3. Development to Mature Biofilm
What are the main features required for a biofilm to mature? Prior to microcolony formation,
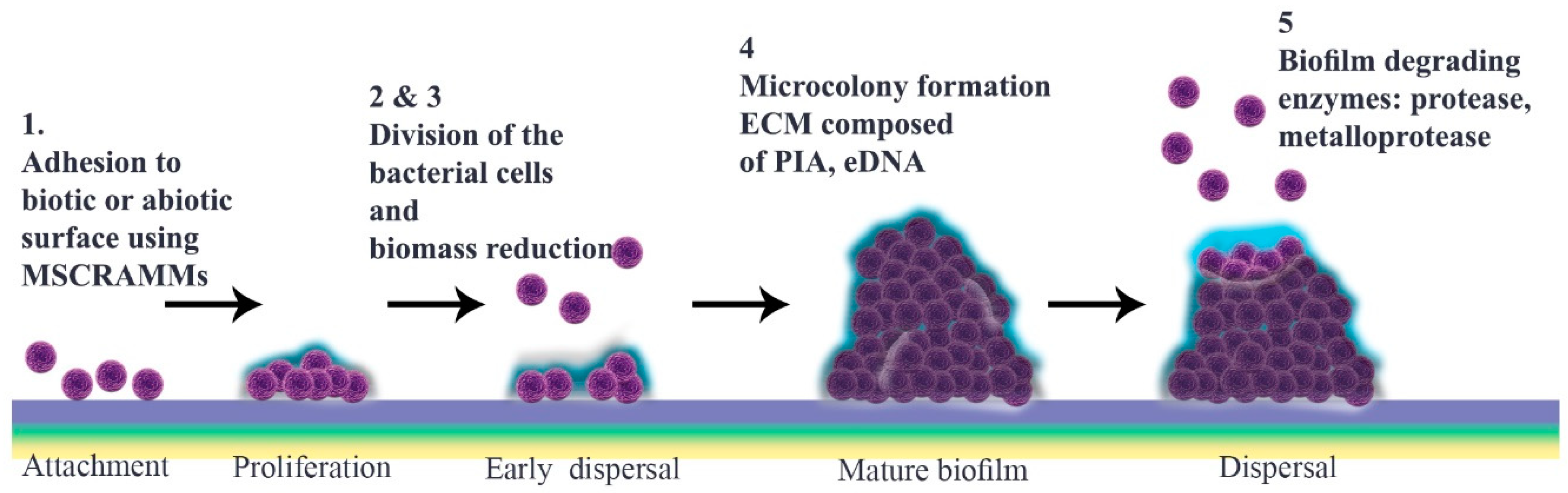
S. aureus cell attachment is followed by a dispersal stage that is independent of final detachment. The initial proliferation that takes place during biofilm formation requires strengthening intercellular binding, which involves various virulence factors including MSCRAMMs, SasG, Bap and protein A. Once cells multiply, they start to disseminate, a stage that is defined as “early dispersal”, in order to restructure the biofilm. This process is aided by nucleases. Microcolonies, which are characteristic of “mature biofilm”, form only after biomass reduction. Bacterial vulnerability results in multiplication as a strategy to enhance cell interactions prior to the “exodus” stage, the role of which is not yet elucidated
[56,57,58,59,60,61,62][27][28][29][30][31][32][33].
The most notable structural component of biofilm is ECM, in which bacterial cells embed. This comprises polymeric molecules secreted from daughter cells composed of proteins, polysaccharide-intercellular adhesins (PIA) and/or extracellular DNA (eDNA). During multiplication, cells are protease-sensitive, indicative of the fact that ECM is composed mostly of protein components like those that bind to eDNA to stabilize this “early biofilm”. These proteins are degraded by nuclease enzymes secreted from bacterial cells at the early dispersal stage. At this time, a protein/DNA-based ECM is predominant
[56,63][27][34].
Among polymeric molecules involved in ECM, PIA (also known as poly-N-acetylglucosamine; PNAG), is characteristic of
Staphylococcus and has a cationic nature that can facilitate attachment
[57,59,64][28][30][35]. Enzymes encoded by the ica operon catalyze production of PIA. While this operon exists in most
S. aureus isolates, its expression is affected by levels of glucose, anaerobicity, osmotic stress and CO
2. PIA increases biofilm retention and its resistance to antimicrobial peptides (AMPs) through deacetylation. Additionally, it is believed that the ica operon is under phase variation, which has a role in slipped strand mispairing and leads to an on/off switch for expression of the products
[65,66,67][36][37][38].
In the subsequent stage, a three-dimensional “mature biofilm” forms. This has two towers either side of a central channel
[68][39]. Different models are described for “microcolony” formation, which is a cue for maturation. Mature biofilm has a diverse and metabolically distinct structure that makes it resistant to unwanted environmental and stressful drivers. Interestingly, distinct gene patterns are responsible for coding these microcolonies at different rates
[57,69,70][28][40][41]. EPS, which contain several components including polysaccharides, glycolipids, protein, glycoproteins, PIA and eDNA, are thought to constitute around 90% of the microcolony structure
[71,72,73][42][43][44]. Inside this, not only can bacteria exchange nutrients and waste but they can also be dispersed over far distances
[34,72][7][43]. Through phenol-soluble modulin (PSM)-mediated dispersal, alpha-helical peptides can break up channels from thick biofilm cells or from those cells belonging to different foci in the basal layer that have remained after the so-called ‘exodus’
[69][40] (
Figure 1).
Treatment of an infected individual during this mature stage is extremely challenging, as it is the most stable form of biofilm
[74][45]. It presents several recognized barriers to the effective action of antibiotics. The EPS matrix can reduce antibiotic efficiency by providing an obstacle to diffusion and a storage for enzymes. This natural defense can lessen phage recognition that depends on EPS. This is an important consideration when determining treatment targets. Similarly, eDNA can diminish antibiotic performance by bolstering the cellular structure. Quorum sensing, a distinctive feature of biofilm, controls production of virulence factors and thereby promotes antimicrobial resistance. Persister cells offer another potential therapeutic target
[23,26,75,76][46][47][48][49]. Antibodies can either target MSCRAMMs to prevent attachment, or cover host cell surfaces to heighten clearance of bacteria. Regarding vaccine design against biofilm-producing bacteria, PIA is a potential target
[77][50]. ClfA, ClfB, FnBPA and FnBPB are also good candidate antigens as their expression is ubiquitous among
S. aureus strains and each participates in biofilm formation
[78,79][51][52].
Physical removal by surgery and debridement for currently embedded bacteria, antibiotic regimens and application of ECM-degrading enzymes are notable therapies
[80,81,82][53][54][55]. Although justified experimentally, these methods are not entirely practical to translate to large-scale clinical use. In some cases, antibiotic therapy should follow physical approaches to enhance efficacy because bacteria embedded in biofilm are more resistant than planktonic cells
[83,84][56][57].
4. Detachment
There are several proposals to explain how biofilm is dispersed (
Figure 1), including isolating new cells from growing ones, reducing biofilm mass, quorum sensing and triggering by insufficient nutrient levels
[85][58]. It is thought that matrix composition determines physical forces that can propel this stage through erosion, sloughing or abrasion
[8][1]. Different enzymes, typically proteases, can weaken protein-dependent biofilm and thereby facilitate its degradation.
S. aureus and
S. epidermidis produce various proteases including serine/cysteine protease and metalloprotease
[86][59]. Similarly, nuclease and nuclease 2 (NUC and NUC2) play important roles by disrupting neutrophils and altering biofilm formation as well as targeting eDNA in the matrix
[58,87,88][29][60][61]. Furthermore, P3 promoter expression in accessory gene regulator (
agr) quorum sensing has a function in detachment of cells, which can initiate dispersal by autoinducer peptide addition or through glucose depletion. Production of proteases is under the control of the
agr quorum sensing system, following activation of which autoinducing peptides (AIPs) are detectable, implicating the next stage as dispersal
[89,90,91,92][62][63][64][65].
Regarding treatment, antibiotic efficacies have increased by using enzymes as dispersal agents. In terms of prevention, utilizing dispersal agents for pretreatment of medical devices not only suppresses proliferation, but also facilitates biofilm purgation. While these appear to be promising therapeutic advances, some concerns have been expressed. For instance, a chronic infection occurs if the administered dose of some antibiotics is unable to permeate the biofilm as sub-inhibitory concentrations can drive
agr activation or eDNA release. Moreover, embolism as a consequence of degrading matrix components is a possible adverse reaction
[11][66].
5. Quorum Sensing
Quorum sensing plays a substantial role during different stages of biofilm formation including attachment and detachment. This cell-to-cell signaling is under the control of
agr quorum sensing or an accessory gene regulator
[69,93,94,95][40][67][68][69]. There are four loci, namely
agr D,
agr B,
agr C and agr A, which encode the central system and between each of which there is a close relationship.
S. aureus has only one copy of each locus, but this is not proven for other species. Via this system, bacteria communicate by producing hormone-like AIPs. Once the rate of signal generation reaches a threshold level signal transduction is activated. The fluctuation in cell density provides the main stimulus for this gene regulation. Bacterial AIPs are responsible for such key activities as biofilm formation, antibiotic resistance, conjugation, and virulence. Therefore, quorum sensing is considered a potential target for therapy and infection control
[95,96][69][70]. Different types of quorum sensing are used by Gram-positive and Gram-negative bacteria or are common to both
[97,98][71][72].
Although results from
in vitro studies are not altogether consistent the consensus view is that quorum sensing is a requirement for biofilm formation and that detachment is controlled at the AIP level. Not only does the
agr system propel detachment of
S. aureus by adding AIP or glucose to a mature biofilm, it is also necessary to suppress biofilm. In one study, 78% of
S. aureus that formed biofilm was
agr-negative. Such findings strengthen the argument that the quorum sensing system may be harnessed as a biofilm blocker. Moreover, it seems that proteases, an important propeller in biofilm dispersal, are under
agr regulation
[89,90,94,99,100][62][63][68][73][74].