Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria do Carmo Pereira | -- | 1317 | 2022-04-06 12:02:57 | | | |
| 2 | Camila Xu | + 3 word(s) | 1320 | 2022-04-07 04:04:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pereira, M.D.C.; Nunes, D.; Andrade, S.; Ramalho, M.J.; Loureiro, J. Nanoparticles-Loaded Hydrogel System. Encyclopedia. Available online: https://encyclopedia.pub/entry/21398 (accessed on 28 February 2026).
Pereira MDC, Nunes D, Andrade S, Ramalho MJ, Loureiro J. Nanoparticles-Loaded Hydrogel System. Encyclopedia. Available at: https://encyclopedia.pub/entry/21398. Accessed February 28, 2026.
Pereira, Maria Do Carmo, Débora Nunes, Stéphanie Andrade, Maria João Ramalho, Joana Loureiro. "Nanoparticles-Loaded Hydrogel System" Encyclopedia, https://encyclopedia.pub/entry/21398 (accessed February 28, 2026).
Pereira, M.D.C., Nunes, D., Andrade, S., Ramalho, M.J., & Loureiro, J. (2022, April 06). Nanoparticles-Loaded Hydrogel System. In Encyclopedia. https://encyclopedia.pub/entry/21398
Pereira, Maria Do Carmo, et al. "Nanoparticles-Loaded Hydrogel System." Encyclopedia. Web. 06 April, 2022.
Copy Citation
Hydrogels are three-dimensional porous structures produced with hydrophilic polymers through physical or chemical cross-linking methods and can be prepared from a wide range of natural and synthetic polymers. Nanoparticles (NPs) are colloidal structures designed and produced to transport drugs across biological barriers. Their optimal size range is approximately between 100 and 200 nm. They protect drugs from degradation, increasing their half-life, improving drugs’ bioavailability, and providing a sustained and localized release.
nanomaterials
polymers
drug release
local delivery
1. Introduction
The physiological barriers of the human body challenge the traditional delivery of drugs, limiting drug access to the desired organs and tissues. Furthermore, the efficacy and retention of drugs in the target tissue are affected by their bioavailability, pharmacokinetic, and pharmacodynamic parameters. These challenges lead to the need for higher dosages and more frequent administrations to reach effective treatment doses, which induces undesirable side effects and toxicity in the other tissues [1].
Over the last years, due to considerable advances in the nanotechnology field, several nanomaterials have been developed as drug delivery systems (DDS). These systems have emerged to overcome the drawbacks of drug administration by improving the drug’s solubility and bioavailability, decreasing drug degradation, and extending the drug’s half-life [2][3]. DDS can provide a specific and targeted therapy, reducing the required dose to achieve a therapeutic effect. DDS capabilities make therapies more accurate, effective, and less invasive by preventing systemic toxicity and unwanted side effects [2][3][4][5]. Among the potential DDS approaches under development, the design of nanoparticles (NPs), hydrogels, and, more recently, NPs-loaded hydrogel (NLH) systems for drug release applications are gaining attention.
The growing advances in the use of nanomaterials led to the need to establish guidelines and regulations for their usage in medicine. Updates in the future are expected to guarantee the quality, effectiveness, and security of these DDS for human use [6]. Besides, scale-up manufacturing is another important condition for clinical use and commercialization of DDS. The clinical application of the DDS faces some concerns regarding their production. When it comes to large-scale production, the procedures should ensure the preservation of the DDS physicochemical characteristics for the desired application since these properties directly affect the efficiency, safety, and drug delivery capabilities of the developed DDS. Simultaneously, the methods must be scalable, reproducible, and cost-effective [7]. Good manufacturing practices verification is required to ensure conformance in large-scale production [6]. In addition, to evaluate whether DDS large-scale production influences clinical performance, comprehensive quality controls of drug carriers are essential [8]. Finally, clinical trials are mandatory to determine the ratio between benefits and risks of the developed DDS [6].
As in vitro studies can only give a partial indication of potential toxicity, in vivo studies are fundamental to evaluate the efficacy and safety of these DDS [4]. In this sense, this entry aims to discuss the most recently developed polymeric NLH systems for biomedical applications focusing on their in vivo performance for different administration routes, including parenteral and topical administration. Polymeric NLH systems integrate NPs into a hydrogel, synergistically combining their individual functionalities and benefits. The resulting system presents increased performance and can be successfully employed as DDS for biomedical applications, including tissue engineering, bacterial applications (e.g., wounds or eye infections), and contact lenses [9][10].
2. Nanoparticles (NPs)-Loaded Hydrogel System
NPs are colloidal structures designed and produced to transport drugs across biological barriers. Their optimal size range is approximately between 100 and 200 nm. They protect drugs from degradation, increasing their half-life, improving drugs’ bioavailability, and providing a sustained and localized release [11]. Moreover, their surface could be modified for targeted delivery, reducing drugs’ toxicity resultant from the systemic spread and, consequently, side effects [12]. Among them, polymeric NPs have been widely chosen as DDS in several biomedical applications due to their high stability, water solubility, biocompatibility, biodegradability, and non-immunogenicity [13][14]. Another advantage of these NPs is their capacity to encapsulate both hydrophilic and hydrophobic drugs [12]. Moreover, their loading capacity, drug release kinetics, and biological performance could be regulated by adjusting their composition or surface charge. However, these NPs are quite predisposed to premature burst release of drugs [11][14], their permanency on the target site until complete drug release is unpredictable [11][15]. When in contact with the biological environment, NPs can present instability or clearance by the immune system [5].
Hydrogels are three-dimensional porous structures produced with hydrophilic polymers through physical or chemical cross-linking methods [16] and can be prepared from a wide range of natural and synthetic polymers. Natural polymers include alginate, chitosan, gelatin, collagen, hydroxypropyl methylcellulose (HPMC), and hyaluronic acid (HA); in contrast, synthetic polymers could be polyacrylamide (PAM), poly(hydroxyethyl methacrylate) (PHEMA), polyvinylpyrrolidone (PVP), poly(vinyl alcohol) (PVA), poly(ethylene glycol) (PEG), and poly-ε-caprolactone (PCL) [17]. Hydrogels are materials with great solute permeability, and a high-water retention capability [9][17][18][19]. Depending on the polymers employed in their production, hydrogels can be biocompatible, biodegradable, and present minimal toxicity. They can encapsulate molecules in an effective amount, protecting and releasing them over time while increasing their local concentration and reducing their toxicity in the remaining tissues [20]. Moreover, hydrogels kinetics can respond to biological, chemical, or physical external stimuli. Biological triggers include antibodies or enzymes; chemical factors comprising pH, type of ions, or organic solvents; and physical stimuli include temperature, light, and magnetic or electrical fields [17]. Thermo-sensitive hydrogels are the most commonly used variety of hydrogels for medical use due to their sol-gel transition behavior at body temperature. While at room temperature hydrogels are in the form of an aqueous suspension that can be easily injected; at body temperature, the solution rapidly transits to a stable gel [17][21]. Their administration is minimally invasive since most of them can be administered without the need for a surgical procedure [22]. Besides, thermo-sensitive hydrogels manage to mold themselves perfectly to the shape of the place where they are administered, creating a drug depot for a localized and sustained release [1]. On the other hand, hydrogels have a few drawbacks limiting their application as DDS. Hydrogels have weak mechanical properties; for example, their mechanical strength decreases after swelling [23]. Drug release from hydrogels depends on their network structure, rearrangement, size, materials employed in their production, and drugs’ physicochemical properties [18]. Most of the time, hydrogels present an initial burst release of drugs when in contact with the release medium due to their high-water content [24][25]. Another problem of hydrogels’ hydrophilic nature is the integration of poorly soluble drugs, which are rapidly released through diffusion [11][26].
Individual limitations of NPs and hydrogels could be addressed with their combination into a single platform (Figure 1). As NPs can be physically or covalently integrated into hydrogels, the development of these DDS has emerged, taking advantage of their benefits synergistically combined in one system [18].
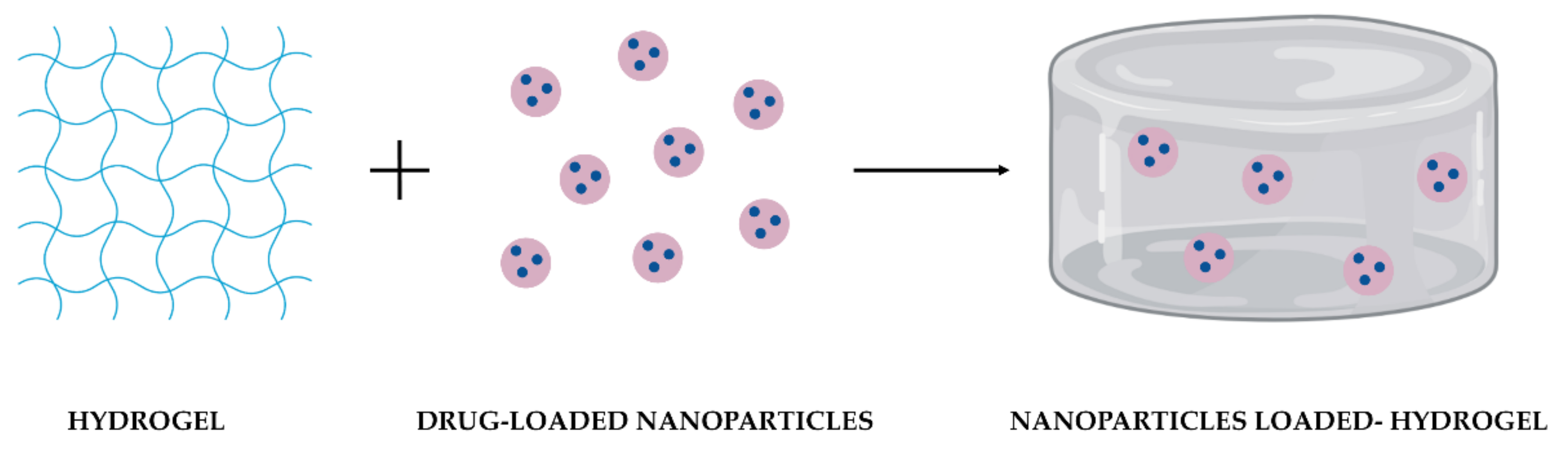 Figure 1. Schematic representation of the combination of drug-loaded NPs and a hydrogel as DDS.
Figure 1. Schematic representation of the combination of drug-loaded NPs and a hydrogel as DDS.Besides the biocompatibility, biodegradability, and non-toxicity of both NPs and hydrogels as individual DDS, their combination provides benefits that none of them could achieve independently (Table 1).
Table 1. Benefits of polymeric NPs, hydrogels, and polymeric NLH as DDS. (+) and (–) indicate advantages and disadvantages, respectively.
| Polymeric NPs | Hydrogel | Polymeric NLH | Refs. | |
|---|---|---|---|---|
| Multiple drug loading | + | + | Improved | [27][12][20] |
| Hydrophobic drugs loading | + | − | Maintained | [11][27][12][26] |
| Controlled and sustained release | + | +/− | Improved | [11][20] |
| Drug bioavailability improvement | + | + | Improved | [11] |
| Targeted drug delivery | + | − | Maintained | [12] |
| Local retention of drug | − | + | Maintained | [1][24][25][28] |
| Stimuli-responsive behavior | + | + | Improved | [29][17][21] |
NPs and hydrogels can both provide multiple drug loading. When combined, the hydrogel protects the NPs from degradation, prevents their aggregation [30], and promotes the local delivery of drugs [24][25]. Incorporating NPs into hydrogels can improve hydrogel mechanical properties, such as strength, stiffness, and degradation, in a concentration-dependent relationship [11]. NPs may also act as a hydrogel crosslinking and fortify the stimuli-responsive behavior of the hydrogel [29]. Although NPs and hydrogels can improve drugs’ bioavailability and release them over time [11], their combination forms a depot at the administration site for prolonged local drug retention [28]. This dual delivery system provides a double encapsulation of drugs, regulates drug release kinetics, and prevents the initial burst release. It can also encapsulate both hydrophobic and hydrophilic drugs [11][27]. These benefits work together to minimize side effects and systemic toxicity [11] and improve the therapeutic effect of treatment and patients’ compliance [24][25].
References
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable thermoresponsive hydrogels as drug delivery system for the treatment of central nervous system disorders: A review. J. Control. Release 2020, 329, 16–35.
- El-Sayed, A.; Kamel, M. Advances in nanomedical applications: Diagnostic, therapeutic, immunization, and vaccine production. Environ. Sci. Pollut. Res. 2019, 27, 19200–19213.
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500.
- Nobile, L.; Nobile, S. Recent advances of nanotechnology in medicine and engineering. AIP Conf. Proc. 2016, 1736, 20058.
- Saxena, S.K.; Nyodu, R.; Kumar, S.; Maurya, V.K. Current Advances in Nanotechnology and Medicine. NanoBioMedicine 2020, 3–16.
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part I—Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU. Pharmaceutics 2020, 12, 146.
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130.
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int. J. Pharm. 2021, 605, 120807.
- Wang, K.; Hao, Y.; Wang, Y.; Chen, J.; Mao, L.; Deng, Y.; Chen, J.; Yuan, S.; Zhang, T.; Ren, J.; et al. Functional Hydrogels and Their Application in Drug Delivery, Biosensors, and Tissue Engineering. Int. J. Polym. Sci. 2019, 2019, 1–14.
- Wahid, F.; Zhao, X.J.; Jia, S.R.; Bai, H.; Zhong, C. Nanocomposite hydrogels as multifunctional systems for biomedical applications: Current state and perspectives. Compos. Part B Eng. 2020, 200, 108208.
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle–hydrogel superstructures for biomedical applications. J. Control. Release 2020, 324, 505–521.
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403.
- Adhikari, C. Polymer nanoparticles-preparations, applications and future insights: A concise review. Polym. Technol. Mater. 2021, 1–29.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124.
- Bernal-Chávez, S.A.; Alcalá-Alcalá, S.; Cerecedo, D.; Ganem-Rondero, A. Platelet lysate-loaded PLGA nanoparticles in a thermo-responsive hydrogel intended for the treatment of wounds. Eur. J. Pharm. Sci. 2020, 146, 105231.
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23.
- Chang, D.; Park, K.; Famili, A. Hydrogels for sustained delivery of biologics to the back of the eye. Drug Discov. Today 2019, 24, 1470–1482.
- Wang, Y.; Li, Q.; Zhou, J.-E.; Tan, J.; Li, M.; Xu, N.; Qu, F.; Chen, J.; Li, J.; Wang, J.; et al. A Photopolymerized Semi-Interpenetrating Polymer Networks-Based Hydrogel Incorporated with Nanoparticle for Local Chemotherapy of Tumors. Pharm. Res. 2021, 38, 669–680.
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. App. Polym. Sci. 2021, 138, 50376.
- Sun, Z.; Wang, X.; Liu, J.; Wang, Z.; Wang, W.; Kong, D.; Leng, X. ICG/l-Arginine Encapsulated PLGA Nanoparticle-Thermosensitive Hydrogel Hybrid Delivery System for Cascade Cancer Photodynamic-NO Therapy with Promoted Collagen Depletion in Tumor Tissues. Mol. Pharm. 2021, 18, 928–939.
- Boffito, M.; Gioffredi, E.; Chiono, V.; Calzone, S.; Ranzato, E.; Martinotti, S.; Ciardelli, G. Novel polyurethane-based thermosensitive hydrogels as drug release and tissue engineering platforms: Design and in vitro characterization. Polym. Int. 2016, 65, 756–769.
- Mellati, A.; Hasanzadeh, E.; Gholipourmalekabadi, M.; Enderami, S.E. Injectable nanocomposite hydrogels as an emerging platform for biomedical applications: A review. Mater. Sci. Eng. C 2021, 131, 112489.
- Welch, V.; Petticrew, M.; Petkovic, J.; Moher, D.; Waters, E.; White, H.; Tugwell, P.; Atun, R.; Awasthi, S.; Barbour, V.; et al. Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): Explanation and elaboration. J. Clin. Epidemiol. 2015, 70, 68–89.
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061.
- Ren, Y.; Li, X.; Han, B.; Zhao, N.; Mu, M.; Wang, C.; Du, Y.; Wang, Y.; Tong, A.; Liu, Y.; et al. Improved anti-colorectal carcinomatosis effect of tannic acid co-loaded with oxaliplatin in nanoparticles encapsulated in thermosensitive hydrogel. Eur. J. Pharm. Sci. 2018, 128, 279–289.
- Cao, D.; Zhang, X.; Akabar, M.D.; Luo, Y.; Wu, H.; Ke, X.; Ci, T. Liposomal doxorubicin loaded PLGA-PEG-PLGA based thermogel for sustained local drug delivery for the treatment of breast cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 181–191.
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid Nanoparticles and Their Hydrogel Composites for Drug Delivery: A Review. Pharmaceuticals 2018, 11, 118.
- Brachi, G.; Ruiz-Ramírez, J.; Dogra, P.; Wang, Z.; Cristini, V.; Ciardelli, G.; Rostomily, R.C.; Ferrari, M.; Mikheev, A.M.; Blanco, E.; et al. Intratumoral injection of hydrogel-embedded nanoparticles enhances retention in glioblastoma. Nanoscale 2020, 12, 23838–23850.
- Biondi, M.; Borzacchiello, A.; Mayol, L.; Ambrosio, L. Nanoparticle-Integrated Hydrogels as Multifunctional Composite Materials for Biomedical Applications. Gels 2015, 1, 162–178.
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
07 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





