
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zahra Sedarat | -- | 1930 | 2022-04-04 20:12:51 | | | |
| 2 | Andrew W. Taylor-Robinson | + 251 word(s) | 2181 | 2022-04-05 07:32:13 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2181 | 2022-04-06 04:00:08 | | |
Video Upload Options
Biofilm is a complicated bacterial structure that was first recognized by the Dutch microscopist Anton Van Leeuwenhoek in dental plaque during the 1670s. Until around 50 years ago, very few studies had been performed on biofilm properties. Following the invention of the electron microscopy, it was revealed that biofilm is a microbial community composed of bacteria that is protected by the barrier of an exopolysaccharide matrix. Within this unique structure, microorganisms possess multicellular behavior that is distinct from that of simple planktonic cells, and they are typically at least 500 times more resistant to antibacterial agents. The enclosed environment is beneficial to bacterial survival for extended periods and is thus considered a self-defense measure to safeguard against unfavorable conditions. This drives chronic infection by opportunistic pathogens in which the bacterial community is resistant to antibacterial agents and to host immunity. For instance, the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in clinical specimens is closely associated with a potent ability to produce biofilm.
1. Introduction
Staphylococcus aureus is the most common cause of biofilm formation of public health relevance and that which is the most studied. This typically commensal Gram-positive bacterium is a leading source of opportunistic infections, including those relating to skin, osteoarticular pathology, endocarditis, and contaminated introduced devices [9]. Medical implants and host tissues can be covered by this bacterium, when the biofilm so formed plays a pivotal role in chronic, difficult-to-treat infections. The principles of biofilm formation by S. aureus, as described here, apply to other pathogenic biofilm-forming bacteria that contribute to the increasing global challenge of antimicrobial resistance. These include, but are not restricted to, Bacillus subtilis, Enterococcus faecalis, Escherichia coli, Helicobacter pylori, Klebsiella pneumoniae, Listeria monocytogenes, Pseudomonas aeruginosa, Salmonella enterica serovars and Streptococcus mutans.
2. Microbial Surface Adhesion
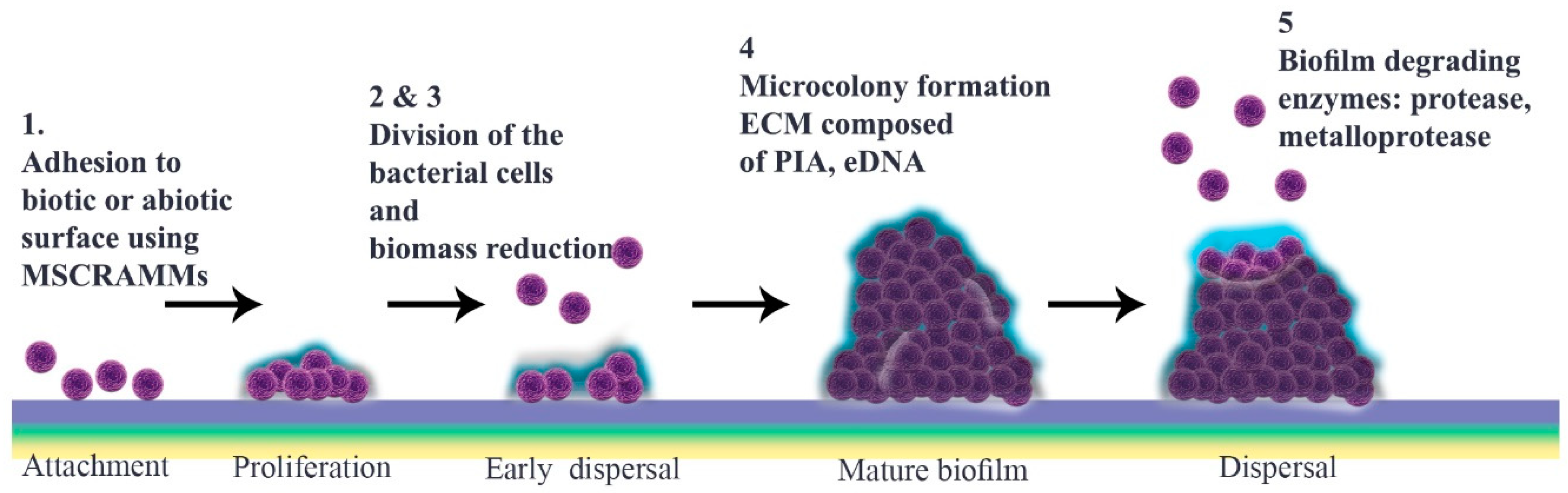
3. Development to Mature Biofilm
4. Detachment
5. Quorum Sensing
References
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890.
- Serra, D.O.; Hengge, R. Stress responses go three dimensional—The spatial order of physiological differentiation in bacterial macrocolony biofilms. Environ. Microbiol. 2014, 16, 1455–1471.
- Fletcher, M.; Loeb, G.I. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl. Environ. Microbiol. 1979, 37, 67–72.
- Bendinger, B.; Rijnaarts, H.H.; Altendorf, K.; Zehnder, A.J. Physicochemical cell surface and adhesive properties of coryneform bacteria related to the presence and chain length of mycolic acids. Appl. Environ. Microbiol. 1993, 59, 3973–3977.
- Power, P.M.; Jennings, M.P. The genetics of glycosylation in Gram-negative bacteria. FEMS Microbiol. Lett. 2003, 218, 211–222.
- Costerton, J.W.; Cheng, K.-J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987, 41, 435–464.
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108.
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633.
- Otto, M. Bacterial evasion of antimicrobial peptides by biofilm formation. Curr. Top. Microbiol. Immunol. 2006, 306, 251–258.
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.; Kathju, S.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007.
- Taylor, P.K.; Yeung, A.T.Y.; Hancock, R.E.W. Antibiotic resistance in Pseudomonas aeruginosa biofilms: Towards the development of novel anti-biofilm therapies. J. Biotechnol. 2014, 191, 121–130.
- Otto, M. Staphylococcal biofilms. Microbiol. Spectr. 2018, 6.
- McCarty, S.; Woods, E.; Percival, S.L. Biofilms: From concept to reality. In Biofilms in Infection Prevention and Control; Percival, S.L., Randle, J., Cooper, T., Williams, D.W., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 143–163.
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, pathogenesis and prevention—A journey to break the wall: A review. Arch. Microbiol. 2016, 198, 1–15.
- Navarre, W.W.; Schneewind, O. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol. Microbiol. 1994, 14, 115–121.
- O’Neill, E.; Pozzi, C.; Houston, P.; Smyth, D.; Humphreys, H.; Robinson, D.A.; O’Gara, J.P. Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J. Clin. Microbiol. 2007, 45, 1379–1388.
- Corrigan, R.M.; Miajlovic, H.; Foster, T.J. Surface proteins that promote adherence of Staphylococcus aureus to human desquamated nasal epithelial cells. BMC Microbiol. 2009, 9, 22.
- McDevitt, D.; Francois, P.; Vaudaux, P.; Foster, T.J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 1994, 11, 237–248.
- Zong, Y.; Xu, Y.; Liang, X.; Keene, D.R.; Höök, A.; Gurusiddappa, S.; Höök, M.; Narayana, S.V. A ‘Collagen Hug’ model for Staphylococcus aureus CNA binding to collagen. EMBO J. 2005, 24, 4224–4236.
- Nguyen, T.; Ghebrehiwet, B.; Peerschke, E.I. Staphylococcus aureus protein A recognizes platelet gC1qR/p33: A novel mechanism for staphylococcal interactions with platelets. Infect. Immun. 2000, 68, 2061–2068.
- Cucarella, C.; Solano, C.; Valle, J.; Amorena, B.; Lasa, I.; Penadés, J.R. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 2001, 183, 2888–2896.
- Corrigan, R.M.; Rigby, D.; Handley, P.; Foster, T.J. The role of Staphylococcus aureus surface protein SasG in adherence and biofilm formation. Microbiology 2007, 153, 2435–2446.
- Kennedy, C.A.; O’Gara, J.P. Contribution of culture media and chemical properties of polystyrene tissue culture plates to biofilm development by Staphylococcus aureus. J. Med. Microbiol. 2004, 53, 1171–1173.
- Gross, M.; Cramton, S.E.; Götz, F.; Peschel, A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 2001, 69, 3423–3426.
- Biswas, R.; Voggu, L.; Simon, U.K.; Hentschel, P.; Thumm, G.; Götz, F. Activity of the major staphylococcal autolysin Atl. FEMS Microbiol. Lett. 2006, 259, 260–268.
- Speziale, P.; Pietrocola, G.; Foster, T.J.; Geoghegan, J.A. Protein-based biofilm matrices in staphylococci. Front. Cell. Infect. Microbiol. 2014, 4, 171.
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376.
- Mann, E.E.; Rice, K.C.; Boles, B.R.; Endres, J.L.; Ranjit, D.; Chandramohan, L.; Tsang, L.H.; Smeltzer, M.S.; Horswill, A.R.; Bayles, K.W. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS ONE 2009, 4, e5822.
- Kiedrowski, M.R.; Kavanaugh, J.S.; Malone, C.L.; Mootz, J.M.; Voyich, J.M.; Smeltzer, M.S.; Bayles, K.W.; Horswill, A.R. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS ONE 2011, 6, e26714.
- Ma, L.; Conover, M.; Lu, H.; Parsek, M.R.; Bayles, K.; Wozniak, D.J. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 2009, 5, e1000354.
- Rohde, H.; Burandt, E.C.; Siemssen, N.; Frommelt, L.; Burdelski, C.; Wurster, S.; Scherpe, S.; Davies, A.P.; Harris, L.G.; Horstkotte, M.A.; et al. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials 2007, 28, 1711–1720.
- Pozzi, C.; Waters, E.M.; Rudkin, J.K.; Schaeffer, C.R.; Lohan, A.J.; Tong, P.; Loftus, B.J.; Pier, G.B.; Fey, P.D.; Massey, R.C.; et al. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog. 2012, 8, e1002626.
- Wireman, J.W.; Dworkin, M. Developmentally induced autolysis during fruiting body formation by Myxococcus xanthus. J. Bacteriol. 1977, 129, 798–802.
- Sugimoto, S.; Sato, F.; Miyakawa, R.; Chiba, A.; Onodera, S.; Hori, S.; Mizunoe, Y. Broad impact of extracellular DNA on biofilm formation by clinically isolated methicillin-resistant and -sensitive strains of Staphylococcus aureus. Sci. Rep. 2018, 8, 2254.
- Mack, D.; Fischer, W.; Krokotsch, A.; Leopold, K.; Hartmann, R.; Egge, H.; Laufs, R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: Purification and structural analysis. J. Bacteriol. 1996, 178, 175–183.
- Vuong, C.; Kocianova, S.; Voyich, J.M.; Yao, Y.; Fischer, E.R.; DeLeo, F.R.; Otto, M. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J. Biol. Chem. 2004, 279, 54881–54886.
- Fitzpatrick, F.; Humphreys, H.; O’Gara, J.P. Evidence for icaADBC-independent biofilm development mechanism in methicillin-resistant Staphylococcus aureus clinical isolates. J. Clin. Microbiol. 2005, 43, 1973–1976.
- Brooks, J.L.; Jefferson, K.K. Phase variation of poly-N-acetylglucosamine expression in Staphylococcus aureus. PLoS Pathog. 2014, 10, e1004292.
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79.
- Periasamy, S.; Joo, H.-S.; Duong, A.C.; Bach, T.-H.L.; Tan, V.Y.; Chatterjee, S.S.; Cheung, G.Y.; Otto, M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1281–1286.
- Moormeier, D.E.; Endres, J.L.; Mann, E.E.; Sadykov, M.R.; Horswill, A.R.; Rice, K.C.; Fey, P.D.; Bayles, K.W. Use of microfluidic technology to analyze gene expression during Staphylococcus aureus biofilm formation reveals distinct physiological niches. Appl. Environ. Microbiol. 2013, 79, 3413–3424.
- Simões, M.; Simões, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. LWT Food Sci. Technol. 2010, 43, 573–583.
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210.
- Flemming, H.-C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947.
- Liaqat, I.; Liaqat, M.; Ali, S.; Ali, N.M.; Haneef, U.; Mirza, S.M.; Tahir, H.M. Biofilm formation, maturation and prevention: A review. J. Bacteriol. Mycol. 2019, 6, 1092.
- Lewis, K. Persister cells. Annu. Rev. Microbiol. 2010, 64, 357–372.
- Ferriol-González, C.; Domingo-Calap, P. Phages for biofilm removal. Antibiotics 2020, 9, 268.
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008, 4, e1000213.
- Van Gennip, M.; Christensen, L.D.; Alhede, M.; Phipps, R.; Jensen, P.Ø.; Christophersen, L.; Pamp, S.J.; Moser, C.; Mikkelsen, P.J.; Koh, A.Y.; et al. Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. APMIS 2009, 117, 537–546.
- Harro, J.M.; Peters, B.M.; O’May, G.A.; Archer, N.; Kerns, P.; Prabhakara, R.; Shirtliff, M.E. Vaccine development in Staphylococcus aureus: Taking the biofilm phenotype into consideration. FEMS Immunol. Med. Microbiol. 2010, 59, 306–323.
- Raafat, D.; Otto, M.; Reppschläger, K.; Iqbal, J.; Holtfreter, S. Fighting Staphylococcus aureus biofilms with monoclonal antibodies. Trends Microbiol. 2019, 27, 303–322.
- Hong, Q.; Huo, S.; Tang, H.; Qu, X.; Yue, B. Smart nanomaterials for treatment of biofilm in orthopedic implants. Front. Bioeng. Biotechnol. 2021, 9, 694635.
- Kathju, S.; Nistico, L.; Tower, I.; Lasko, L.A.; Stoodley, P. Bacterial biofilms on implanted suture material are a cause of surgical site infection. Surg. Infect. 2014, 15, 592–600.
- Post, V.; Wahl, P.; Richards, R.G.; Moriarty, T.F. Vancomycin displays time-dependent eradication of mature Staphylococcus aureus biofilms. J. Orthop. Res. 2017, 35, 381–388.
- Ibberson, C.B.; Parlet, C.P.; Kwiecinski, J.; Crosby, H.A.; Meyerholz, D.K.; Horswill, A.R. Hyaluronan modulation impacts Staphylococcus aureus biofilm infection. Infect. Immun. 2016, 84, 1917–1929.
- Urish, K.L.; DeMuth, P.W.; Craft, D.W.; Haider, H.; Davis, C.M., 3rd. Pulse lavage is inadequate at removal of biofilm from the surface of total knee arthroplasty materials. J. Arthroplast. 2014, 29, 1128–1132.
- Donelli, G.; Francolini, I.; Romoli, D.; Guaglianone, E.; Piozzi, A.; Ragunath, C.; Kaplan, J.B. Synergistic activity of dispersin B and cefamandole nafate in inhibition of staphylococcal biofilm growth on polyurethanes. Antimicrob. Agents Chemother. 2007, 51, 2733–2740.
- Gilbert, P.; Evans, D.J.; Brown, M.R. Formation and dispersal of bacterial biofilms in vivo and in situ. J. Appl. Bacteriol. 1993, 74 (Suppl. S22), 67S–78S.
- Otto, M. Staphylococcus Epidermidis—The ‘Accidental’ Pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567.
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487.
- Tang, J.; Zhou, R.; Shi, X.; Kang, M.; Wang, H.; Chen, H. Two thermostable nucleases coexisted in Staphylococcus aureus: Evidence from mutagenesis and in vitro expression. FEMS Microbiol. Lett. 2008, 284, 176–183.
- Boles, B.R.; Horswill, A.R. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008, 4, e1000052.
- Yarwood, J.M.; Bartels, D.J.; Volper, E.M.; Greenberg, E.P. Quorum sensing in Staphylococcus aureus biofilms. J. Bacteriol. 2004, 186, 1838–1850.
- Thoendel, M.; Kavanaugh, J.S.; Flack, C.E.; Horswill, A.R. Peptide signaling in the staphylococci. Chem. Rev. 2011, 111, 117–151.
- Peschel, A.; Otto, M. Phenol-soluble modulins and staphylococcal infection. Nat. Rev. Microbiol. 2013, 11, 667–673.
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 178.
- Xie, H.; Cook, G.S.; Costerton, J.W.; Bruce, G.; Rose, T.M.; Lamont, R.J. Intergeneric communication in dental plaque biofilms. J. Bacteriol. 2000, 182, 7067–7069.
- Vuong, C.; Saenz, H.L.; Götz, F.; Otto, M. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J. Infect. Dis. 2000, 182, 1688–1693.
- Kavanaugh, J.S.; Horswill, A.R. Impact of environmental cues on staphylococcal quorum sensing and biofilm development. J. Biol. Chem. 2016, 291, 12556–12564.
- Reen, F.J.; Gutiérrez-Barranquero, J.A.; Parages, M.L.; O’Garra, F. Coumarin: A novel player in microbial quorum sensing and biofilm formation inhibition. Appl. Microbiol. Biotechnol. 2018, 102, 2063–2073.
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199.
- Reading, N.C.; Sperandio, V. Quorum sensing: The many languages of bacteria. FEMS Microbiol. Lett. 2006, 254, 1–11.
- Banat, I.M.; De Rienzo, M.A.; Quinn, G.A. Microbial biofilms: Biosurfactants as antibiofilm agents. Appl. Microbiol. Biotechnol. 2014, 98, 9915–9929.
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.H.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-independent target gene control by the agr quorum-sensing system: Insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158.




