Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Karine Elena Rodriguez Fernandez | -- | 2412 | 2022-04-07 10:42:48 | | | |
| 2 | Amina Yu | -152 word(s) | 2260 | 2022-04-08 04:13:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rodriguez Fernandez, K.E.; Mangas-Sanjuan, V.; Merino-Sanjuán, M.; , . Monoclonal Antibodies in the Management of Psoriasis. Encyclopedia. Available online: https://encyclopedia.pub/entry/21449 (accessed on 07 March 2026).
Rodriguez Fernandez KE, Mangas-Sanjuan V, Merino-Sanjuán M, . Monoclonal Antibodies in the Management of Psoriasis. Encyclopedia. Available at: https://encyclopedia.pub/entry/21449. Accessed March 07, 2026.
Rodriguez Fernandez, Karine Elena, Victor Mangas-Sanjuan, Matilde Merino-Sanjuán, . "Monoclonal Antibodies in the Management of Psoriasis" Encyclopedia, https://encyclopedia.pub/entry/21449 (accessed March 07, 2026).
Rodriguez Fernandez, K.E., Mangas-Sanjuan, V., Merino-Sanjuán, M., & , . (2022, April 07). Monoclonal Antibodies in the Management of Psoriasis. In Encyclopedia. https://encyclopedia.pub/entry/21449
Rodriguez Fernandez, Karine Elena, et al. "Monoclonal Antibodies in the Management of Psoriasis." Encyclopedia. Web. 07 April, 2022.
Copy Citation
Psoriasis is a chronic autoimmune and inflammatory skin disease associated with physical and psychological burdens characterized by erythematic plaques with adherent shiny scales.
psoriasis
monoclonal antibodies
therapeutic drug monitoring
1. Introduction
Psoriasis is a chronic autoimmune and inflammatory skin disease associated with physical and psychological burdens characterized by erythematic plaques with adherent shiny scales [1]. The country-specific prevalence of psoriasis varies from 0.14% (95% uncertainty interval 0.05% to 0.40%) in east Asia to 1.99% (0.64% to 6.60%) in Australasia. Additionally, the prevalence is high in western Europe (1.92%, 1.07% to 3.46%), central Europe (1.83%, 0.62% to 5.32%), and North America (1.50%, 0.63% to 3.60%). Its age of onset shows a bimodal distribution, with peaks at 30–39 years and 60–69 years in men, and 10 years earlier in women [2]. The phenotypes of this disease are plaque psoriasis or psoriasis vulgaris, guttate psoriasis, inverse psoriasis, and erythrodermic psoriasis, which differ in terms of their clinical and morphological characteristics [3][4][5]. In addition, nail psoriasis is reported to affect more than half of the patients [6].
1.1. Pathophysiology of Psoriasis
A complex and not fully understood pathogenesis is exhibited in psoriasis. External factors can trigger an interaction between skin cells, pro-inflammatory immunocytes (i.e., tumor necrosis factor (TNF)-α and interferon (IFN)-α), and biologic signaling molecules in genetically predisposed individuals [7][8]. This interaction stimulates the myeloid dendritic cells (mDC) in the lymph nodes to release interleukin (IL)-12 and IL-23 to promote the cellular immune response of T helper lymphocytes (Th) type 1 (Th1), 17 (Th17), and 22 (Th22) T cells. Activated Th migrate to the skin guided by a gradient of chemokine and produce abundant psoriatic cytokines (for example, IL-17, IFN-γ, TNF-α, and IL-22). The cytokine-mediated effects on keratinocytes influence typical psoriatic inflammation [9][10][11][12][13]. Molecular and genetic ones in specific psoriasis phenotypes have identified different inflammatory pathways that may coexist and evolve over time. The identification of the main inflammatory pathways through individual molecular descriptors represents a future step to guide personalized therapy [14]. In this sense, different classes of possible biomarkers have been explored in psoriasis, but further replication and validation are required [15][16][17].
1.2. Clinical Endpoints of Psoriasis
The severity of psoriasis will be determined by the extent of the disease, the location of the lesions, the degree of inflammation, and the impact on quality of life. According to the most important clinical guidelines , the evaluation of psoriasis severity and the levels of its treatment responses is generally based on the percentage of the total Body Surface Area (BSA) affected, Psoriasis Area Severity Index (PASI), Physician Global Assessment (PGA), and Dermatologic Life Quality Index (DLQI) [18][19].
2. Pharmacokinetic/Pharmacodynamic Properties of Monoclonal Antibodies in Psoriasis
Despite the increasing number of therapeutic monoclonal antibodies (mAb) on the market and in the drug development process for psoriasis treatment, the pharmacokinetic (PK) and pharmacodynamic (PD) properties of these molecules are more specific. In this regard, non-linear mixed-effects modeling allows for the accurate quantification of the central tendency and the different sources of the variability of mAb by considering data from all individuals simultaneously.
2.1. Pharmacokinetic Properties
Monoclonal antibodies are heterodimeric glycoprotein macromolecules of type-G immunoglobulin recognizing a single epitope on a target antigen in a bivalent manner [20]. They are produced and engineered by hybridoma technology, developed for the first time by Köhler and Milstein in 1975 [21]. Due to their molecular size and their three-dimensional conformation, the PK and PD properties of mAbs are considerably different compared with those related to small-molecule drugs (SDM) [22].
The low permeability and high degradation of mAbs throughout the gastrointestinal tract lead to intravenous, subcutaneous, or intramuscular administration [23], and no significant improvement has been published to overcome the limitations of the oral administration of mAbs. Consequently, the most frequent routes of the administration of mAb in psoriasis follow intravenous (IV) or subcutaneous (SC) injections [24]. Regarding the distribution and tissue infiltration, mAbs can easily move from the SC space most probably via diffusion and/or convection through lymphatic capillaries, and they can be able to reach the intracellular space of targets beyond systemic circulation by pinocytosis or by receptor-mediated endocytosis [25].
The large size and physicochemical properties (charge and hydrophobicity) explain the distribution of mAbs mainly in the vascular and interstitial fluids. Usually, tissue distribution represents 5 to 15% of the total amount of mAb, and distribution into the brain is quite restricted (0.1%) [26]. A significant fraction of mAb in the body may be found if mAb-tissue target binding occurs with high affinity. Therefore, large apparent volumes of distribution in steady state (Vss) could be estimated for mAbs. In cases where the binding capacity of tissue is limited, nonlinear distribution is more probable and Vss decreases in a dose or concentration-dependent manner [27].
In regard to mAbs metabolism and excretion, the impacts of the renal and biliary pathways are insignificant [28]. Due to the null role of enzymatic processes related to the metabolism and excretion of mAb, the interaction with other substrates of these enzymes is negligible [29][30][31][32]. mAbs exhibit specific and non-specific types of binding, depending on the fragment of the antibody. The first one occurs when the antigen-binding fragment (Fab) attaches to the target antigen. The second one appears after the fragment crystallizable (Fc) region binds to cell surface receptors, such as the Fcγ receptor (FcγR) on the immune effector cells and the neonatal Fc receptor (FcRn) on different cell types, as well as components of the complement system (for example, complement C1q) [20]. For such reasons, mAb distribution can be directly influenced by the density and expression of the target antigen. The two parallel metabolic pathways, for example, specific and non-specific. are involved in mAb disposition, and their impact changes over time based on the available free mAb in the plasma and the dose administered. Metabolism through the reticuloendothelial system via pinocytosis/proteolysis represents the linear and non-specific clearance, which may be relevant at certain dose levels due to the larger endothelial surface area in the gut, muscle, and skin [33]. The specific pathway is initiated after the internalization of the receptor–drug complex, which allows the drug to enter the cell and then be inactivated by cytoplasmic endosomes. However, FcRn can bind IgG and mAbs at the acidic pH conditions of the lysosome, escape from proteolysis, and be directed back to the cell membrane [34][35][36]. Both pathways have been mechanistically described in population PK models through a target-mediated drug disposition (TMDD) approach and its quasi-equilibrium or rapid binding approximations, quasi-steady-state approximation, and even simpler Michaelis–Menten kinetics.
One more key aspect in the PK of mAb is the rescue from lysosomal degradation by binding to FcRn in endothelial cells, which is crucial for the long half-life and low clearance rate reported for most therapeutic mAbs [37][38]. These mechanisms result ones to partially explain inter-individual differences in mAb exposure, such as FcRn and FcγR gene expression, genetic polymorphism, target properties, and covariates associated with increased clearance, such as the generation of antidrug antibodies (ADA), low serum albumin and high serum C-reactive protein levels (CRP), gender, and high body weight (BW) [22][25].
2.2. Pharmacodynamic Properties
The mAbs for treating psoriasis are designed to block either the specific receptors or soluble mediators of the main pathways in the progress and chronicity of psoriasis, including TNF-α, IL-12/23, and IL-17 [11] (Figure 1). The PD effect of mAb is delayed to the time course of its plasma concentrations, which has been described using PK/PD models, such as indirect responses and transduction models, in order to describe the exposure–response (E–R) relationship [39][40]. The following parameters are mostly determined: kin, formation rate of psoriatic skin lesions; kout, remission rate of psoriatic skin lesions; Emax, maximum mAb effect; and EC50 or IC50, serum mAb concentration causing 50% of the maximum effect.
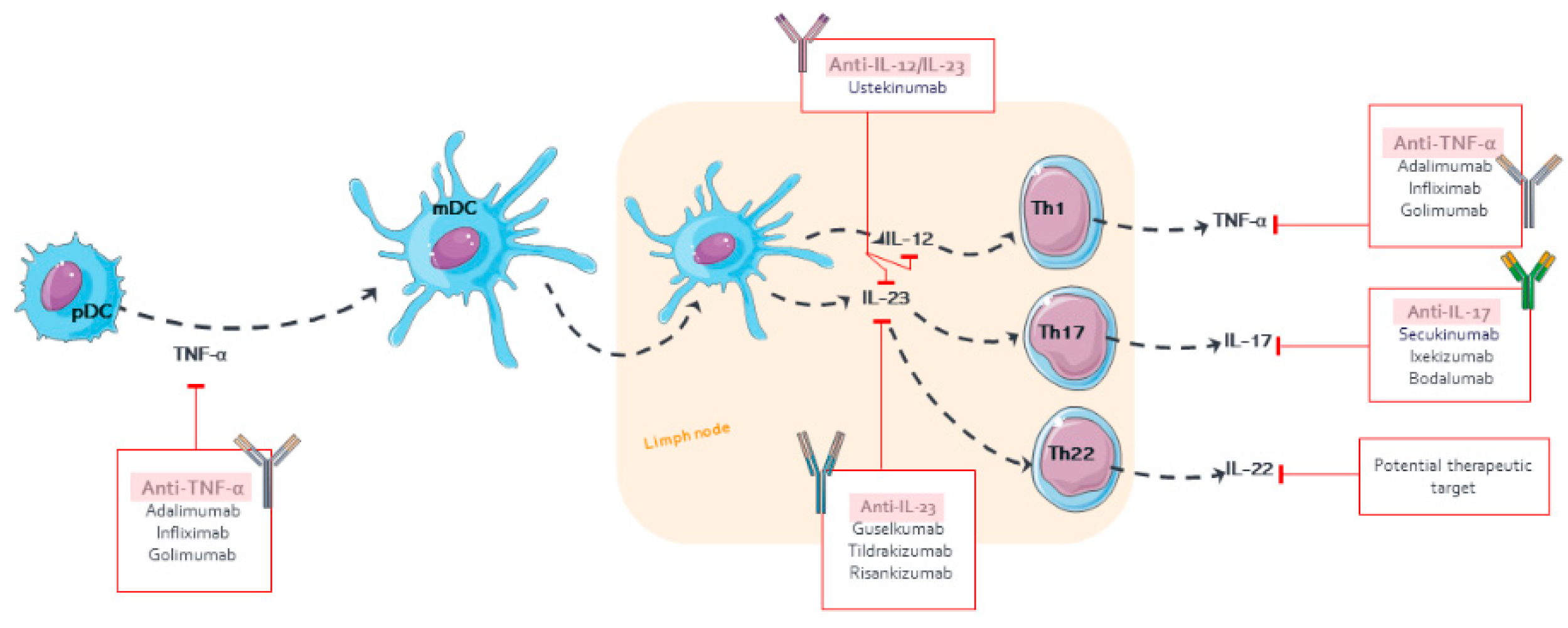
Figure 1. Pharmacological targets of monoclonal antibodies in psoriasis.
Compared with SDM, mAbs offer therapeutic exclusivity, a higher safety profile, and an increase in clinical efficacy [41][42]. The relative change in the PASI of patients receiving novel mAbs (guselkumab and brodalumab) has reached 90–100% PASI reduction, which has led to an adjustment of the primary endpoint of PASI75 to PASI90 or PASI100 in clinical trials [43].
3. Monoclonal Antibody Approved for Psoriasis
The selection of the optimal treatment for psoriasis depends on the severity of the disease [44]. Mild or limited-extent psoriasis is managed by topical treatment, while the moderate to severe types usually require a combination of phototherapy and systemic therapies [45]. Biological agents, such as mAb, have been the most successful approach in the management of this disease in the last decade.
The use of mAbs is indicated in psoriasis when (i) effective control of psoriasis is not achieved with oral and phototherapy treatments, (ii) in patients who have rapid regrowth (3 months or less) after suspending any treatment, (iii) when higher doses of conventional systemic drugs are required with the increased associated risk of adverse effects, (iv) in patients with comorbidities for which the use of systemic agents, such as methotrexate or cyclosporine, are contraindicated, (v) when a patient is unable to tolerate the traditional systemic therapy, or (vi) the patient is at high risk of toxicity with methotrexate, cyclosporine, acitretin, or phototherapy, even in the absence of analytical alterations [46].
4. Therapeutic Drug Monitoring of Monoclonal Antibodies in Psoriasis
Treatments for an immune-mediated inflammatory disease, such as psoriasis, have been enhanced with the development of biologics. However, some patients are not able to achieve an adequate clinical response to mAb-based therapy. Some patients present an insufficient response in the induction phase of the treatment, which is called primary non-response, or after initial clinical benefit, they lose the ability to respond, which is called secondary non-response [47][48]. The IIV of the clinical response to standard biologic doses in patients with psoriasis may be explained by differences in the amount of drug available at the target tissue, which in turn is induced by adherence, physiological and genetic mechanisms, and PK covariates, such as BW and drug immunogenicity [49][50]. Increasing evidence indicates that a way to explain all these concerns about mAb could be TDM.
The term TDM was defined in 1997 by Watson et al. as the measurement of a prescribed xenobiotic in serum or biological fluids at a single or multiple time points, with a view to influencing prescription and individualizing the dosage regimen to achieve maximal clinical efficacy and minimize adverse effects [51]. Distinction should be made between reactive and proactive TDM. Reactive TDM is performed in patients failing treatment in order to guide decision-making, whereas proactive TDM is performed in responding patients to optimize therapy and potentially prevent future flare-ups and loss–of–response [52]. The implementation of TDM is essential to define the optimal dose ranges for each patient for a given biologic in psoriasis. The TDM for biological agents in immune-mediated inflammatory diseases involves the measurement of drug levels and ADA. Dose increase, interval shortening, and/or the addition of an immunomodulator are proposed, with subsequent re-evaluation of the drug concentration until the therapeutic goals are achieved [36].
In the last decade, the data in favor of TDM in psoriasis are growing. Based on the distribution of a survey among dermatologists who participated in Belgian Dermatology Days 2019 and Skin Inflammation & Psoriasis International Network Congress 2019, Schots et al. [53] indicated that 70% of the total study cohort admitted the need for TDM, implying the necessity in the daily dermatology routine for active interaction about the accessibility, utility, and application of TDM assays. However, over the years, there has been much confusion about what exposure metrics are informative in patients with psoriasis. Most have been measured drug levels, but very little information has been used to evaluate the relationship between the mAb levels and clinical response to treatment [54]. Therefore, the selection of TDM in mAb for psoriasis may be beneficial due to the large IIV observed in clinical trials, its chronic administration that leads to the appearance of time-dependent changes in PK or PD parameters, and the role of disease progression in the increase of clearance and decrease in the response over time.
The attempts to establish therapeutic ranges and the incidence of ADA of some mAbs employed for the treatment of psoriasis are shown. Takahashi et al. [55] identified the infliximab Ctrough for responder patients at 0.92 μg/mL. Recently, the NORwegian DRUg Monitoring study was published [56] to assess the efficacy of TDM in patients on infliximab treatment regarding the achievement of remission, as well as to maintain immune-mediated inflammatory disease control. Additionally, among patients with immune-mediated inflammatory diseases undergoing maintenance therapy with infliximab, proactive TDM was more effective than treatment without TDM in sustaining disease control without disease worsening [57]. For adalimumab, Menting et al. [58] defined a window based on Ctrough from 3.51 to 7.00 μg/mL corresponding to the optimal clinical response. This window was confirmed by the Psoriasis Stratification to Optimize Relevant Therapy (PSORT) consortium in a large multicenter prospective study [54]. Other studies have shown how early measurement of the adalimumab Ctrough levels could help to predict the possibilities of responses [54][59][60].
A pilot study estimated a negative correlation between PASI and the trough secukinumab concentrations during maintenance therapy, suggesting no clinically relevant relationship between Ctrough and PASI. On the other hand, a minimal effective Ctrough of 33.2 μm/mL for achieving PASI ≤ 2 was proposed based on receiver operating characteristic curve analysis [61]. Menting et al. [62] reported low and variable trough concentration levels of ustekinumab, which were not correlated with clinical response. However, the studies by Toro-Montecinos et al. [63] and Van Den Berghe et al. [64] found an inverse correlation between the absolute PASI score and ustekinumab serum concentrations measured at week six and week four, respectively. These contradictory results have not made it possible to reach a consensus for the ustekinumab concentration–response relationship. Nevertheless, it has been demonstrated how early serum ustekinumab levels post-injection monitoring contribute to timely identifying under-exposed patients who might benefit from treatment optimization [65][64][66]. E–R association data in psoriasis is limited for certolizumab pegol, brodalumab, ixekizumab [67][68][69], guselkumab [70], tildrakizumab [71], and risankizumab [72].
References
- Di Meglio, P.; Villanova, F.; Nestle, F.O. Psoriasis. Cold Spring Harb. Perspect. Med. 2014, 4, a015354.
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ 2020, 369, m1590.
- Menter, A.; Gottlieb, A.; Feldman, S.R.; Van Voorhees, A.S.; Leonardi, C.L.; Gordon, K.B.; Lebwohl, M.; Koo, J.Y.; Elmets, C.A.; Korman, N.J. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 2008, 58, 826–850.
- Schadler, E.D.; Ortel, B.; Mehlis, S.L. Biologics for the primary care physician: Review and treatment of psoriasis. Dis. Mon. DM 2018, 65, 51–90.
- Todke, P.; Shah, V.H. Psoriasis: Implication to disease and therapeutic strategies, with an emphasis on drug delivery approaches. Int. J. Dermatol. 2018, 57, 1387–1402.
- Salomon, J.; Szepietowski, J.C.; Proniewicz, A. Psoriatic nails: A prospective clinical study. J. Cutan. Med. Surg. 2003, 7, 317–321.
- Mahil, S.K.; Capon, F.; Barker, J.N. Genetics of psoriasis. Dermatol. Clin. 2015, 33, 1–11.
- Zeng, J.; Luo, S.; Huang, Y.; Lu, Q. Critical role of environmental factors in the pathogenesis of psoriasis. J. Dermatol. 2017, 44, 863–872.
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509.
- Perera, G.K.; Di Meglio, P.; Nestle, F.O. Psoriasis. Annu. Rev. Pathol. 2012, 7, 385–422.
- Kim, J.; Krueger, J.G. The immunopathogenesis of psoriasis. Dermatol. Clin. 2015, 33, 13–23.
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082.
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960.
- Conrad, C.; Gilliet, M. Psoriasis: From Pathogenesis to Targeted Therapies. Clin. Rev. Allergy Immunol. 2018, 54, 102–113.
- Rashmi, R.; Rao, K.S.; Basavaraj, K.H. A comprehensive review of biomarkers in psoriasis. Clin. Exp. Dermatol. 2009, 34, 658–663.
- Villanova, F.; Di Meglio, P.; Nestle, F.O. Biomarkers in psoriasis and psoriatic arthritis. Ann. Rheum. Dis. 2013, 72 (Suppl. S2), ii104–ii110.
- Aydin, B.; Arga, K.Y.; Karadag, A.S. Omics-Driven Biomarkers of Psoriasis: Recent Insights, Current Challenges, and Future Prospects. Clin. Cosmet. Investig. Dermatol. 2020, 13, 611–625.
- Spuls, P.I.; Lecluse, L.L.; Poulsen, M.L.; Bos, J.D.; Stern, R.S.; Nijsten, T. How good are clinical severity and outcome measures for psoriasis?: Quantitative evaluation in a systematic review. J. Investig. Dermatol. 2010, 130, 933–943.
- Bożek, A.; Reich, A. The reliability of three psoriasis assessment tools: Psoriasis area and severity index, body surface area and physician global assessment. Adv. Clin. Exp. Med. 2017, 26, 851–856.
- Ryman, J.T.; Meibohm, B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 576–588.
- KÖHler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497.
- Mould, D.R.; Green, B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies. BioDrugs 2010, 24, 23–39.
- Regazzi, M.; Joseè, G.; Molinaro, M. Monoclonal Antibody Monitoring: Clinically Relevant Aspects, A Systematic Critical Review. Ther. Drug Monit. 2019, 42, 45–56.
- Zhao, L.; Shang, E.Y.; Sahajwalla, C.G. Application of pharmacokinetics–pharmacodynamics/clinical response modeling and simulation for biologics drug development. J. Pharm. Sci. 2012, 101, 4367–4382.
- Thomas, V.A.; Balthasar, J.P. Understanding Inter-Individual Variability in Monoclonal Antibody Disposition. Antibodies 2019, 8, 56.
- Posner, J.; Barrington, P.; Brier, T.; Datta-Mannan, A. Monoclonal Antibodies: Past, Present and Future. In Concepts and Principles of Pharmacology: 100 Years of the Handbook of Experimental Pharmacology; Barrett, J.E., Page, C.P., Michel, M.C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 81–141.
- Lobo, E.D.; Hansen, R.J.; Balthasar, J.P. Antibody pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 2004, 93, 2645–2668.
- Wang, W.; Wang, E.Q.; Balthasar, J.P. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin. Pharmacol. Ther. 2008, 84, 548–558.
- Zinner, R.G.; Glisson, B.S.; Fossella, F.V.; Pisters, K.M.; Kies, M.S.; Lee, P.M.; Massarelli, E.; Sabloff, B.; Fritsche, H.A., Jr.; Ro, J.Y.; et al. Trastuzumab in combination with cisplatin and gemcitabine in patients with Her2-overexpressing, untreated, advanced non-small cell lung cancer: Report of a phase II trial and findings regarding optimal identification of patients with Her2-overexpressing disease. Lung Cancer 2004, 44, 99–110.
- Gaudreault, J.; Shiu, V.; Bricarello, A.; Christian, B.J.; Zuch, C.L.; Mounho, B. Concomitant administration of bevacizumab, irinotecan, 5-fluorouracil, and leucovorin: Nonclinical safety and pharmacokinetics. Int. J. Toxicol. 2005, 24, 357–363.
- Ettlinger, D.E.; Mitterhauser, M.; Wadsak, W.; Ostermann, E.; Farkouh, A.; Schueller, J.; Czejka, M. In vivo disposition of irinotecan (CPT-11) and its metabolites in combination with the monoclonal antibody cetuximab. Anticancer Res. 2006, 26, 1337–1341.
- Xu, L.; Zuch, C.L.; Lin, Y.S.; Modi, N.B.; Lum, B.L. Pharmacokinetics and safety of bevacizumab administered in combination with cisplatin and paclitaxel in cynomolgus monkeys. Cancer Chemother. Pharmacol. 2008, 61, 607–614.
- Wright, A.; Sato, Y.; Okada, T.; Chang, K.; Endo, T.; Morrison, S. In vivo trafficking and catabolism of IgG1 antibodies with Fc associated carbohydrates of differing structure. Glycobiology 2000, 10, 1347–1355.
- Israel, E.J.; Wilsker, D.F.; Hayes, K.C.; Schoenfeld, D.; Simister, N.E. Increased clearance of IgG in mice that lack beta 2-microglobulin: Possible protective role of FcRn. Immunology 1996, 89, 573–578.
- Junghans, R.P.; Anderson, C.L. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 5512–5516.
- Liu, L. Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell 2018, 9, 15–32.
- Dirks, N.L.; Meibohm, B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharm. 2010, 49, 633–659.
- Lucas, A.T.; Robinson, R.; Schorzman, A.N.; Piscitelli, J.A.; Razo, J.F.; Zamboni, W.C. Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients. Antibodies 2019, 8, 3.
- Friberg, L.E.; Henningsson, A.; Maas, H.; Nguyen, L.; Karlsson, M.O. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 4713–4721.
- Dayneka, N.L.; Garg, V.; Jusko, W.J. Comparison of four basic models of indirect pharmacodynamic responses. J. Pharmacokinet. Biopharm. 1993, 21, 457–478.
- Molinelli, E.; Campanati, A.; Ganzetti, G.; Offidani, A. Biologic Therapy in Immune Mediated Inflammatory Disease: Basic Science and Clinical Concepts. Curr. Drug Saf. 2016, 11, 35–43.
- Lebwohl, M. Psoriasis. Ann. Intern. Med. 2018, 168, Itc49–Itc64.
- Kaushik, S.B.; Lebwohl, M.G. Review of safety and efficacy of approved systemic psoriasis therapies. Int. J. Dermatol. 2019, 58, 649–658.
- Gisondi, P.; Del Giglio, M.; Girolomoni, G. Treatment Approaches to Moderate to Severe Psoriasis. Int. J. Mol. Sci. 2017, 18, 2427.
- Boehncke, W.-H. Etiology and pathogenesis of psoriasis. Rheum. Dis. Clin. 2015, 41, 665–675.
- Daudén, E.; Puig, L.; Ferrándiz, C.; Sánchez-Carazo, J.L.; Hernanz-Hermosa, J.M. Consensus document on the evaluation and treatment of moderate-to-severe psoriasis: Psoriasis Group of the Spanish Academy of Dermatology and Venereology. J. Eur. Acad. Dermatol. Venereol. JEADV 2016, 30 (Suppl. S2), 1–18.
- Papamichael, K.; Vogelzang, E.H.; Lambert, J.; Wolbink, G.; Cheifetz, A.S. Therapeutic drug monitoring with biologic agents in immune mediated inflammatory diseases. Expert Rev. Clin. Immunol. 2019, 15, 837–848.
- Liau, M.M.; Oon, H.H. Therapeutic drug monitoring of biologics in psoriasis. Biol. Targets Ther. 2019, 13, 127–132.
- Karczewski, J.; Poniedziałek, B.; Rzymski, P.; Adamski, Z. Factors affecting response to biologic treatment in psoriasis. Dermatol. Ther. 2014, 27, 323–330.
- Imamura, C.K. Therapeutic drug monitoring of monoclonal antibodies: Applicability based on their pharmacokinetic properties. Drug Metab. Pharm. 2019, 34, 14–18.
- Watson, I.; Potter, J.; Yatscoff, R.; Fraser, A.; Himberg, J.J.; Wenk, M. Editorial. Ther. Drug Monit. 1997, 19, 125.
- Vermeire, S.; Dreesen, E.; Papamichael, K.; Dubinsky, M.C. How, When, and for Whom Should We Perform Therapeutic Drug Monitoring? Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 1291–1299.
- Schots, L.; Grine, L.; Soenen, R.; Lambert, J. Dermatologists on the medical need for therapeutic drug monitoring of biologics in psoriasis: Results of a structured survey. J. Dermatol. Treat. 2020, 15, 1–9.
- Wilkinson, N.; Tsakok, T.; Dand, N.; Bloem, K.; Duckworth, M.; Baudry, D.; Pushpa-Rajah, A.; Griffiths, C.E.M.; Reynolds, N.J.; Barker, J.; et al. Defining the Therapeutic Range for Adalimumab and Predicting Response in Psoriasis: A Multicenter Prospective Observational Cohort Study. J. Investig. Dermatol. 2019, 139, 115–123.
- Takahashi, H.; Tsuji, H.; Ishida-Yamamoto, A.; Iizuka, H. Plasma trough levels of adalimumab and infliximab in terms of clinical efficacy during the treatment of psoriasis. J. Dermatol. 2013, 40, 39–42.
- Syversen, S.W.; Goll, G.L.; Jørgensen, K.K.; Olsen, I.C.; Sandanger, Ø.; Gehin, J.E.; Warren, D.J.; Sexton, J.; Mørk, C.; Jahnsen, J.; et al. Therapeutic drug monitoring of infliximab compared to standard clinical treatment with infliximab: Study protocol for a randomised, controlled, open, parallel-group, phase IV study (the NOR-DRUM study). Trials 2020, 21, 13.
- Syversen, S.W.; Jørgensen, K.K.; Goll, G.L.; Brun, M.K.; Sandanger, Ø.; Bjørlykke, K.H.; Sexton, J.; Olsen, I.C.; Gehin, J.E.; Warren, D.J.; et al. Effect of Therapeutic Drug Monitoring vs. Standard Therapy During Maintenance Infliximab Therapy on Disease Control in Patients With Immune-Mediated Inflammatory Diseases: A Randomized Clinical Trial. JAMA 2021, 326, 2375–2384.
- Menting, S.P.; Coussens, E.; Pouw, M.F.; van den Reek, J.M.; Temmerman, L.; Boonen, H.; de Jong, E.M.; Spuls, P.I.; Lambert, J. Developing a Therapeutic Range of Adalimumab Serum Concentrations in Management of Psoriasis: A Step Toward Personalized Treatment. JAMA Dermatol. 2015, 151, 616–622.
- Mahil, S.K.; Arkir, Z.; Richards, G.; Lewis, C.M.; Barker, J.N.; Smith, C.H. Predicting treatment response in psoriasis using serum levels of adalimumab and etanercept: A single-centre, cohort study. Br. J. Dermatol. 2013, 169, 306–313.
- Carrascosa, J.M.; Toro Montecinos, M.; Ballescá, F.; Teniente Serra, A.; Martínez Cáceres, E.; Ferrándiz, C. Correlation between trough serum levels of adalimumab and absolute PASI score in a series of patients with psoriasis. J. Dermatol. Treat. 2018, 29, 140–144.
- Soenen, R.; Meulewaeter, E.; Grine, L.; Van den Berghe, N.; Brouwers, E.; Speeckaert, R.; Lanssens, S.; Temmerman, L.; Lambert, J.; Gils, A. Defining a Minimal Effective Serum Trough Concentration of Secukinumab in Psoriasis: A Step toward Personalized Therapy. J. Investig. Dermatol. 2019, 139, 2232–2235.e2231.
- Menting, S.P.; van den Reek, J.M.; Baerveldt, E.M.; de Jong, E.M.; Prens, E.P.; Lecluse, L.L.; Wolbink, G.J.; Van der Kleij, D.; Spuls, P.I.; Rispens, T. The correlation of clinical efficacy, serum trough levels and antidrug antibodies in ustekinumab-treated patients with psoriasis in a clinical-practice setting. Br. J. Dermatol. 2015, 173, 855–857.
- Toro-Montecinos, M.; Ballescá, F.; Ferrandiz, C.; Teniente-Serra, A.; Martinez-Caceres, E.; Carrascosa, J.M. Usefulness and correlation with clinical response of serum ustekinumab levels measured at 6 weeks versus 12 weeks. J. Dermatol. Treat. 2019, 30, 35–39.
- Van den Berghe, N.; De Keyser, E.; Soenen, R.; Meuleman, L.; Lanssens, S.; Gils, A.; Lambert, J. Clinical response correlates with 4-week postinjection ustekinumab concentrations in patients with moderate-to-severe psoriasis. Br. J. Dermatol. 2019, 182, 390–397.
- Pan, S.; Tsakok, T.; Dand, N.; Lonsdale, D.O.; Loeff, F.C.; Bloem, K.; de Vries, A.; Baudry, D.; Duckworth, M.; Mahil, S.; et al. Using Real-World Data to Guide Ustekinumab Dosing Strategies for Psoriasis: A Prospective Pharmacokinetic-Pharmacodynamic Study. Clin. Transl. Sci. 2020, 13, 400–409.
- Tsakok, T.; Wilson, N.; Dand, N.; Loeff, F.C.; Bloem, K.; Baudry, D.; Duckworth, M.; Pan, S.; Pushpa-Rajah, A.; Standing, J.F.; et al. Association of Serum Ustekinumab Levels With Clinical Response in Psoriasis. JAMA Dermatol. 2019, 155, 1235–1243.
- Tham, L.-S.; Tang, C.-C.; Choi, S.-L.; Satterwhite, J.H.; Cameron, G.S.; Banerjee, S. Population exposure-response model to support dosing evaluation of ixekizumab in patients with chronic plaque psoriasis. J. Clin. Pharm. 2014, 54, 1117–1124.
- Chigutsa, E.; Velez de Mendizabal, N.; Chua, L.; Heathman, M.; Friedrich, S.; Jackson, K.; Reich, K. Exposure-Response Modeling to Characterize the Relationship Between Ixekizumab Serum Drug Concentrations and Efficacy Responses at Week 12 in Patients with Moderate to Severe Plaque Psoriasis. J. Clin. Pharm. 2018, 58, 1489–1500.
- Reich, K.; Jackson, K.; Ball, S.; Garces, S.; Kerr, L.; Chua, L.; Muram, T.M.; Blauvelt, A. Ixekizumab Pharmacokinetics, Anti-Drug Antibodies, and Efficacy through 60 Weeks of Treatment of Moderate to Severe Plaque Psoriasis. J. Investig. Dermatol. 2018, 138, 2168–2173.
- Hu, C.; Yao, Z.; Chen, Y.; Randazzo, B.; Zhang, L.; Xu, Z.; Sharma, A.; Zhou, H. A comprehensive evaluation of exposure-response relationships in clinical trials: Application to support guselkumab dose selection for patients with psoriasis. J. Pharmacokinet. Pharmacodyn. 2018, 45, 523–535.
- Papp, K.; Thaçi, D.; Reich, K.; Riedl, E.; Langley, R.G.; Krueger, J.G.; Gottlieb, A.B.; Nakagawa, H.; Bowman, E.P.; Mehta, A.; et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br. J. Dermatol. 2015, 173, 930–939.
- Pang, Y.; Khatri, A.; Suleiman, A.A.; Othman, A.A. Clinical Pharmacokinetics and Pharmacodynamics of Risankizumab in Psoriasis Patients. Clin. Pharm. 2019, 59, 311–326.
More
Information
Subjects:
Pharmacology & Pharmacy; Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
08 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





