
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Asparuh Nikolov | -- | 1689 | 2022-04-06 15:14:14 | | | |
| 2 | Rita Xu | Meta information modification | 1689 | 2022-04-07 05:09:27 | | |
Video Upload Options
Accumulating evidence indicates that two major proteins are responsible for the structural coherence of bounding cardiomyocytes. These biomolecules are known as myocardial fibrillar collagen type I (COL1) and type III (COL3). In addition, fibronectin, laminin, fibrillin, elastin, glycoproteins, and proteoglycans take part in the formation of cardiac extracellular matrix (ECM). In physiological conditions, collagen synthesis and degradation in human cardiac ECM are well-regulated processes, but they can be impaired in certain cardiovascular diseases, such as heart failure (HF). Myocardial remodeling is part of the central mechanism of HF and involves cardiomyocyte injury and cardiac fibrosis due to increased fibrillar collagen accumulation.
1. Introduction
2. Type I and Type III Collagen Characteristics
3. Cardiac Extracellular Matrix: Structure and Function
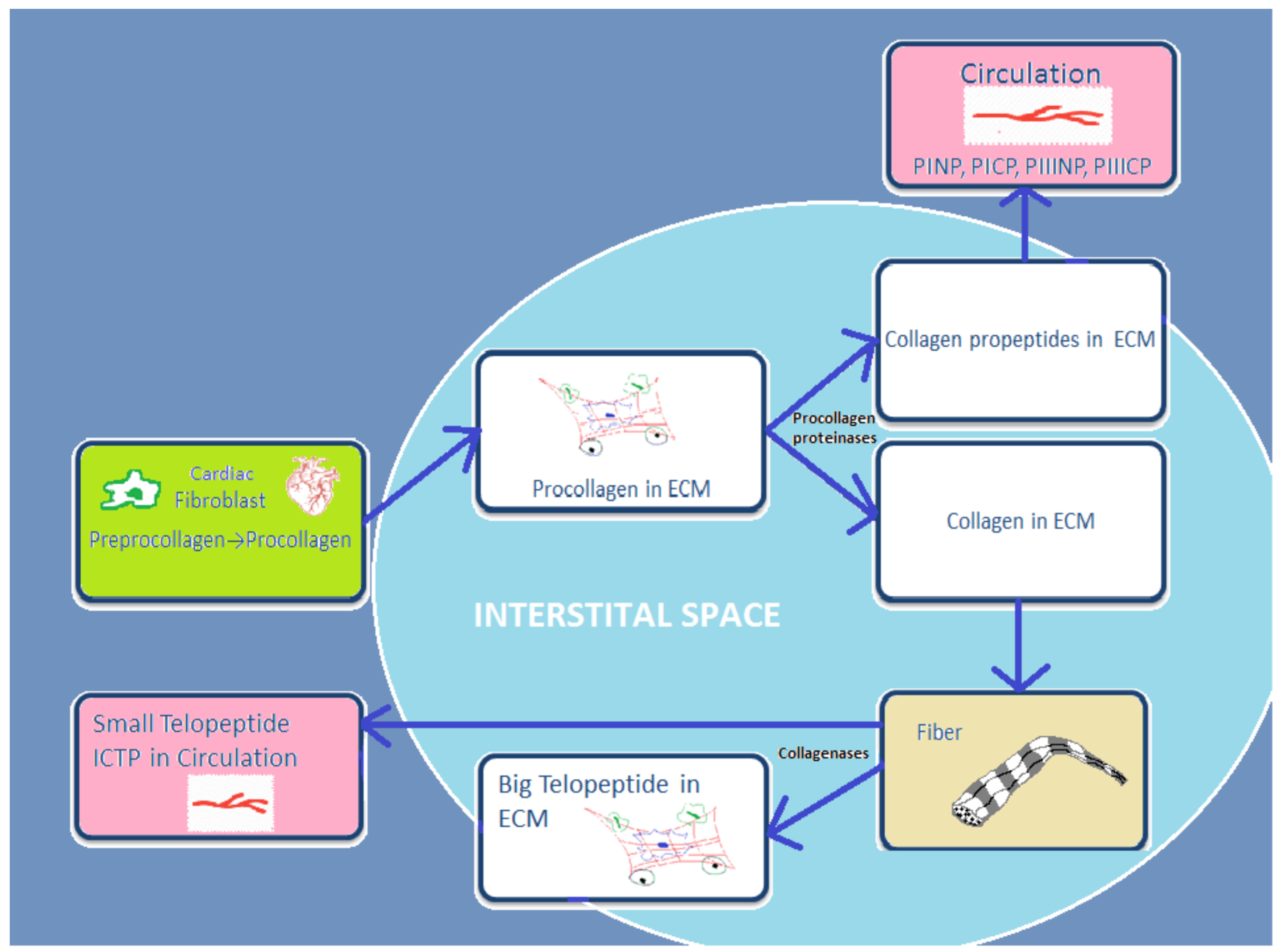
4. General Concepts of Abnormal Cardiac Extracellular Matrix Changes in Heart Failure
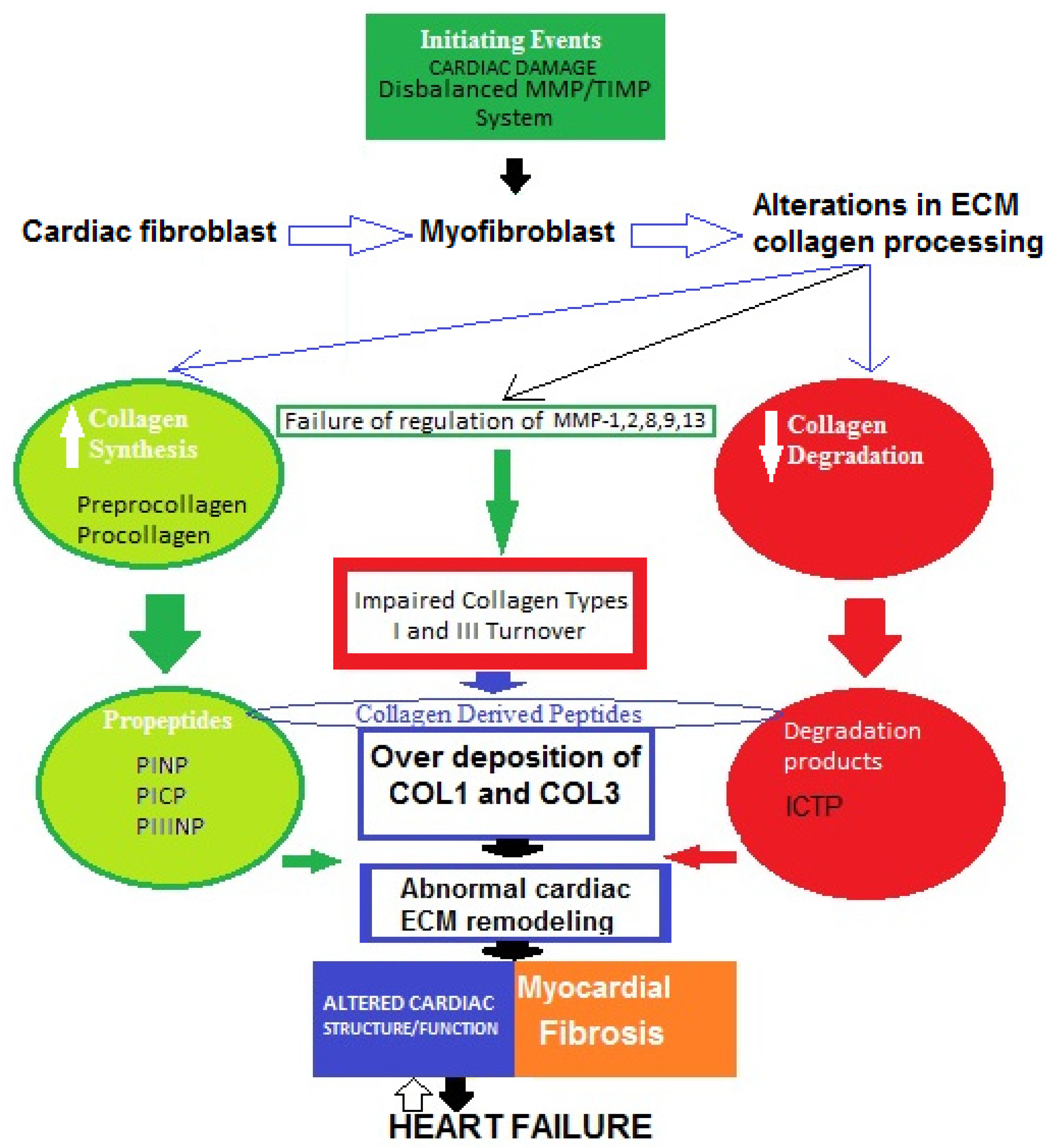
5. Basic Underlying Mechanisms of Myocardial Fibrosis in Heart Failure: Role of Impaired Type I and III Collagen Turnover
References
- GBD 2015. Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study. Lancet 2015, 388, 10053.
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.; Ponikowski, P.; Poole-Wilson, P.A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. Heart J. 2018, 29, 2388–2442.
- Metra, M.; Teerlink, J.R. Heart failure. Lancet 2017, 390, 1981–1995.
- Mann, D.L.; Chakinala, M. Harrison’s Principles of Internal Medicine: Heart Failure and Cor Pulmonale, 18th ed.; McGraw-Hill: New York, NY, USA, 2012; Chapter 234; pp. 1–11.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726.
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140.
- De Wever, O.; Demetter, P.; Mareel, M.; Bracke, M. Stromal myofibroblasts are drivers of invasive cancer growth. Int. J. Cancer 2008, 123, 2229–2238.
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200.
- Henriksen, K.; Karsdal, M.A. Type I Collagen. In Biochemistry of Collagens, Laminins and Elastin Structure, Function and Biomarkers, 1st ed.; Karsdal, M.A., Ed.; Academic Press: Cambridge, MA, USA, 2016; Chapter 1; pp. 1–11.
- Han, S.; Makareeva, E.; Kuznetsova, N.V. Molecular mechanism of type I collagen homotrimer resistance to mammalian collagenases. J. Biol. Chem. 2010, 285, 22276–22281.
- Von Der Mark, K. Localization of collagen types in tissues. Int. Rev. Connect. Tissue Res. 1981, 9, 265–324.
- Nielsen, M.J.; Karsdal, M.A. Type III Collagen. In Biochemistry of Collagens, Laminins and Elastin Structure, Function and Biomarkers, 1st ed.; Karsdal, M.A., Ed.; Academic Press: Cambridge, MA, USA, 2016; Chapter 3; pp. 21–30.
- Jarvelainen, H.; Sainio, A.; Koulu, M.; Wight, T.N.; Penttinen, R. Extracellular matrix molecules: Potential targets in pharmacotherapy. Pharmacol. Rev. 2009, 61, 198–223.
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Science: London, UK, 2007.
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell Mol. Life Sci. 2014, 71, 549–574.
- Anderson, K.R.; Sutton, M.G.; Lie, J.T. Histopathological types of cardiac fibrosis in myocardial disease. J. Pathol. 1978, 128, 79–85.
- Eghbali, M.; Czaja, M.J.; Zeydel, M.; Weiner, F.; Zern, M.A.; Seifter, S.; Blumenfefd, O.O. Colllagen chain mRNAs in isolated heart cells from young and adult rats. J. Mol. Cell. Cardiol. 1988, 20, 267–276.
- Lijnen, P.; Petrov, V.; Fagard, R. Induction of cardiac fibrosis by transforming growth factor-β1. Mol. Gen. Metab. 2000, 71, 418–435.
- Cohn, J.N.; Ferrari, R.; Sharpe, N. Cardiac remodeling-concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J. Am. Coll. Cardiol. 2000, 35, 569–582.
- Hockman, J.S.; Bulkley, B.H. Expansion of acute myocardial infarction: An experimental study. Circulation 1982, 65, 1446–1450.
- Pfeffer, M.A.; Braunwald, E. Ventricular remodeling after myocardial infarction: Experimental observations and clinical implications. Circulation 1990, 81, 1161–1172.
- Gaasch, W.H. Left ventricular radius to wall thickness ratio. Am. J. Cardiol. 1979, 43, 1189–1194.
- Sayer, G.; Bhat, G. The renin-angiotensin-aldosterone system and heart failure. Cardiol. Clin. 2014, 32, 21–32.
- Florea, V.G.; Cohn, J.N. The autonomic nervous system and heart failure. Circ. Res. 2014, 114, 1815–1826.
- Tan, L.B.; Jalil, J.E.; Pick, R. Cardiac myocyte necrosis induced by angiotensin II. Circ. Res. 1991, 69, 1185–1195.
- Sharov, V.G.; Sabbah, H.N.; Shimoyama, H. Evidence of cardiocyte apoptosis in myocardium of dogs with chronic heart failure. Am. J. Pathol. 1996, 148, 141–149.
- Teiger, E.; Dam, T.V.; Richard, L. Apoptosis in pressure overload—Induced heart hypertrophy in the rat. J. Clin. Investig. 1996, 97, 2891–2897.
- Olivetti, G.; Abbi, R.; Quaini, F. Apoptosis in the failing human heart. N. Engl. J. Med. 1997, 336, 1131–1141.
- Villarreal, F.J.; Kim, N.N.; Ungab, G.D. Identification of functional angiotensin II receptors on rat cardiac fibroblastos. Circulation 1993, 88, 2849–2861.
- Weber, K.T.; Pick, R.; Silver, M.A. Fibrillar collagen and remodeling of dilated canine left ventricle. Circulation 1990, 82, 1387–1401.
- Douglas, L.; Mann, M.D.; Spinale, F.G. Activation of Matrix Metalloproteinases in the Failing Human Heart Breaking the Tie That Binds. Circulation 1998, 98, 1699–1702.
- Alla, F. Early changes in serum markers of cardiac extra-cellular matrix turnover in patients with uncomplicated hypertension and type II diabetes. Eur. J. Heart Fail. 2006, 8, 147–153.
- Diez, J.; Querejeta, R.; Lopez, B. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 2002, 105, 2512–2517.
- Laviades, C.; Varo, N.; Fernandez, J. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation 1998, 98, 535–540.
- Fan, D.; Takawale, A.; Lee, J. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 2012, 5, 5–15.
- Eghbali, M. Cardiac fibroblasts: Function, regulation of gene expression, and phenotypic modulation. Basic Res. Cardiol. 1992, 87 (Suppl. S2), 183–189.
- Moore, L.; Fan, D.; Basu, R. Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart Fail Rev. 2012, 17, 693–706.
- Gyöngyösi, M.; Winkler, J.; Ramos, I. Myocardial fibrosis: Biomedical research from bench to bedside. Eur. J. Heart Fail. 2017, 19, 177–191.
- Ravassa, S.; Lopez, B.; Querejeta, R.; Echegaray, K.; San-Jose, G.; Moreno, M.U.; Beaumont, F.J.; Gonzalez, A.; Diez, J. Phenotyping of myocardial fibrosis in hypertensive patients with heart failure. Influence on clinical outcome. J. Hypertens. 2017, 35, 853–861.
- Hinderer, S.; Schenke-Layland, K. Cardiac fibrosis—A short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 77–82.
- Bing, R.; Dweck, M.R. Myocardial fibrosis: Why image, how to image and clinical implications. Heart 2019, 105, 1832–1840.
- Lopez, B.; Ravassa, S.; Moreno, M.U.; San José, G.; Beaumont, J.; González, A.; Díez, J. Diffuse myocardial fibrosis: Mechanisms, diagnosis and therapeutic approaches. Nat. Rev. Cardiol. 2021, 18, 479–498.
- Espeland, T.; Lunde, I.G.; Amundsen, B.H.; Gullestad, L.; Aakhus, S. Myocardial fibrosis. Tidsskriftet 2018, 138.
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac fibrosis: The fibroblast awakens. Circ. Res. 2016, 118, 1021–1040.
- Liu, T.; Song, D.; Dong, J.; Zhu, P.; Liu, J.; Liu, W.; Ma, X.; Zhao, L.; Ling, S. Current unserstandings of the pathophysiology of myocardial fibrosis and its quantitative assessment in heart failure. Front. Physiol. 2017, 8, 238.




