Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Maria do Carmo Pereira and Version 2 by Camila Xu.
Hydrogels are three-dimensional porous structures produced with hydrophilic polymers through physical or chemical cross-linking methods [24] and can be prepared from a wide range of natural and synthetic polymers. Nanoparticles (NPs)Ps are colloidal structures designed and produced to transport drugs across biological barriers. Their optimal size range is approximately between 100 and 200 nm. They protect drugs from degradation, increasing their half-life, improving drugs’ bioavailability, and providing a sustained and localized release.
- nanomaterials
- polymers
- drug release
- local delivery
1. Introduction
The physiological barriers of the human body challenge the traditional delivery of drugs, limiting drug access to the desired organs and tissues. Furthermore, the efficacy and retention of drugs in the target tissue are affected by their bioavailability, pharmacokinetic, and pharmacodynamic parameters. These challenges lead to the need for higher dosages and more frequent administrations to reach effective treatment doses, which induces undesirable side effects and toxicity in the other tissues [1].
Over the last years, due to considerable advances in the nanotechnology field, several nanomaterials have been developed as drug delivery systems (DDS). These systems have emerged to overcome the drawbacks of drug administration by improving the drug’s solubility and bioavailability, decreasing drug degradation, and extending the drug’s half-life [2][3][2,3]. DDS can provide a specific and targeted therapy, reducing the required dose to achieve a therapeutic effect. DDS capabilities make therapies more accurate, effective, and less invasive by preventing systemic toxicity and unwanted side effects [2][3][4][5][2,3,4,5]. Among the potential DDS approaches under development, the design of nanoparticles (NPs), hydrogels, and, more recently, NPs-loaded hydrogel (NLH) systems for drug release applications are gaining attention.
The growing advances in the use of nanomaterials led to the need to establish guidelines and regulations for their usage in medicine. Updates in the future are expected to guarantee the quality, effectiveness, and security of these DDS for human use [6]. Besides, scale-up manufacturing is another important condition for clinical use and commercialization of DDS. The clinical application of the DDS faces some concerns regarding their production. When it comes to large-scale production, the procedures should ensure the preservation of the DDS physicochemical characteristics for the desired application since these properties directly affect the efficiency, safety, and drug delivery capabilities of the developed DDS. Simultaneously, the methods must be scalable, reproducible, and cost-effective [7]. Good manufacturing practices verification is required to ensure conformance in large-scale production [6]. In addition, to evaluate whether DDS large-scale production influences clinical performance, comprehensive quality controls of drug carriers are essential [8]. Finally, clinical trials are mandatory to determine the ratio between benefits and risks of the developed DDS [6].
As in vitro studies can only give a partial indication of potential toxicity, in vivo studies are fundamental to evaluate the efficacy and safety of these DDS [4]. In this sense, this revientryw aims to discuss the most recently developed polymeric NLH systems for biomedical applications focusing on their in vivo performance for different administration routes, including parenteral and topical administration. Polymeric NLH systems integrate NPs into a hydrogel, synergistically combining their individual functionalities and benefits. The resulting system presents increased performance and can be successfully employed as DDS for biomedical applications, including tissue engineering, bacterial applications (e.g., wounds or eye infections), and contact lenses [9][10][9,10].
2. Nanoparticles (NPs)-Loaded Hydrogel System
NPs are colloidal structures designed and produced to transport drugs across biological barriers. Their optimal size range is approximately between 100 and 200 nm. They protect drugs from degradation, increasing their half-life, improving drugs’ bioavailability, and providing a sustained and localized release [11][13]. Moreover, their surface could be modified for targeted delivery, reducing drugs’ toxicity resultant from the systemic spread and, consequently, side effects [12][20]. Among them, polymeric NPs have been widely chosen as DDS in several biomedical applications due to their high stability, water solubility, biocompatibility, biodegradability, and non-immunogenicity [13][14][21,22]. Another advantage of these NPs is their capacity to encapsulate both hydrophilic and hydrophobic drugs [12][20]. Moreover, their loading capacity, drug release kinetics, and biological performance could be regulated by adjusting their composition or surface charge. However, these NPs are quite predisposed to premature burst release of drugs [11][14][13,22], their permanency on the target site until complete drug release is unpredictable [11][15][13,23]. When in contact with the biological environment, NPs can present instability or clearance by the immune system [5]. Hydrogels are three-dimensional porous structures produced with hydrophilic polymers through physical or chemical cross-linking methods [16][24] and can be prepared from a wide range of natural and synthetic polymers. Natural polymers include alginate, chitosan, gelatin, collagen, hydroxypropyl methylcellulose (HPMC), and hyaluronic acid (HA); in contrast, synthetic polymers could be polyacrylamide (PAM), poly(hydroxyethyl methacrylate) (PHEMA), polyvinylpyrrolidone (PVP), poly(vinyl alcohol) (PVA), poly(ethylene glycol) (PEG), and poly-ε-caprolactone (PCL) [17][25]. Hydrogels are materials with great solute permeability, and a high-water retention capability [9][17][18][19][9,25,26,27]. Depending on the polymers employed in their production, hydrogels can be biocompatible, biodegradable, and present minimal toxicity. They can encapsulate molecules in an effective amount, protecting and releasing them over time while increasing their local concentration and reducing their toxicity in the remaining tissues [20][28]. Moreover, hydrogels kinetics can respond to biological, chemical, or physical external stimuli. Biological triggers include antibodies or enzymes; chemical factors comprising pH, type of ions, or organic solvents; and physical stimuli include temperature, light, and magnetic or electrical fields [17][25]. Thermo-sensitive hydrogels are the most commonly used variety of hydrogels for medical use due to their sol-gel transition behavior at body temperature. While at room temperature hydrogels are in the form of an aqueous suspension that can be easily injected; at body temperature, the solution rapidly transits to a stable gel [17][21][25,29]. Their administration is minimally invasive since most of them can be administered without the need for a surgical procedure [22][11]. Besides, thermo-sensitive hydrogels manage to mold themselves perfectly to the shape of the place where they are administered, creating a drug depot for a localized and sustained release [1]. On the other hand, hydrogels have a few drawbacks limiting their application as DDS. Hydrogels have weak mechanical properties; for example, their mechanical strength decreases after swelling [23][19]. Drug release from hydrogels depends on their network structure, rearrangement, size, materials employed in their production, and drugs’ physicochemical properties [18][26]. Most of the time, hydrogels present an initial burst release of drugs when in contact with the release medium due to their high-water content [24][25][15,30]. Another problem of hydrogels’ hydrophilic nature is the integration of poorly soluble drugs, which are rapidly released through diffusion [11][26][13,31]. Individual limitations of NPs and hydrogels could be addressed with their combination into a single platform (Figure 12). As NPs can be physically or covalently integrated into hydrogels, the development of these DDS has emerged, taking advantage of their benefits synergistically combined in one system [18][26].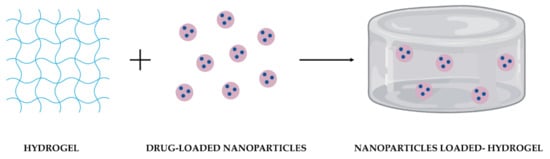
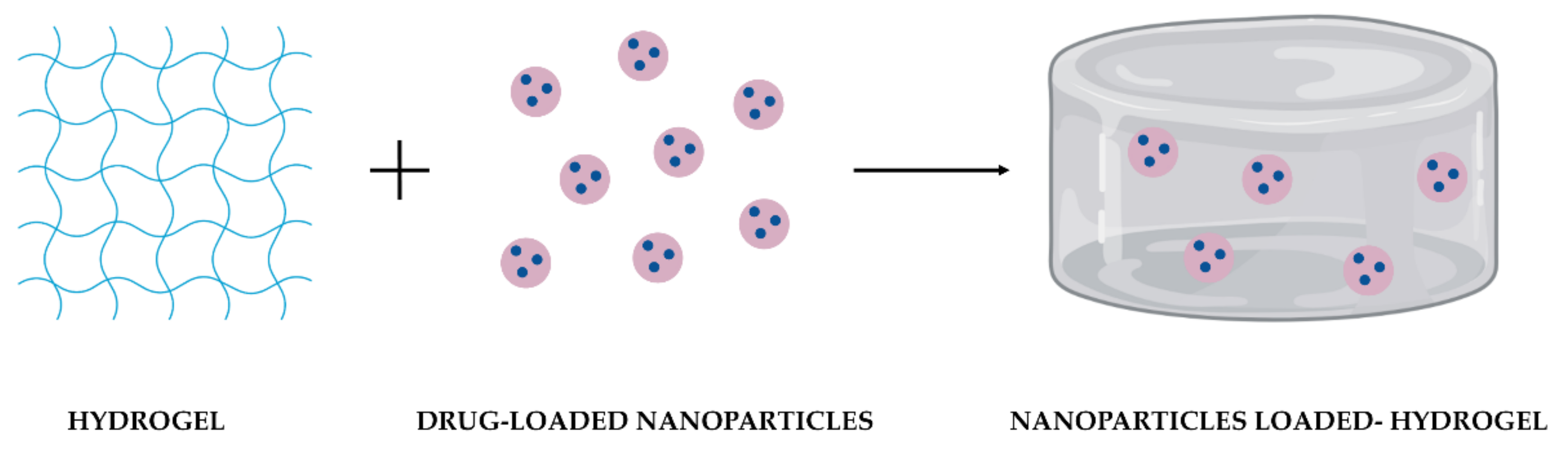
Figure 12. Schematic representation of the combination of drug-loaded NPs and a hydrogel as DDS.
Table 1. Benefits of polymeric NPs, hydrogels, and polymeric NLH as DDS. (+) and (–) indicate advantages and disadvantages, respectively.
| Polymeric NPs | Hydrogel | Polymeric NLH | Refs. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Multiple drug loading | + | + | Improved | [27][12][20] | [17,20,28] | ||||||
| Hydrophobic drugs loading | + | − | Maintained | [11][27][12][26] | [13,17,20,31] | ||||||
| Controlled and sustained release | + | +/− | Improved | [11][20] | [13,28] | ||||||
| Drug bioavailability improvement | + | + | Improved | [11] | [13] | ||||||
| Targeted drug delivery | + | − | Maintained | [12] | [20] | ||||||
| Local retention of drug | − | + | Maintained | [1][24][25] | [1 | [ | ,15 | 28 | ,30 | ] | ,32] |
| Stimuli-responsive behavior | + | + | Improved | [29][17][21] | [12,25,29] |
