Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Farzad Salehpour | -- | 2052 | 2022-04-06 14:35:23 | | | |
| 2 | Dean Liu | Meta information modification | 2052 | 2022-04-07 03:00:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Salehpour, F.; Di Duro, J.; Khademi, M.; Bragin, D. Brain Lymphatic Drainage System. Encyclopedia. Available online: https://encyclopedia.pub/entry/21412 (accessed on 08 February 2026).
Salehpour F, Di Duro J, Khademi M, Bragin D. Brain Lymphatic Drainage System. Encyclopedia. Available at: https://encyclopedia.pub/entry/21412. Accessed February 08, 2026.
Salehpour, Farzad, Joseph Di Duro, Mahsa Khademi, Denis Bragin. "Brain Lymphatic Drainage System" Encyclopedia, https://encyclopedia.pub/entry/21412 (accessed February 08, 2026).
Salehpour, F., Di Duro, J., Khademi, M., & Bragin, D. (2022, April 06). Brain Lymphatic Drainage System. In Encyclopedia. https://encyclopedia.pub/entry/21412
Salehpour, Farzad, et al. "Brain Lymphatic Drainage System." Encyclopedia. Web. 06 April, 2022.
Copy Citation
In 2012, Iliff et al., for the first time, identified a novel structure in the brain called the glymphatic system. This system is considered as a crucial fluid-clearance system in the brain.
photobiomodulation
near-infrared light
glymphatic system
meningeal lymphatic vessels
1. The System, Its Components, and Pathways
Based on physiological findings of communication among different parts of the brain, the existence of a specific lymphatic drainage system in the brain of vertebrates has been suggested [1][2]. In 2012, Iliff et al., for the first time, identified a novel structure in the brain called the glymphatic system [3]. This system is considered as a crucial fluid-clearance system in the brain [4][5]. Studies on mouse models using different fluorescent tracers constructed this glymphatic drainage pathway in the brain [6][7].
This system consists of five main functional components, each facilitating the movement of CSF and ISF (Figure 1). The first compartment of the glymphatic system consists of the production of CSF by epithelial cells of the choroid plexus in the cerebral ventricles and circulation of CSF in the subarachnoid space, followed by the second, periarterial influx of CSF into the brain parenchyma. In fact, periarterial influx refers to the entrance of CSF into the periarterial spaces surrounding the arteries and its penetration deep into the brain tissue. Arterial pulsation caused by smooth muscle cells intensifies CSF movement inward along the periarterial space [8]. Exchange of CSF and ISF is the third component of this system, which occurs in the interstitial space of the brain parenchyma (Figure 2).
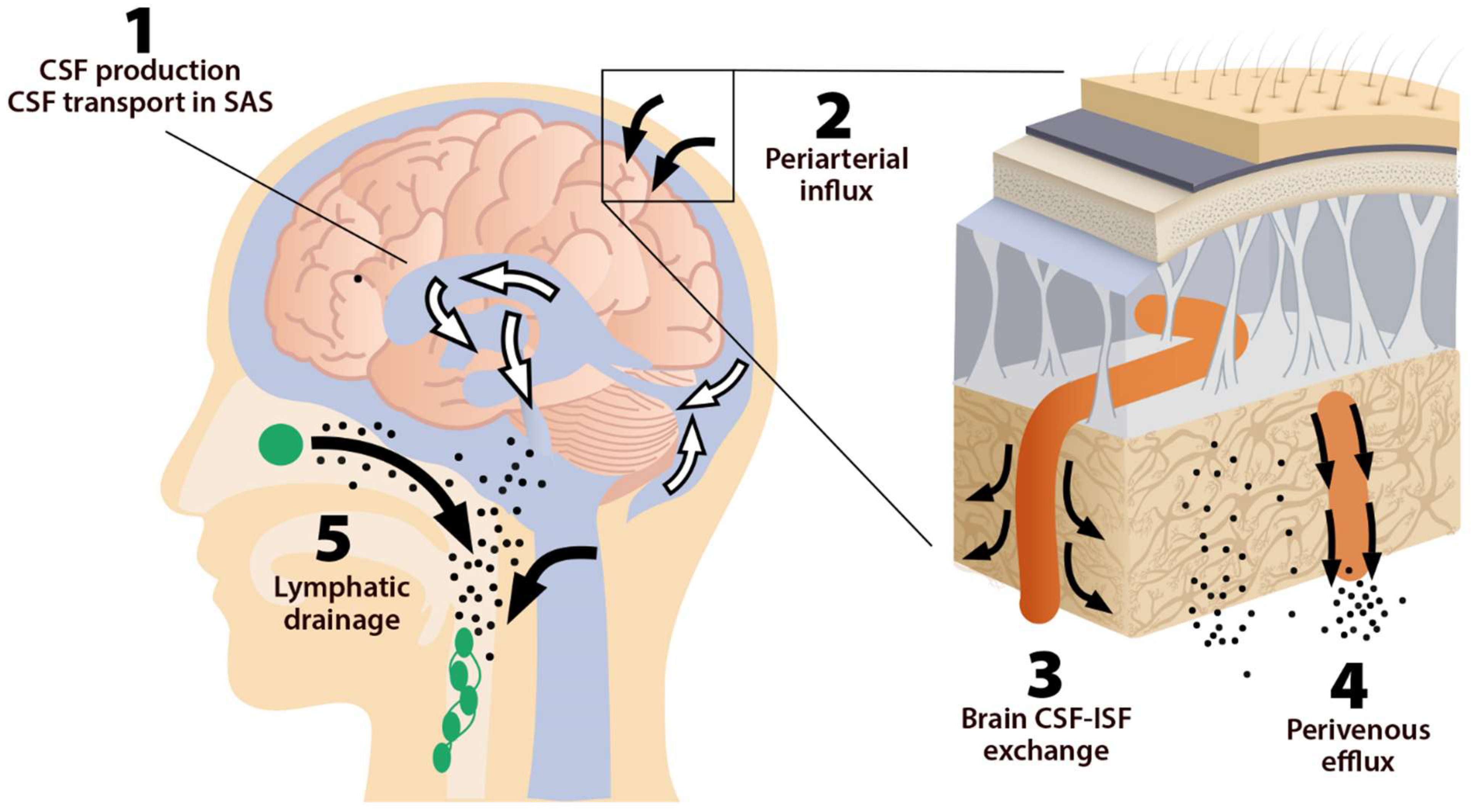
Figure 1. The five components of the glymphatic system. The fluid transport pathway is divided into five distinct segments: (1) cerebrospinal fluid (CSF) is produced by the choroid plexus and likely by extrachoroidal sources (capillary influx and metabolic water production); (2) arterial wall pulsatility drives CSF deep into brain along perivascular spaces; (3) CSF enters the brain parenchyma supported by aquaporin-4 (AQP4) water channels and disperses within the neuropil; interstitial fluid (ISF) mixes with CSF, (4) accumulates in the perivenous space, and drains out of the brain via (5) meningeal and cervical lymphatic vessels, as well as along cranial and spinal nerves Fluids from both the brain and the cribriform plate drain into the cervical lymphatic vessels, which then empty into the venous system at the level of the subclavian veins. The olfactory/cervical lymphatic drainage route is the primary bulk flow pathway.
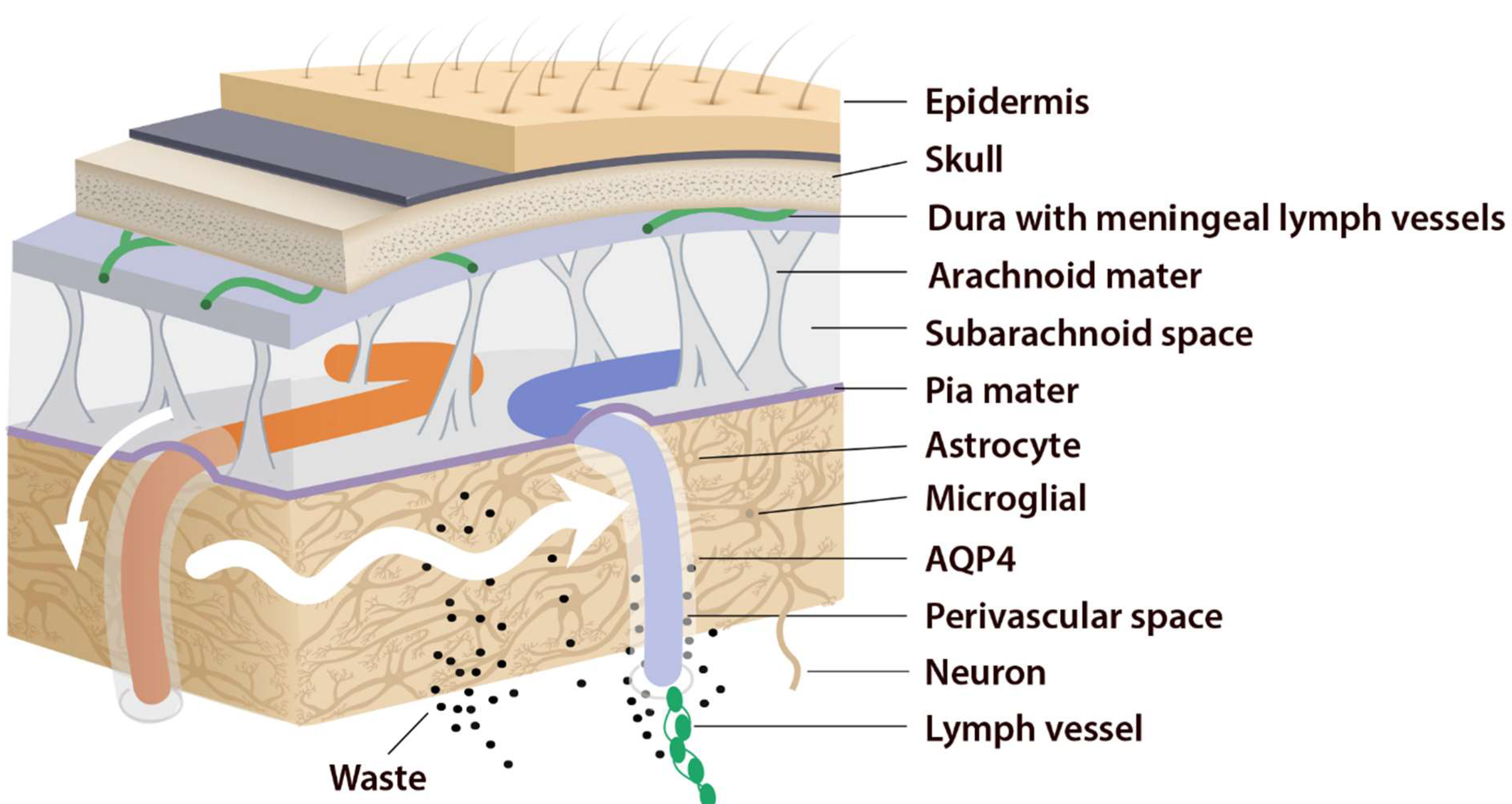
Figure 2. Periarterial influx of CSF into the brain tissue (small white arrow). CSF–ISF exchange supported by AQP4 channels in the vascular end feet plastered along the arterioles. From here, the fluid leaves the axons and moves towards the perivenous space in a path supported by astrocytes. Astrocytic AQP4 water channels facilitate this perivenous efflux of interstitial fluid, which drains to the dural lymphatic vessels.
Astrocytes are believed to facilitate the fluid movement between periarterial spaces and the interstitium through water channels such as aquaporins-4 (AQP4) [3][9]. The fourth component is the glymphatic efflux, which consists of drainage of ISF into the perivenous spaces. The meningeal lymphatic system is the fifth component and final downstream clearance of the glymphatic system. MLVs drain waste products and other solutes from the CNS [10]. This ISF then flows towards the leptomeningeal arteries located at the cortical surface (sulci) and ultimately moves into the cervical lymphatics [3].
Indeed, this system was named “glymphatic” based on the involvement of glial cells “gl” and its similar function with the “lymphatic system” [11][12]. The brain glymphatic system has several essential physiological functions such as drainage of ISF from the parenchymal section of the brain to nearby lymph nodes. It is also involved in communication with the immune system, which regulates and monitors brain responses to neuroinflammation [13]. Moreover, the glymphatic system possesses numerous physiological functions in addition to solute clearance [14]. It is hypothesized that the glymphatic system has a role in rapid lipid transportation across the blood–brain barrier (BBB) and promote glial signaling [15]. Additionally, CSF is involved in the transportation of apolipoprotein E, essential for cholesterol transport, and most notably, synaptic plasticity [16]. CSF influx is also a vehicle for glucose and other vital nutrients that are necessary for the metabolism of astrocytes and neurons [14].
Lifestyle factors, genetics, and pathological conditions can modulate brain clearance and influence the risk of developing neurodegenerative diseases [17]. Several factors such as genetic phenotypes, body posture, aging, and the sleep–wake cycle could influence these physiological functions [6] so that an impaired cerebral lymphatic system is counted as a risk factor for neurodegenerative [18], neuroinflammatory [19], and neurovascular diseases [20] and tumors, as well as impaired recovery from brain injuries [19] (Figure 3). Pathological conditions can strongly affect the brain lymphatic systems. In various vascular disorders including hypertension, atherosclerosis, and small vessel diseases [21], any alteration in the composition of the constituent proteins can result in a significant decline in vascular plasticity and decrease cerebral blood flow (CBF) into the perivascular pathways. In arterial stenosis (either cervical or intracranial), blockage of CBF and obstruction of perivascular or paravascular channels are observed [20], leading to reduced ISF flow resulting in loss of CSF clearance from the brain. Glymphatic system dysfunction has been demonstrated to be associated with many neurological diseases such as AD and PD [22][12]. The glymphatic system has been described as the “final common pathway” for neurodegenerative diseases [23].
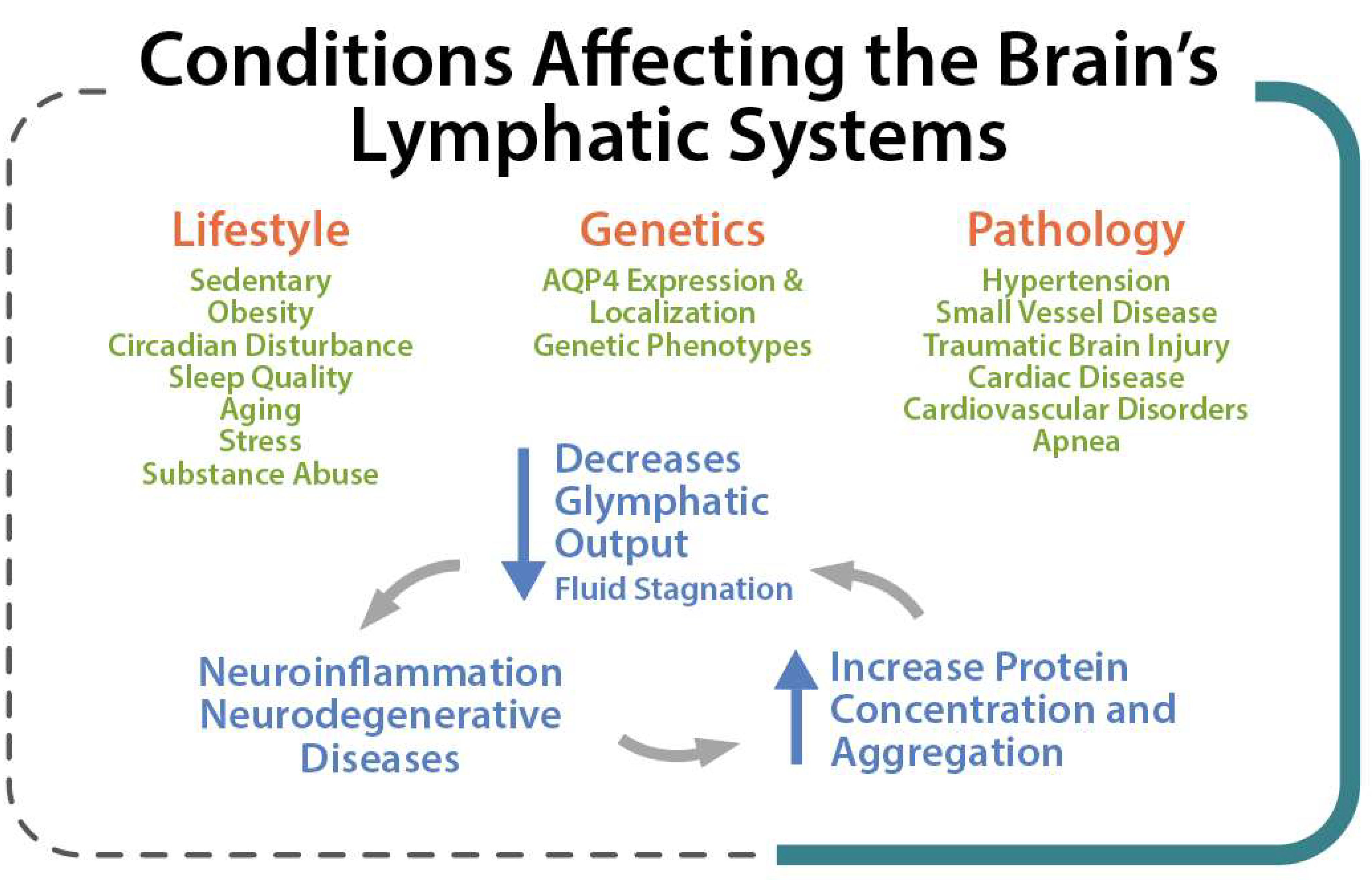
Figure 3. Lifestyle, Genetic and Pathological conditions that can strongly affect the brain lymphatic systems.
2. MLVs, Olfactory/Cervical Lymphatic Drainage Route, and Their Association with CSF Circulation
The absence of a conventional lymphatic vasculature in the CNS prompted a series of studies on rodents and human brains to identify MLVs as the lymphatic system of the CNS [24][25]. MLVs seem to provide a critical route for drainage of ISF and CSF. Various macromolecules and immune cells pass from CNS into the lymph nodes located in the deep cervical area [26][27][28]. More recently, strong evidence demonstrated that MLVs might be associated with the regulation of immune responses and also involved in the pathogenesis of neuroinflammatory diseases [29]. Animal studies have also shown impaired meningeal lymphatic function in AD [10] and PD [22]. In a neuroimaging study using a dynamic contrast-enhanced MRI, patients with idiopathic PD exhibited markedly decreased flow through the MLVs along the superior sagittal sinus and sigmoid sinus, as well as a significant delay in deep cLNs (dcLNs) perfusion [30].
Under normal physiological conditions, the olfactory/cervical lymphatic drainage route serves the primary bulk flow drainage pathway. The ethmoid bone and particularly the cribriform plate located at the anterior aspect of the brain (between the anterior cranial fossa and the nasal cavity) is considered a critical extracranial site of CSF outflow [31]. CSF in subarachnoid space passes through the cribriform plate along the olfactory nerves to the nasal lymphatics and cLNs. At the end of the route, CSF is deposited into the extracranial lymphatic system [20]. The continuous circulation and drainage of CSF are critical for removing CSF metabolic products and maintaining normal neural functions. The outflow routes of CSF are the arachnoid villi of the dural superior sagittal sinus [32], olfactory nerves, across the cribriform plate, and into the cervical lymphatic pathway [33].
The cribriform plate is a fenestrated bony plate of the ethmoid that separates the cranial and nasal cavities (Figure 4). Even though there are lymphatic vessels in the meninges [34], it has been demonstrated that CSF can drain through the cribriform plate in both humans and other mammals [35]. The main pathway by which CSF is removed from the skull is through the cribriform plate associated with the olfactory nerves [36]. The CSF is absorbed by lymphatic vessels located in the submucosa of the olfactory epithelium, in the nasal mucosa after passing the cribriform plate, and then drained into the cLNs. Any damage to the cribriform plate (by traumatic brain injuries or surgical methods) can lead to acute blockage of CSF outflow and, as a result, increase in resting intracranial pressure (ICP) and outflow resistance, emphasizing that the olfactory pathway represents the leading site for the CSF drainage [37]. There is a space between the olfactory sensory axons that provides a conduit for the outflow of CSF. Any damage to these nerves can also diminish the outflow of CSF through the cribriform plate [36] (Figure 4).
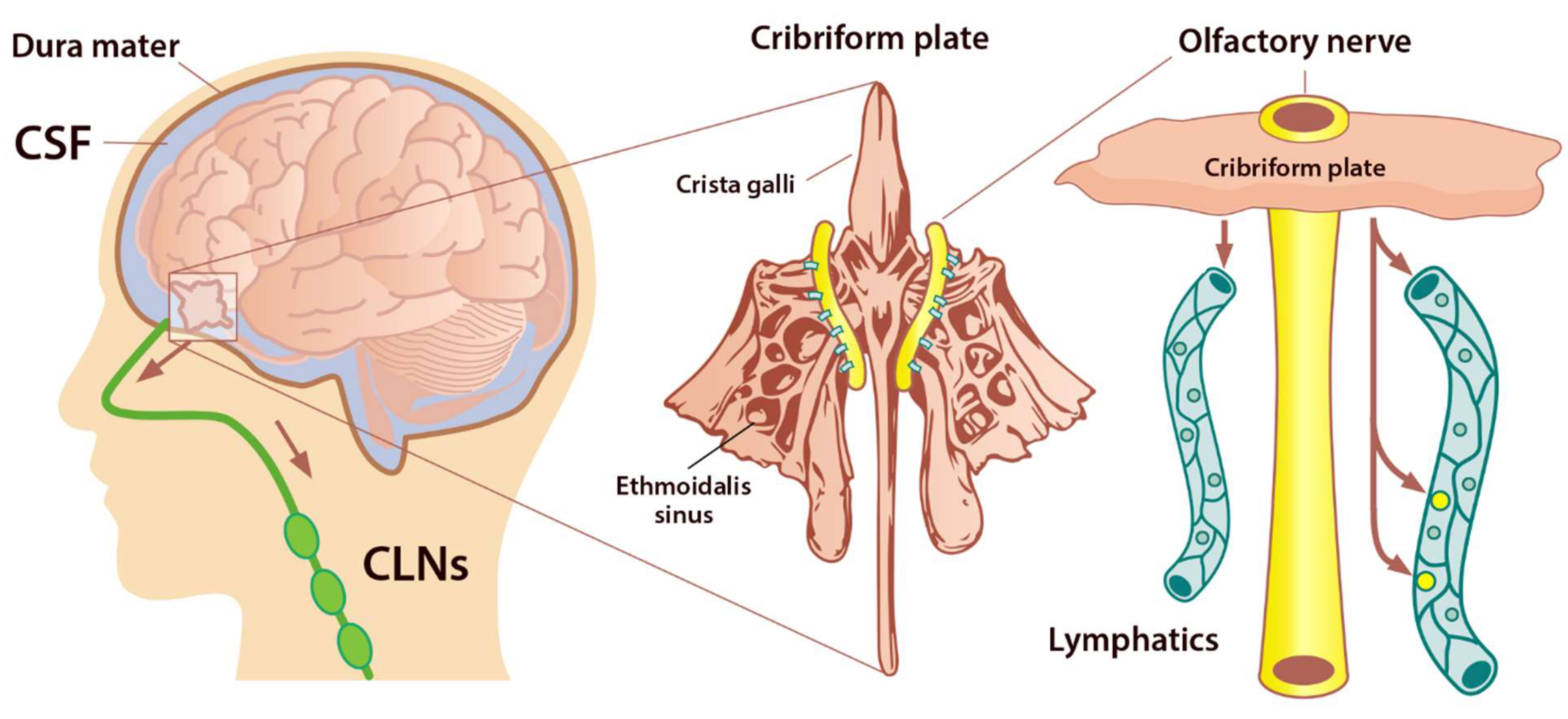
Figure 4. Perineural space surrounding olfactory nerve penetrates the nasal mucosa through the cribriform plate. The cribriform plate of the ethmoid bone is considered the key extracranial site of CSF outflow. CSF in SAS passes across the cribriform plate along olfactory nerves to nasal lymphatics and enters cervical lymph nodes (adapted from Semyachkina-Glushkovskaya et al. 2021).
AQP are a family of small integral membrane proteins that significantly boost the permeability of cells to water and facilitate the movement of fluid down the pressure gradient in various tissues, including the brain [38]. So far, 13 AQPs have been found in mammals (AQP0–12). AQP1 maintains CSF production by the choroid plexus, and it is also expressed along the periphery of the olfactory bulb, nerve junction, and lining the foramina of the cribriform plate. Moreover, there are high levels of AQP1, 3, and 5 within the nasal cavity. These AQPs facilitate the flow of fluid out of the olfactory bulb and subarachnoid space into the nasal cavity via the extensive network of lymphatic vessels, which play an essential role in moving fluid throughout the body. AQPs are found in the meninges and at the cribriform plate and olfactory bulb junction [39]. These vessels crossing the cribriform plate play a key role in transporting fluid from the cranial cavity to the nasal cavity olfactory sensory nerves. Following CSF absorption by lymphatics, it is conveyed in larger ducts through numerous lymph nodes and eventually is deposited into the body’s lymphatic system. Evidence has also shown that aging decreases the elimination rate of CSF from the nasal/cribriform plate region [40][41].
3. Sleep and Clearance of the Brain
The glymphatic system uses convective flow between the CSF and ISF to remove toxic metabolites in/from the brain. CSF enters the brain parenchyma (functional parts) along a paraarterial route and exchanges with the ISF [42]. The ISF carries extracellular solutes from the interstitial (extracellular) space in the brain along paravenous drainage pathways (Figure 1). This activity is dramatically boosted during sleep and is related to increased interstitial volume, possibly by shrinkage of astroglial cells [43]. Emerging evidence shows that sleep is the primary driver of glymphatic clearance and is essential for the maintenance of brain function via the discharge of metabolites and neurotoxic wastes from the brain, which accumulates in the highly active brain during waking hours [23][44].
Comparing the brain ISF volume during deep sleep to wakefulness, the volume of the brain’s ISF increases by 40–60% [45]. Astrocytic AQP4 water channels that encircle the brain’s vasculature contribute to this increase in ISF. This increase in ISF is required for proper glymphatic function and facilitates the clearance of soluble proteins, waste products, and excess extracellular fluid. ISF increase leads to a 2-fold faster removal of neurotoxic waste products such as lactate and Aβ from the brain. This increase in the clearance of brain waste happens during non-rapid eye movement (NREM) sleep [46], and the majority of glymphatic activity occurs during deep, slow-wave sleep. Poor sleep quality and short sleep duration result in an increased amount of Aβ in the CSF as well as a risk of Aβ plaque formation [47]. In addition, tau levels have been shown to be increased in the ISF of the hippocampus following sleep deprivation [48]. Evidently, these neurobiological mechanisms can support the fact that neurodegenerative diseases such as AD, PD, Huntington disease, and frontotemporal dementias are strongly linked to sleep disturbances [49]. With the glymphatic system in mind, it is of interest to note that sleep quality decreases as a function of normal aging, and individuals over 60 years old rarely enter deep NREM (stages 3). The effectiveness of glymphatic fluid transport is directly linked to the prevalence of slow-wave activity. Therefore, the age-related impairment in sleep quality can cause a catastrophic drop in the clearance of brain waste and potentially increase the incidence risk of neurodegenerative diseases [23].
Evidence revealed that endocytosis occurs across the BBB during sleep, and inhibition of this process causes the need for more sleep [50]. In addition, several studies have reported that sleep deprivation can increase the activity of several pro-inflammatory mediators such as C-reactive protein, interleukin (IL)-1β, IL-6, IL-17, interferon-γ (IFN-γ), and tumor necrosis factor-alpha (TNF-α). These mediators suppress astrocytic maintenance of the BBB, causing an increase in its permeability [51][52]. Sleep deprivation has been shown to decrease influx efficiency along the perivascular space, thus impairing the function of the glymphatic system and disturbing AQP4 polarization in a mouse model [53].
References
- Weller, R.O.; Djuanda, E.; Yow, H.-Y.; Carare, R.O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009, 117, 1–14.
- Matsumae, M.; Sato, O.; Hirayama, A.; Hayashi, N.; Takizawa, K.; Atsumi, H.; Sorimachi, T. Research into the physiology of cerebrospinal fluid reaches a new horizon: Intimate exchange between cerebrospinal fluid and interstitial fluid may contrib-ute to maintenance of homeostasis in the central nervous system. Neurol. Med. Chir. 2016, 56, 416–441.
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111.
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 2018, 65, 106–119.
- Bakker, E.; Bacskai, B.J.; Arbel-Ornath, M.; Aldea, R.; Bedussi, B.; Morris, A.; Weller, R.O.; Carare, R.O. Lymphatic Clearance of the Brain: Perivascular, Paravascular and Significance for Neurodegenerative Diseases. Cell. Mol. Neurobiol. 2016, 36, 181–194.
- Engelhardt, B.; Carare, R.O.; Bechmann, I.; Flügel, A.; Laman, J.D.; Weller, R.O. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol. 2016, 132, 317–338.
- Carare, R.O.; Bernardes-Silva, M.; Newman, T.A.; Page, A.M.; Nicoll, J.A.R.; Perry, V.H.; Weller, R.O. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 2008, 34, 131–144.
- Mestre, H.; Tithof, J.; Du, T.; Song, W.; Peng, W.; Sweeney, A.M.; Olveda, G.; Thomas, J.H.; Nedergaard, M.; Kelley, D.H. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 2018, 9, 1–9.
- Silva, I.; Silva, J.; Ferreira, R.; Trigo, D. Glymphatic system, AQP4, and their implications in Alzheimer’s disease. Neurol. Res. Pract. 2021, 3, 1–9.
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.C.; Kingsmore, K.M.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018, 560, 185–191.
- Zhou, W.; Shen, B.; Shen, W.-Q.; Chen, H.; Zheng, Y.-F.; Fei, J.-J. Dysfunction of the Glymphatic System Might Be Related to Iron Deposition in the Normal Aging Brain. Front. Aging Neurosci. 2020, 12, 445.
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024.
- Chen, J.; Wang, L.; Xu, H.; Wang, Y.; Liang, Q. The lymphatic drainage system of the CNS plays a role in lymphatic drain-age, immunity, and neuroinflammation in stroke. J. Leukoc. Biol. 2021, 110, 283–291.
- Wang, Q.; Sawyer, I.A.; Sung, M.-H.; Sturgill, D.; Shevtsov, S.P.; Pegoraro, G.; Hakim, O.; Baek, S.; Hager, G.L.; Dundr, M. Cajal bodies are linked to genome conformation. Nat. Commun. 2016, 7, 10966.
- Thrane, V.R.; Thrane, A.S.; Plog, B.A.; Thiyagarajan, M.; Iliff, J.J.; Deane, R.; Nagelhus, E.A.; Nedergaard, M. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci. Rep. 2013, 3, srep02582.
- Achariyar, T.M.; Li, B.; Peng, W.; Verghese, P.B.; Shi, Y.; McConnell, E.; Benraiss, A.; Kasper, T.; Song, W.; Takano, T.; et al. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol. Neurodegener. 2016, 11, 1–20.
- Kylkilahti, T.M.; Berends, E.; Ramos, M.; Shanbhag, N.C.; Töger, J.; Bloch, K.M.; Lundgaard, I. Achieving brain clearance and preventing neurodegenerative diseases—A glymphatic perspective. Br. J. Pharmacol. 2021, 41, 2137–2149.
- Baranello, R.J.; Bharani, K.L.; Padmaraju, V.; Chopra, N.; Lahiri, D.K.; Greig, N.H.; Pappolla, M.A.; Sambamurti, K. Amy-loid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 32–46.
- Dunn, G.P.; Okada, H. Principles of immunology and its nuances in the central nervous system: Figure 1. Neuro-Oncology 2015, 17, vii3–vii8.
- Sun, B.-L.; Wang, L.-H.; Yang, T.; Sun, J.-Y.; Mao, L.-L.; Yang, M.-F.; Yuan, H.; Colvin, R.A.; Yang, X.-Y. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Prog. Neurobiol. 2018, 163–164, 118–143.
- Gupta, A.; Iadecola, C. Impaired Aβ clearance: A potential link between atherosclerosis and Alzheimer’s disease. Front. Aging Neurosci. 2015, 7, 115.
- Zou, W.; Pu, T.; Feng, W.; Lu, M.; Zheng, Y.; Du, R.; Xiao, M.; Hu, G. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated α-synuclein. Transl. Neurodegener. 2019, 8, 1–17.
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56.
- Lord, C.C.; Wyler, S.C.; Wan, R.; Castorena, C.M.; Ahmed, N.; Mathew, D.; Lee, S.; Liu, C.; Elmquist, J.K. The atypical anti-psychotic olanzapine causes weight gain by targeting serotonin receptor 2C. J. Clin. Investig. 2017, 127, 3402–3406.
- Ahn, J.H.; Cho, H.; Kim, J.-H.; Kim, S.H.; Ham, J.-S.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.-H.; Hong, Y.-K.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66.
- Louveau, A.; Herz, J.; Alme, M.N.; Salvador, A.F.; Dong, M.Q.; Viar, K.E.; Herod, S.G.; Knopp, J.; Setliff, J.C.; Lupi, A.; et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 2018, 21, 1380–1391.
- King, H.H. Lymphatic Vessels Found in the Brain—Osteopathic Considerations. J. Am. Osteopat. Assoc. 2015, 115, 627.
- Dupont, G.; Iwanaga, J.; Yilmaz, E.; Tubbs, R.S. Connections between amyloid beta and the meningeal lymphatics as a possi-ble route for clearance and therapeutics. Lymphat. Res. Biol. 2020, 18, 2–6.
- Wang, X.; Tian, H.; Liu, H.; Liang, D.; Qin, C.; Zhu, Q.; Wang, X. Impaired Meningeal Lymphatic Flow in NMOSD Patients with Acute Attack. Front. Immunol. 2021, 12, 2239.
- Ding, X.-B.; Wang, X.-X.; Xia, D.-H.; Liu, H.; Tian, H.-Y.; Fu, Y.; Chen, Y.-K.; Qin, C.; Wang, J.-Q.; Xiang, Z.; et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat. Med. 2021, 27, 411–418.
- Ghandili, M.; Munakomi, S. Neuroanatomy, Putamen. StatPearls . 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542170/ (accessed on 25 December 2021).
- Tripathi, R. Tracing the bulk outflow route of cerebrospinal fluid by transmission and scanning electron microscopy. Brain Res. 1974, 80, 503–506.
- Kida, S.; Pantazis, A.; Weller, R.O. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol. 1993, 19, 480–488.
- De Leon, M.J.; Li, Y.; Okamura, N.; Tsui, W.H.; Saint-Louis, L.A.; Glodzik, L.; Osorio, R.; Fortea, J.; Butler, T.; Pirraglia, E.; et al. Cerebrospinal Fluid Clearance in Alzheimer Disease Measured with Dynamic PET. J. Nucl. Med. 2017, 58, 1471–1476.
- Ma, Q.; Ries, M.; Decker, Y.; Müller, A.; Riner, C.; Bücker, A.; Fassbender, K.; Detmar, M.; Proulx, S.T. Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol. 2019, 137, 151–165.
- Norwood, J.N.; Zhang, Q.; Card, D.; Craine, A.; Ryan, T.M.; Drew, P.J. Anatomical basis and physiological role of cerebro-spinal fluid transport through the murine cribriform plate. elife 2019, 8, e44278.
- Wostyn, P. COVID-19 and chronic fatigue syndrome: Is the worst yet to come? Med. Hypotheses 2021, 146, 110469.
- Rash, J.E.; Davidson, K.G.V.; Kamasawa, N.; Yasumura, T.; Kamasawa, M.; Zhang, C.; Michaels, R.; Restrepo, D.; Ottersen, O.P.; Olson, C.O.; et al. Ultrastructural localization of connexins (Cx36, Cx43, Cx45), glutamate receptors and aquaporin-4 in rodent olfactory mucosa, olfactory nerve and olfactory bulb. J. Neurocytol. 2005, 34, 307–341.
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341.
- Brady, M.; Rahman, A.; Combs, A.; Venkatraman, C.; Kasper, R.T.; McQuaid, C.; Kwok, W.-C.E.; Wood, R.W.; Deane, R. Cerebrospinal fluid drainage kinetics across the cribriform plate are reduced with aging. Fluids Barriers CNS 2020, 17, 1–16.
- Ma, Q.; Ineichen, B.V.; Detmar, M.; Proulx, S.T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 2017, 8, 1–13.
- Hauglund, N.L.; Pavan, C.; Nedergaard, M. Cleaning the sleeping brain–the potential restorative function of the glymphatic system. Curr. Opin. Physiol. 2020, 15, 1–6.
- Benveniste, H.; Heerdt, P.M.; Fontes, M.; Rothman, D.L.; Volkow, N.D. Glymphatic System Function in Relation to Anesthesia and Sleep States. Anesthesia Analg. 2019, 128, 747–758.
- Fultz, N.E.; Bonmassar, G.; Setsompop, K.; Stickgold, R.A.; Rosen, B.R.; Polimeni, J.R.; Lewis, L.D. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019, 366, 628–631.
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342, 373–377.
- Reddy, O.C.; Van Der Werf, Y.D. The sleeping brain: Harnessing the power of the glymphatic system through lifestyle choic-es. Brain Sci. 2020, 10, 868.
- Semyachkina-Glushkovskaya, O.; Abdurashitov, A.; Dubrovsky, A.; Klimova, M.; Agranovich, I.; Terskov, A.; Shirokov, A.; Vinnik, V.; Kuzmina, A.; Lezhnev, N.; et al. Photobiomodulation of lymphatic drainage and clearance: Perspective strategy for augmentation of meningeal lymphatic functions. Biomed. Opt. Express 2020, 11, 725–734.
- Holth, J.K.; Fritschi, S.K.; Wang, C.; Pedersen, N.P.; Cirrito, J.R.; Mahan, T.E.; Finn, M.B.; Manis, M.; Geerling, J.C.; Fuller, P.M.; et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 2019, 363, 880–884.
- Raggi, A.; Ferri, R. Sleep disorders in neurodegenerative diseases. Eur. J. Neurol. 2010, 17, 1326–1338.
- Cuddapah, V.; Zhang, S.L.; Sehgal, A. Regulation of the Blood–Brain Barrier by Circadian Rhythms and Sleep. Trends Neurosci. 2019, 42, 500–510.
- Yehuda, S.; Sredni, B.; Carasso, R.L.; Kenigsbuch-Sredni, D. REM sleep deprivation in rats results in inflammation and inter-leukin-17 elevation. J. Interferon Cytokine Res. 2009, 29, 393–398.
- Hurtado-Alvarado, G.; Becerril-Villanueva, E.; De Oca, A.C.-M.; Domínguez-Salazar, E.; Salinas-Jazmín, N.; Pérez-Tapia, S.; Pavon, L.; Velázquez-Moctezuma, J.; Gómez-González, B. The yin/yang of inflammatory status: Blood-brain barrier regula-tion during sleep. Brain Behav. Immun. 2018, 69, 154–166.
- Liu, D.-X.; He, X.; Wu, D.; Zhang, Q.; Yang, C.; Liang, F.-Y.; He, X.-F.; Dai, G.-Y.; Pei, Z.; Lan, Y.; et al. Continuous theta burst stimulation facilitates the clearance efficiency of the glymphatic pathway in a mouse model of sleep deprivation. Neurosci. Lett. 2017, 653, 189–194.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.7K
Revisions:
2 times
(View History)
Update Date:
07 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




