiu, M. Advances of blood cell-based drug delivery systems. Eur. J. Pharm. Sci. 2017, 96, 115–128.
- Su, J.; Zhang, R.; Lian, Y.; Kamal, Z.; Cheng, Z.; Qiu, Y.; Qiu, M. Preparation and characterization of erythrocyte membrane-camouflaged berberine hydrochloride-loaded gelatin nanoparticles. Pharmaceutics 2019, 11, 93.
- Liu, W.; Ruan, M.; Wang, Y.; Song, R.; Ji, X.; Xu, J.; Dai, J.; Xue, W. Light-Triggered Biomimetic Nanoerythrocyte for Tumor-Targeted Lung Metastatic Combination Therapy of Malignant Melanoma. Small 2018, 14, e1801754.
- Malhotra, S.; Dumoga, S.; Sirohi, P.; Singh, N. Red Blood Cells-Derived Vesicles for Delivery of Lipophilic Drug Camptothecin. ACS Appl. Mater. Interfaces 2019, 11, 22141–22151.
- Kamal, Z.; Su, J.; Qiu, M. Erythrocytes modified (coated) gold nanoparticles for effective drug delivery. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Micro and Nanotechnologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–29. ISBN 978-0-12-816960-5.
- Li, L.L.; Xu, J.H.; Qi, G.B.; Zhao, X.; Yu, F.; Wang, H. Core-shell supramolecular gelatin nanoparticles for adaptive and “on-demand” antibiotic delivery. ACS Nano 2014, 8, 4975–4983.
- Pornpattananangkul, D.; Zhang, L.; Olson, S.; Aryal, S.; Obonyo, M.; Vecchio, K.; Huang, C.M.; Zhang, L. Bacterial toxin-triggered drug release from gold nanoparticle-stabilized liposomes for the treatment of bacterial infection. J. Am. Chem. Soc. 2011, 133, 4132–4139.
- Wang, F.; Gao, W.; Thamphiwatana, S.; Luk, B.T.; Angsantikul, P.; Zhang, Q.; Hu, C.M.J.; Fang, R.H.; Copp, J.A.; Pornpattananangkul, D.; et al. Hydrogel retaining toxin-absorbing nanosponges for local treatment of methicillin-resistant Staphylococcus aureus infection. Adv. Mater. 2015, 27, 3437–3443.
- Lin, A.; Liu, Y.; Zhu, X.; Chen, X.; Liu, J.; Zhou, Y.; Qin, X.; Liu, J. Bacteria-Responsive Biomimetic Selenium Nanosystem for Multidrug-Resistant Bacterial Infection Detection and Inhibition. ACS Nano 2019, 13, 13965–13984.
- Huang, P.; Wang, D.; Su, Y.; Huang, W.; Zhou, Y.; Cui, D.; Zhu, X.; Yan, D. Combination of small molecule prodrug and nanodrug delivery: Amphiphilic drug-drug conjugate for cancer therapy. J. Am. Chem. Soc. 2014, 136, 11748–11756.
- Chen, S. Biomimetic Nanoscale Systems for pH-Triggered Intracellular Drug Delivery. Ph.D. Thesis, Imperial College London, London, UK, 2017.
- Chu, M.; Zhang, M.B.; Liu, Y.C.; Kang, J.R.; Chu, Z.Y.; Yin, K.L.; Ding, L.Y.; Ding, R.; Xiao, R.X.; Yin, Y.N.; et al. Role of berberine in the treatment of methicillin-resistant staphylococcus aureus infections. Sci. Rep. 2016, 6, 24748.
- Gao, M.; Liang, C.; Song, X.; Chen, Q.; Jin, Q.; Wang, C.; Liu, Z. Erythrocyte-membrane-enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor hypoxia and enhance cancer radiotherapy. Adv. Mater. 2017, 29, 1701429.
- Zhang, Y.; Zhang, J.; Chen, W.; Angsantikul, P.; Spiekermann, K.A.; Fang, R.H.; Gao, W.; Zhang, L. Erythrocyte membrane-coated nanogel for combinatorial antivirulence and responsive antimicrobial delivery against Staphylococcus aureus infection. J. Control. Release 2017, 263, 185–191.





