Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhuqing Ouyang | -- | 1877 | 2022-04-06 15:37:34 | | | |
| 2 | Dean Liu | Meta information modification | 1877 | 2022-04-12 02:53:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ouyang, Z.; Qi, J. Targeting CDK4/6 for Anticancer Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/21419 (accessed on 02 March 2026).
Ouyang Z, Qi J. Targeting CDK4/6 for Anticancer Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/21419. Accessed March 02, 2026.
Ouyang, Zhuqing, Jiating Qi. "Targeting CDK4/6 for Anticancer Therapy" Encyclopedia, https://encyclopedia.pub/entry/21419 (accessed March 02, 2026).
Ouyang, Z., & Qi, J. (2022, April 06). Targeting CDK4/6 for Anticancer Therapy. In Encyclopedia. https://encyclopedia.pub/entry/21419
Ouyang, Zhuqing and Jiating Qi. "Targeting CDK4/6 for Anticancer Therapy." Encyclopedia. Web. 06 April, 2022.
Copy Citation
Cyclin-dependent kinase 4/6 (CDK4/6) are key regulators of the cell cycle and are deemed as critical therapeutic targets of multiple cancers. Various approaches have been applied to silence CDK4/6 at different levels, i.e., CRISPR to knock out at the DNA level, siRNA to inhibit translation, and drugs that target the protein of interest. Here we summarize the current status in this field, highlighting the mechanisms of small molecular inhibitors treatment and drug resistance.
CDK4/6
PROTAC
small molecular inhibitor
drug resistance
cancer
palbociclib
1. Introduction
Cell division is one of the fundamental biological processes, which functions in various physical and pathological activities [1]. The series of stages sequentially occurring in cell division compose a cell cycle, which includes two successive periods: interphase and mitosis. The former features DNA synthesis in the S phase, before and after which there are G1 and G2 phases, preparing for DNA replication and mitosis, respectively. The latter is marked by sister chromatid segregation. Independent of a cell cycle also exists a dormant G0 phase, where most non-proliferative cells in the human body stay [2].
Normally, the cell cycle is monitored by a quality control system called checkpoints, which consist of G1/S checkpoint (DNA damage checkpoint), G2/M checkpoint, and mitotic spindle checkpoint. These checkpoints detect abnormal cell division and then induce cell cycle arrest, allowing cells to repair defects, thus ensuring proper DNA synthesis and chromosome separation. In addition, whether a cell steps into a cell cycle relies on both extrinsic (e.g., growth factors) and intrinsic (e.g., protein synthesis) signals. The lack of these factors leads cells to enter the G0 phase [1]. The majority of human cells are quiescent, except those in the hematopoietic system or gut epithelium [3].
While in malignant cells, the cell cycle is deregulated, which is characterized by abnormal and uncontrollable cell division. Cancer-related cell cycle defects occur via mutations on multiple proteins essential at different stages of the cell cycle [2]. These genomic dysfunctions dispose of cells to acquire more mutations and numerical aberrations in chromosomes, which reflects abnormal cell division; the accumulated genome mutations result in constitutive mitogenic signals and deficiency of response to anti-mitogenic signals, which brings about unscheduled cell division [4].
The cell cycle has to be tightly regulated owing to its critical role in carcinogenesis. In general, transition through the cell cycle is an orderly process regulated by a series of proteins, of which cyclin-dependent kinases (CDKs) are the most critical ones. CDKs are a family of serine/threonine protein kinases, which form heterodimers with their respective regulatory cyclin subunits [5]. CDKs are generally divided into three groups according to their functions: mitosis-related CDKs (CDK1, CDK2, CDK4, and CDK6), which directly promote cell cycle progression, although there are also other CDKs that work in mammalian cell cycle regulation [4]; transcription-related CDKs (CDK7, CDK8, and CDK9); and atypical CDKs (CDK5, CDK14, CDK15, CDK16, CDK17, and CDK18) [1].
In the cell cycle, CDKs are periodically activated at specific points by cyclins [2]. First, in G1, proliferative signals are sensed by D-type cyclins, which activate CDK4 and CDK6. Subsequently, the expression of E-type cyclins activates CDK2, and the formation of CDK2-cyclin E complex is necessary for G1/S progression. Then in the late S phase, CDK2 is activated by cyclin A, driving transition toward G2. At the end of G2, CDK1-cyclin A complex onsets mitosis. Finally, following nuclear envelope breakdown in prophase, A-type cyclins are degraded, and CDK1-cyclin B promotes cells through mitosis [4]. Besides cyclins, CDKs activity is also regulated by CAK (CDK-activating kinase, i.e., CDK7-cyclin H complex) and CKI (CDK inhibitors), which comprise INK4 (inhibitor of CDK4) proteins and Cip/Kip (CDK-interacting protein/kinase inhibitor protein). Under cell cycle defects, checkpoints can be activated via regulation of CDKs activity and therefore prevent daughter cells from inheriting the faults.
Among various CDKs, CDK4 and CDK6 (CDK4/6) are critical because they play a fundamental role in the G1/S transition. CDKs function via phosphorylating specific substrates [1]. In the G1 phase, the most important substrate for CDK4/6 is retinoblastoma susceptibility protein (Rb), which interacts with the E2F transcriptional family in its hypophosphorylated state, whereby it suppresses transcription of target genes. When a cell senses mitogenic signals, CDK4/6 are activated by cyclin D and then phosphorylate Rb, thus relieving the inhibition [5]. CDK4/6 also contributes to this process by separating Cip/Kip proteins from cyclin E-CDK2 complex, which facilitates CDK2 activation and the following complete phosphorylation of Rb. Sequential phosphorylation of Rb by CDK4/6 and CDK2 results in E2F activation and transcription initiation of genes required for S phase progression (Figure 1). On the other hand, CDK4/6 do not sequester Cip/Kip family under antiproliferative signals; thus, cyclin E-CDK2 is inhibited and cell cycle is arrested at G1 phase.
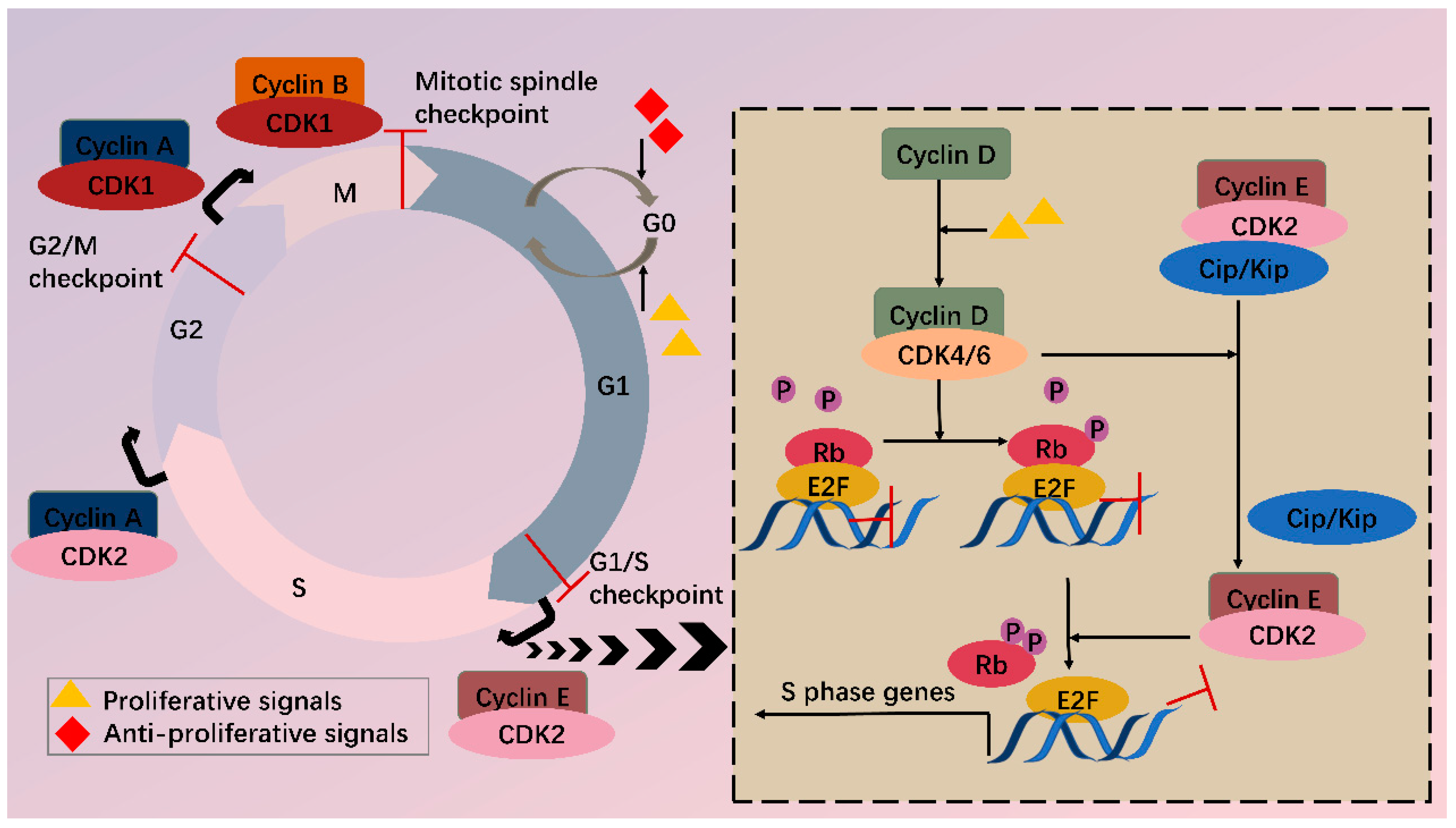
Figure 1. CDKs and cell cycle. Cell cycle consists of G1, S, G2 phase and mitosis. Independent of a cell cycle also exists a dormant G0 phase. Whether a cell steps into a cell cycle relies on balance between proliferative and antiproliferative signals. During a cell cycle, multiple CDKs are sequentially activated. In G1, activated CDK4/6 by cyclin D phosphorylate Rb, partially relieving inhibition of E2F by Rb. Meanwhile, CDK4/6 hijacks Cip/Kip proteins, which stimulate CDK2-cyclin E, facilitating complete phosphorylation of Rb. Thus, E2F activity is totally released, and transcription of S phase genes is initiated. In late G1, CDK2-cyclin E complex is formed, driving transition toward the S phase. Next, CDK2 and CDK1 are successively activated by cyclin A and contribute to S/G2 and G2/M conversion, respectively. Finally, CDK1-cyclin B complex functions during mitosis. Besides CDKs, checkpoints also participate in the cell cycle via regulation of CDKs activity, inducing cell cycle arrest when abnormal cell division is detected.
2. CDK4/6 Are Attractive Targets for Anticancer Treatment
In malignant cells, mutations on and dysregulation of assorted cell cycle regulators, such as CDKs, cyclins, CAK, CKI, CDK substrates, and checkpoint proteins, have been frequently observed [2]. It has been well recognized that the expression levels of CDK4/6 are significantly higher in many tumors [6][7][8][9]. Overexpressed CDK4/6 boost G1/S conversion through directly and indirectly (by stimulating CDK2) phosphorylating Rb, facilitating tumorigenesis. In addition to overexpression, CDK4/6 hyperactivation is common in miscellaneous malignancies (breast cancer, lung cancer, prostate cancer, melanoma, leukemia, lymphoma, glioma, sarcoma, etc.) with differentiated tissue preferences for CDK4/6. CDK4 tends to amplify in epithelial tumors (i.e., in varied cancers) and certain sarcomas, while its homolog favors mesenchymal tissues (including leukemias and sarcomas) [4]. Most human tumors retain wild-type Rb [5], and inhibition of overexpressed or hyperactivated CDK4/6 in these cells can arrest the cell cycle in G1. Even in Rb(-) tumors, CDK4/6 inhibitors also function by reducing cell entry into mitosis or inducing apoptosis in an Rb-independent way [10]. Furthermore, targeting CDK4/6 can also inhibit their cell cycle-independent functions in tumorigenesis. Transcriptomic profiling in breast cancer has uncovered that CDK4 modulates inflammatory cytokine signaling [11]. CDK6 can induce angiogenesis, stem cell activation, immune response, etc. [12].
Moreover, many oncogenes cause cancers by activating the CDK4/6-Rb-E2F pathway and inducing cell proliferation. These include but are not limited to JAK/STAT, PI3K/Akt/mTOR, RAS/RAF/MEK/ERK, BTK/NF-ΚB, and Wnt/β-catenin pathways [13]. In addition, mutations in tumor suppressors such as p53 can also activate the CDK4/6-Rb-E2F pathway by releasing p21CIP1 inhibition. CDK4/6 thus serves as a hub in tumorigenesis pathways. Especially in ER+ breast cancer, there is mutual activation between ER and cyclin D, which will be clarified further (Figure 2). Knockout studies have shown that CDK4/6 are critical for tumor cell growth whereas may be dispensable in normal cells. It is, therefore, safe to kill the enemy without hurting friendly forces [14]. These features together make CDK4/6 appealing and safe targets for anticancer therapy.
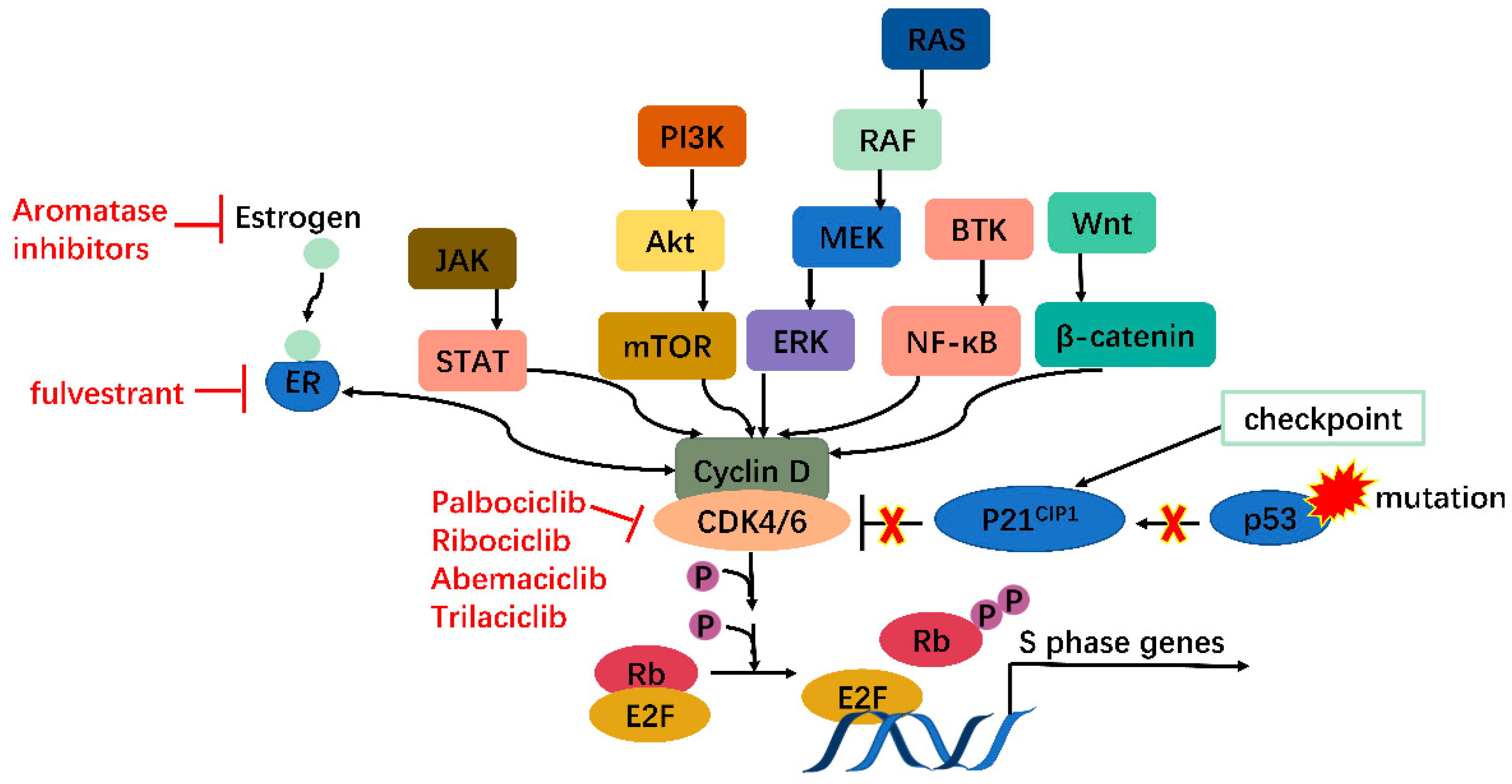
Figure 2. CDK4/6 serves as a hub in tumorigenesis. In cancer, multiple oncogenes may be activated, including those on JAK/STAT, PI3K/Akt/mTOR, RAS/RAF/MEK/ERK, BTK/NF-κB, Wnt/β-catenin pathways, etc., all of which meet at CDK4/6-cyclin D complex. Moreover, mutations on tumor suppressor genes such as p53 can enhance CDK4/6 activity via releasing P21CIP1 inhibition. CDK4/6, therefore, serves as a hub in tumorigenesis.
CDKs, their regulators, and substrates are targets of human cancers. Generally, there are two therapeutic interventions: indirectly targeting CDK regulators or directly focusing on CDKs. The complexity of CDK modulation provides diverse possibilities for indirect tactics, involving lessening or raising the quantity of cyclins or CKI severally, changing activity of those regulators, etc. [2]. Direct policies are attainable at different points of gene expression, which primarily embrace clustered regularly interspaced short palindromic repeats system (CRISPR), RNA interference (RNAi), and protein targeted inhibition or degradation technology. In recent years, direct strategy discovery has been a hot area.
3. CRISPR and RNAi
Silencing CDKs at DNA or RNA level before protein expression is a fascinating approach to cancer therapy. However, very few growth suppression outcomes using CRISPR or RNAi-mediated CDK4/6 or CDK2 deletion have been detected [11][15][16][17]. Contradictorily, there is evidence denying antineoplastic potency of RNAi-based CDK4/6 or CDK2 knockdown [5][18]. In contrast, CRISPR or RNAi treatment targeting other CDKs (CDK7, -8, -9 for CRISPR and CDK1, -10, -12 for RNAi) [19][20][21][22][23] has successfully diminished tumor proliferation.
The disparity between interphase CDKs (CDK2, -4, -6) and CDK1 regarding responsiveness to CRISPR or RNAi is thought-provoking. Among the four CDKs linked to mitosis, CDK1 alone is enough to drive the cell cycle, while CDK2 and CDK4/6 are only indispensable in cell proliferation of specific cells [4][24]. Meanwhile, the lack of cyclin D-CDK4/6 complex can be compensated by cyclin D-CDK1 or cyclin D-CDK2; similarly, cyclin E-CDK 1 or cyclin E-CDK4 can make up for cyclin E-CDK2 absence [5][25][26]; likewise, CDK1 and CDK2 act in a partially redundant way [27]. Hence, the overlapping jobs of mitogenic CDKs and the relatively dispensable position of CDK2 and CDK4/6 compared with CDK1 may partly account for that discrepancy. Namely, loss of function of CDK1 triggered by CRISPR or RNAi cannot be fully offset by interphase CDKs, while it remains possible for CDK1 to fully compensate inhibition of interphase CDKs.
4. Small Molecular Inhibitors
Research on CDK4/6 small molecule inhibitors (SMIs) is an active area. SMIs impede ATP binding to small, determined pockets of CDKs, which holds more potential than hindering large protein-protein interfaces (e.g., interface of cyclin-CDK). However, there are over 500 protein kinases in human genomes, yet ATP binding sites of CDKs are highly conservative [3], so selective drug discovery is challenging. To handle the problem, selectivity determinants outside of the ATP binding pockets are required [28]. The first-generation CDK inhibitors were pan-CDK inhibitors. Due to their poor selectivity and high toxicity, the majority have not been approved for clinical use. The second generation was aimed at improving selectivity for CDK1 and CDK2 and/or total strength [29], and the third generation developed selectivity for CDK4/6 [26]. In addition to poor selectivity and high toxicity, resistance to SMIs cannot be ignored either. Clinical application of CDK4/6 SMIs appears to display weak effects in some tumors (including colorectal cancer, triple-negative breast cancer, melanomas, etc.), where innate or rapid acquisition of resistance may happen [29]. As mentioned, when CDK4/6 are in deficiency, CDK1 or CDK2 can take their roles. Similar events may occur in tumors chronically exposed to CDK4/6 SMIs [5], which may partially explain the mechanisms of resistance, and more reasons will be elucidated hereinafter.
References
- Zhang, M.; Zhang, L.; Hei, R.; Li, X.; Cai, H.; Wu, X.; Zheng, Q.; Cai, C. CDK inhibitors in cancer therapy, an overview of recent development. Am. J. Cancer Res. 2021, 11, 1913–1935.
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149.
- Malumbres, M.; Barbacid, M. To cycle or not to cycle: A critical decision in cancer. Nat. Rev. Cancer 2001, 1, 222–231.
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166.
- Shapiro, G.I. Cyclin-dependent kinase pathways as targets for cancer treatment. J. Clin. Oncol. 2006, 24, 1770–1783.
- Li, M.; Xiao, A.; Floyd, D.; Olmez, I.; Lee, J.; Godlewski, J.; Bronisz, A.; Bhat, K.P.L.; Sulman, E.P.; Nakano, I.; et al. CDK4/6 inhibition is more active against the glioblastoma proneural subtype. Oncotarget 2017, 8, 55319–55331.
- Sheppard, K.E.; McArthur, G.A. The Cell-Cycle Regulator CDK4: An Emerging Therapeutic Target in Melanoma. Clin. Cancer Res. 2013, 19, 5320–5328.
- Malumbres, M. Oncogene-Induced Mitotic Stress: p53 and pRb Get Mad Too. Cancer Cell 2011, 19, 691–692.
- Dall’Acqua, A.; Sonego, M.; Pellizzari, I.; Pellarin, I.; Canzonieri, V.; D’Andrea, S.; Benevol, S.; Sorio, R.; Giorda, G.; Califano, D.; et al. CDK6 protects epithelial ovarian cancer from platinum-induced death via FOXO3 regulation. Embo. Mol. Med. 2017, 9, 1415–1433.
- Alvarez-Fernandez, M.; Malumbres, M. Mechanisms of Sensitivity and Resistance to CDK4/6 Inhibition. Cancer Cell 2020, 37, 514–529.
- Dai, M.; Boudreault, J.; Wang, N.; Poulet, S.; Daliah, G.; Yan, G.; Moamer, A.; Burgos, S.A.; Sabri, S.; Ali, S.; et al. Differential Regulation of Cancer Progression by CDK4/6 Plays a Central Role in DNA Replication and Repair Pathways. Cancer Res. 2021, 81, 1332–1346.
- Nebenfuehr, S.; Kollmann, K.; Sexl, V. The role of CDK6 in cancer. Int. J. Cancer 2020, 147, 2988–2995.
- O’Sullivan, C.C. Overcoming Endocrine Resistance in Hormone-Receptor Positive Advanced Breast Cancer-The Emerging Role of CDK4/6 Inhibitors. Int. J. Cancer Clin. Res. 2015, 2, 29.
- Malumbres, M.; Sotillo, R.; Santamaria, D.; Galan, J.; Cerezo, A.; Ortega, S.; Dubus, P.; Barbacid, M. Mammalian cells cycle without the D-type cyclin-elependent kinases Cdk4 and Cdk6. Cell 2004, 118, 493–504.
- Xing, Z.; Zhang, Y.; Liang, K.; Yan, L.; Xiang, Y.; Li, C.; Hu, Q.; Jin, F.; Putluri, V.; Putluri, N.; et al. Expression of Long Noncoding RNA YIYA Promotes Glycolysis in Breast Cancer. Cancer Res. 2018, 78, 4524–4532.
- Lange, C.; Huttner, W.B.; Calegari, F. Cdk4/CyclinD1 Overexpression in Neural Stem Cells Shortens G1, Delays Neurogenesis, and Promotes the Generation and Expansion of Basal Progenitors. Cell Stem. Cell 2009, 5, 320–331.
- Liu, H.; Li, Z.; Huo, S.; Wei, Q.; Ge, L. Induction of G0/G1 phase arrest and apoptosis by CRISPR/Cas9-mediated knockout of CDK2 in A375 melanocytes. Mol. Clin. Oncol. 2020, 12, 9–14.
- Yoon, S.; Kawasaki, I.; Shim, Y.H. CDC-25.1 controls the rate of germline mitotic cell cycle by counteracting WEE-1.3 and by positively regulating CDK-1 in Caenorhabditis elegans. Cell Cycle 2012, 11, 1354–1363.
- Zhang, Y.; Zhou, L.; Leng, Y.; Dai, Y.; Orlowski, R.Z.; Grant, S. Positive transcription elongation factor b (P-TEFb) is a therapeutic target in human multiple myeloma. Oncotarget 2017, 8, 59476–59491.
- Wang, Y.; Zhang, T.; Kwiatkowski, N.; Abraham, B.J.; Lee, T.I.; Xie, S.; Yuzugullu, H.; Thanh, V.; Li, H.; Lin, Z.; et al. CDK7-Dependent Transcriptional Addiction in Triple-Negative Breast Cancer. Cell 2015, 163, 174–186.
- Fukasawa, K.; Kadota, T.; Horie, T.; Tokumura, K.; Terada, R.; Kitaguchi, Y.; Park, G.; Ochiai, S.; Iwahashi, S.; Okayama, Y.; et al. CDK8 maintains stemness and tumorigenicity of glioma stem cells by regulating the c-MYC pathway. Oncogene 2021, 40, 2803–2815.
- Zhong, S.; Zhang, Y.; Yin, X.; Di, W. CDK7 inhibitor suppresses tumor progression through blocking the cell cycle at the G2/M phase and inhibiting transcriptional activity in cervical cancer. Oncotargets Ther. 2019, 12, 2137–2147.
- Li, Y.; Jiang, F.; Shi, X.; Liu, X.; Yang, H.; Zhang, Z. Identification and Characterization of the Cyclin-Dependent Kinases Gene Family in Silkworm, Bombyx mori. DNA Cell Biol. 2016, 35, 13–23.
- Sherr, C.J.; Roberts, J.M. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004, 18, 2699–2711.
- Tetsu, O.; McCormick, F. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell 2003, 3, 233–245.
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122.
- Mitra, J.; Enders, G.H.; Azizkhan-Clifford, J.; Lengel, K.L. Dual regulation of the anaphase promoting complex in human cells by cyclin A-Cdk2 and cyclin A-Cdk1 complexes. Cell Cycle 2006, 5, 661–666.
- Jiang, B.; Wang, E.S.; Donovan, K.A.; Liang, Y.; Fischer, E.S.; Zhang, T.; Gray, N.S. Development of Dual and Selective Degraders of Cyclin-Dependent Kinases 4 and 6. Angew. Chem.-Int. Ed. 2019, 58, 6321–6326.
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146.
More
Information
Subjects:
Biology; Cell Biology; Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
2 times
(View History)
Update Date:
12 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





