Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Olga Kamanina | -- | 2905 | 2022-04-04 15:48:22 | | | |
| 2 | Camila Xu | Meta information modification | 2905 | 2022-04-06 04:42:55 | | | | |
| 3 | Camila Xu | -1 word(s) | 2904 | 2022-04-06 04:45:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kamanina, O.; Ananikov, V.; , .; Arlyapov, V.; Vereshchagin, A. Hybrid Sol-Gel Materials. Encyclopedia. Available online: https://encyclopedia.pub/entry/21354 (accessed on 03 March 2026).
Kamanina O, Ananikov V, , Arlyapov V, Vereshchagin A. Hybrid Sol-Gel Materials. Encyclopedia. Available at: https://encyclopedia.pub/entry/21354. Accessed March 03, 2026.
Kamanina, Olga, Valentine Ananikov, , Vyacheslav Arlyapov, Anatoly Vereshchagin. "Hybrid Sol-Gel Materials" Encyclopedia, https://encyclopedia.pub/entry/21354 (accessed March 03, 2026).
Kamanina, O., Ananikov, V., , ., Arlyapov, V., & Vereshchagin, A. (2022, April 04). Hybrid Sol-Gel Materials. In Encyclopedia. https://encyclopedia.pub/entry/21354
Kamanina, Olga, et al. "Hybrid Sol-Gel Materials." Encyclopedia. Web. 04 April, 2022.
Copy Citation
Microorganism-cell-based biohybrid materials have attracted considerable attention over the last several decades. They are applied in a broad spectrum of areas, such as nanotechnologies, environmental biotechnology, biomedicine, synthetic chemistry, and bioelectronics. Sol-gel technology allows to obtain a wide range of high-purity materials from nanopowders to thin-film coatings with high efficiency and low cost, which makes it one of the preferred techniques for creating organic-inorganic matrices for biocomponent immobilization.
nanotechnologies
sol-gel
biohybrid
1. Introduction
Over the last few years, the field of creating new hybrid materials has attracted great attention [1][2][3][4][5][6]. Of particular focus are biohybrid materials based on microbial cells. Within this general direction, material scientists study microorganism adaptation strategies to environmental changes. Due to these strategies, microbes can survive even under extremely tough conditions. Biomaterial encapsulation hinders the rapid removal of microorganisms and often their inactivation. Inspired by the versatility and strength of such biomaterials, scientists have developed hybrid materials for application in various areas, from agriculture and (environmental) biotechnology [7], biomedicine, and electrical engineering [8] to food production, synthetic chemistry, and bioelectronics [9][10].
Various approaches and methods are used to create hybrid materials, one of which is the sol-gel process, which allows porous materials to be obtained by converting sol to gel. The most common method of sol-gel synthesis is based on the controlled hydrolysis of alkoxides of silicon, aluminum, and transition metal M(OR)x (such as titanium, zirconium, tungsten, zinc, etc. (Figure 1)) and further polycondensation with the formation of oxoalkoxide derivatives, as described in detail [11].
The stage of condensed form generation during the hydrolysis of precursors determines the structure and morphology of the final products and is extremely important when forming sol-gel materials with desired characteristics. The structure of the forming sol-gel matrices depends on a large number of different factors, such as the presence or absence of substances with nonhydrolyzable MC bonds in the precursors, their concentration and ratio, the pH of the medium, acidic or basic catalyst, the presence of organic components, water-soluble polymers, and microorganism cells in the system [12][13].
The microstructure of the material produced by the sol-gel process depends on hydrolysis and condensation reactions, which are generally controlled by the pH of the solution. When using acid catalysis, hydrolysis proceeds faster than condensation, which usually begins when hydrolysis is completed [16][17]. Under basic catalysis conditions, condensation proceeds faster than hydrolysis, which leads to the formation of highly condensed species [18][19]. The hydrolysis rate of silicon alkoxides is minimal at pH = 7 and increases exponentially both at lower and higher pH values. This contrasts with the condensation rate, which is minimal at pH = 2 and peaks at approximately pH = 7 [20]. By varying the catalyst, it becomes possible to repeatedly influence the gelation time, porosity, density, and volumetric shrinkage during the drying process. The rate of sol-gel processes and the environmental pH directly influence the efficiency of biomaterial immobilization and its catalytic activity after immobilization. The formation of sol-gel matrices around microorganisms is also possible with irreversible transformation of the dispersion of colloidal SiO2 nanosols as a result of the sol-gel transition during freezing (Figure 2). It is important to take this into account when the materials are developed with desired properties. The use of microorganisms in combination with structures synthesized by the sol-gel technique makes it possible to use hybrid materials in medicine, ecology, materials science, and biotechnology.
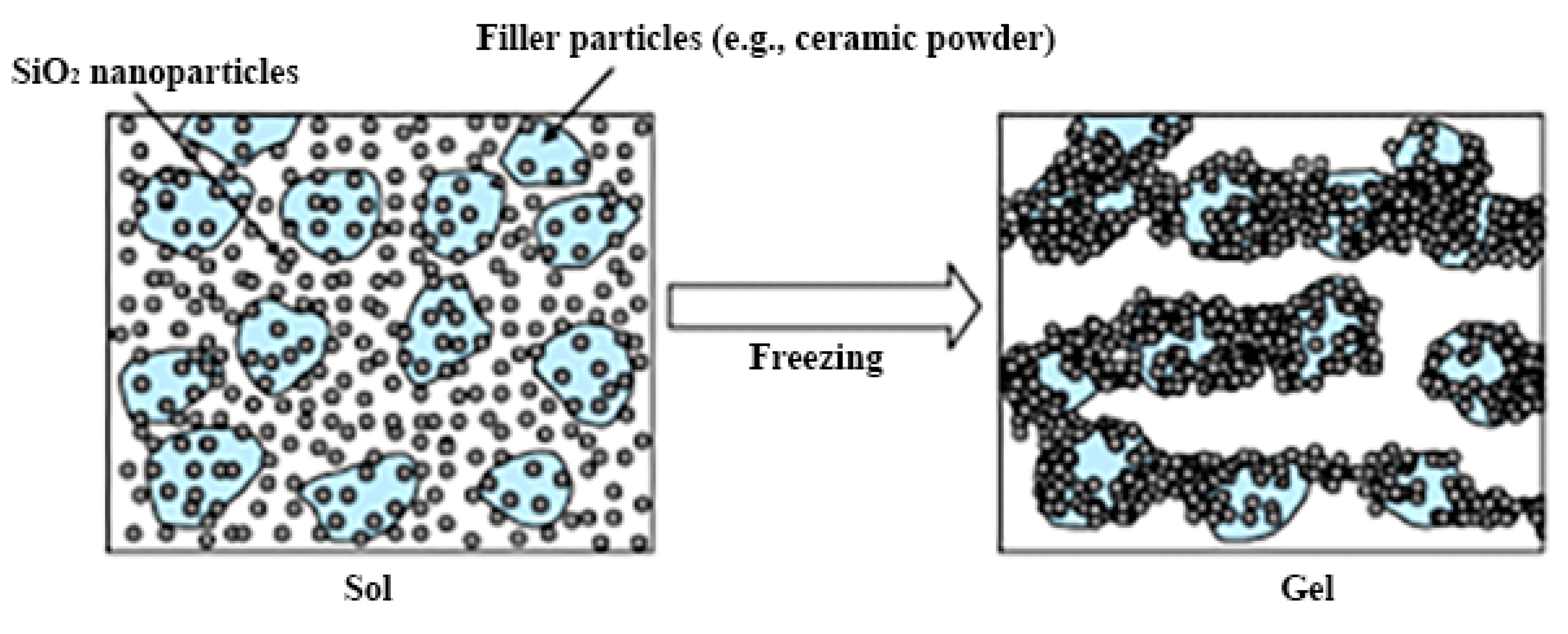 Figure 2. A schematic representation of the colloidal SiO2 nanosol dispersion irreversible transformation by the sol–gel transition caused by freezing. Reprinted with permission from [21].
Figure 2. A schematic representation of the colloidal SiO2 nanosol dispersion irreversible transformation by the sol–gel transition caused by freezing. Reprinted with permission from [21].In the last thirty years, there has been a gradual increase in the complexity of sol-gel processes for the immobilization of microorganisms of various taxonomic groups. This makes it possible to develop new application areas for sol-gel materials obtained by encapsulating microorganisms.
2. The Classification of Hybrid Materials According to the Type of Immobilized Cells
2.1. Material Formation Procedure Optimization
For various fields of chemistry, biotechnology, or medicine, it is most advantageous to utilize living cells immobilized in a stable matrix as biocatalysts. This ensures the effective use of their physiological characteristics for obtaining secondary metabolites or in biotransformation. For industrial application and design of sensors based on whole cells, it is necessary to create their high density in a sufficiently small volume of matter, which can be achieved by encapsulating/incorporating cells into polymer matrices. Polymers containing both inorganic elements and organic components play an important role in the development of encapsulation techniques. Immobilization in such matrices makes it possible to achieve the highest efficiency biocatalysts, which is promising for their practical application in biotechnology.
As a result of the encapsulation process, living microorganism cells are surrounded by the formed silica shells, and the “cell in shell” structure has formed. Because the cells are limited in space, their growth occurs; therefore, the characteristics of the hybrid materials do not change [22][23].
As a result of a two-stage sol-gel process, alcohol toxic to cells is released during hydrolysis. Mild synthesis conditions under which alcohol was removed from the system in the first stage under vacuum on a rotary evaporator [24] or with a gas flow were developed to reduce toxicity and increase biocompatibility. The toxic effects of alcohol can also be eliminated using aqueous precursors such as sodium silicate and colloidal silicon dioxide [25].
Reducing the toxic effects of both acids and alcohol on cells can be achieved using freeze drying. This process consists of freezing the cell suspension with ceramic powder and subsequent lyophilization. The addition of nutrients and cryoprotectants to the system while running the process at the optimum cooling rate improves cell viability. For example, the survival of Rhodococcus ruber was increased from 0.9% to 6.1% by the addition of trehalose solution [26] (Figure 3).
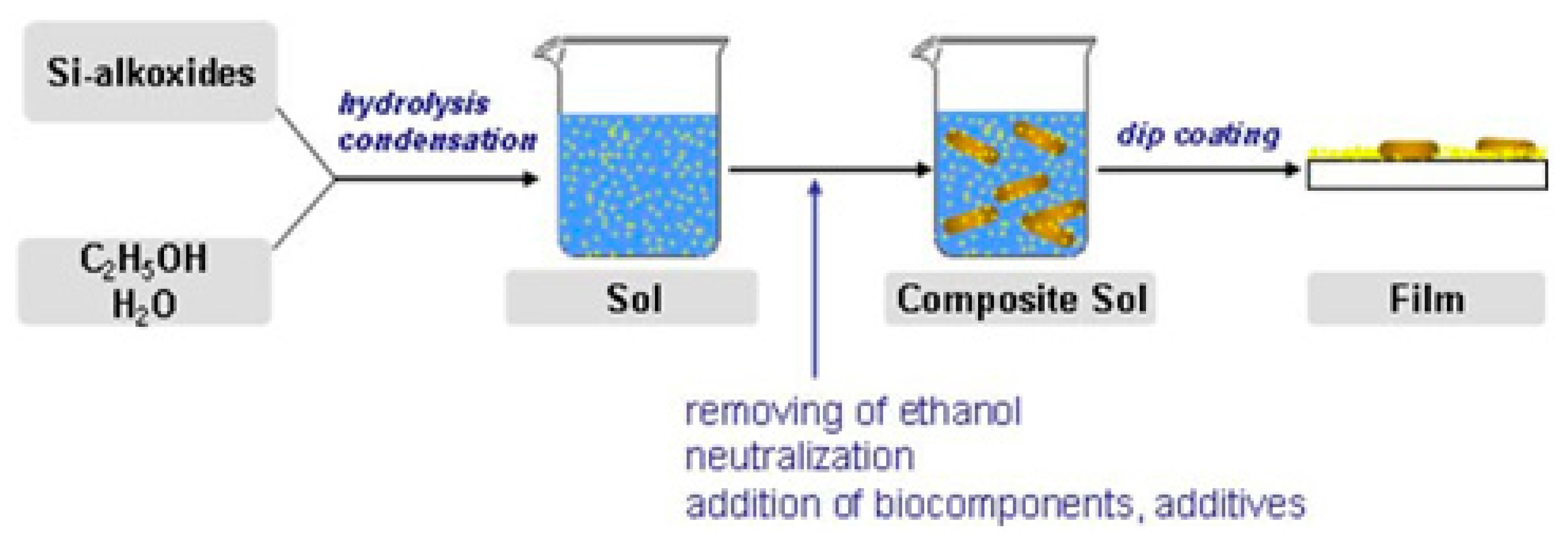 Figure 3. Preparation of silica layers with embedded microorganisms. Reprinted with permission from [26].
Figure 3. Preparation of silica layers with embedded microorganisms. Reprinted with permission from [26].The traditional sol-gel process can be improved by introducing additives such as glycerol, glucose, and other sugars, or natural or synthetic polymers into the system to increase cell survival. They tend to increase the long-term stability of cells, as shown in the case of glycerol [27] and glucose [28]. Simultaneously, the immobilization process remains the same. These additives reduce the transparency of the matrix, which is important in the development of optical sensors.
With the exception of silica-based gels, microorganisms were encapsulated in oxide matrices of alumina, magnetite, titanium oxide, and zirconium [29][30][31][32][33]. Aqueous titanium and zirconium gels and their use for the encapsulation of microorganisms have been described [28][30][34][35][36]. Sols based on metal alkoxide were stabilized by the self-assembly of hydrophilic ligands, which ensured the formation of colorless, transparent aqueous sols. Encapsulated microorganisms were coated with a hydrated oxide and additionally included in the pores of the gel.
2.2. Immobilization of Bacteria by the Sol-Gel Method
The immobilization of bacteria in a sol-gel matrix leads to stabilization of the catalytic activity and makes it possible to repeatedly or continuously use the biocatalyst. The integration of microorganisms into sol-gel structures removes many limitations that arise during the working with free cell systems [21][37][38][39].
Over the last two decades, immobilization in a sol-gel matrix of microorganisms such as Escherichia coli [19][24][29][38], Pseudomonas [40][41][42], Streptococcus [43], and Bacillus [44] has been intensively studied (Figure 6). During the formation of sol-gel materials, the release of lower alcohols (ethanol or methanol) often occurs. This is the main cause of mass mortality of bacterial cells, in contrast to yeast, which is less affected by alcohols [24]. Escherichia coli bacteria have been efficiently encapsulated in organosilicon matrices [45]. Bacteria were isolated from each other in a layer of sol-gel material but still exhibited enzymatic activity against some substrates. However, the long-term stability of bacteria was 1 month, with a survival rate of approximately 10% even with the formation of sol-gel matrices under near physiological conditions at the required temperature, pH and ionic strength of the solution. To increase the viability of bacteria, various organic compounds are added during matrix formation, such as polyvinyl alcohol, gelatin, and glycerol [46]. It was shown that glycerol allowed the maintenance of the metabolic activity of almost 50% of bacteria after 1 month.
Kim et al. immobilized Escherichia coli bacteria in a silica sol-gel matrix and demonstrated their biological activity retention [47]. The study of the obtained material structure was carried out in the presence of various organic components, which increased the long-term performance of the biomaterial. The immobilization of Escherichia coli bacteria is used to explore the stability during storage and long-term continuous processes [48], to study the formation of various structures and the functioning of enzyme preparations during their immobilization [13], to study the effect of the resulting alcohol distillation on increasing cell viability [24][49], and to assess the effect of stress factors on bacterial immobilization [29] (Figure 6).
Immobilization of microorganisms in sol-gel matrices can be considered an alternative for long-term storage of nodule bacteria of the genus Rhyzobium at room temperature [50]. Sodium silicate was used as a precursor by Diazs’ group. In a continuation of the study [46], glycerol was used as the organic component. The bacteria immobilized in the sol-gel matrix retained their viability and catalytic activity for up to 360 days of storage at room temperature. In addition, the silicon matrix has been shown to have the ability to protect bacteria from acid attack.
In view of their high abundance, cyanobacteria (Figure 4) are often used as model objects for studying various processes, including immobilization methods. In addition, they are important in biotechnology in the production of food additives, food, and pharmaceutical compounds and pigments, as well as in the production of biofuels and other products. The study of cyanobacteria encapsulation in a silicate matrix is described in [23].
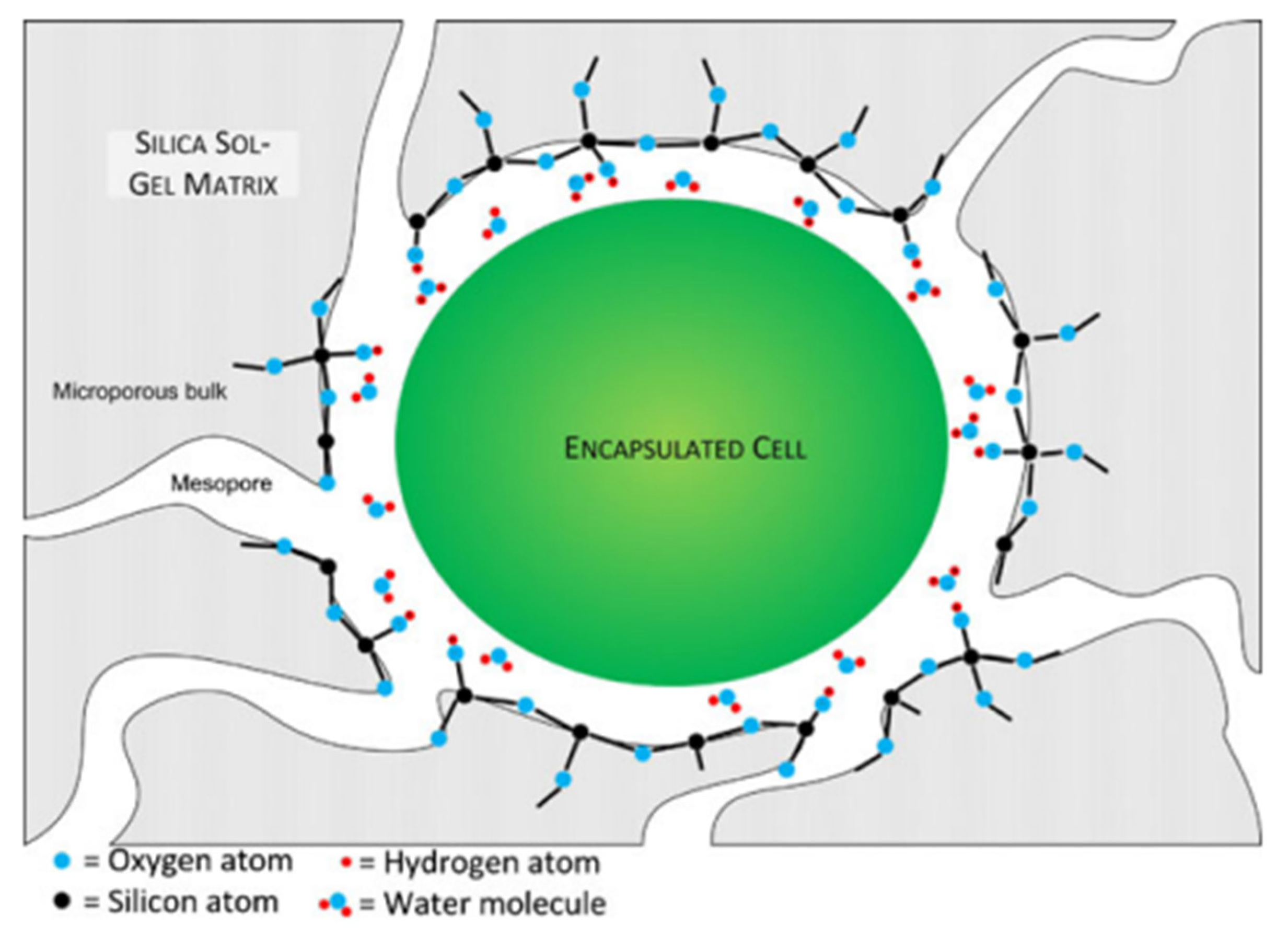 Figure 4. Schematic representation of a cyanobacterial cell encapsulated in silica gel (not to scale). The gel encloses the cell completely within a microporous bulk. The mesopores are large enough to allow diffusion of minerals and nutrients but small enough to contain the encapsulated cell. With alkoxide or aqueous precursors, the surface of the gel is likely composed of hydrophilic condensed silica with some uncondensed hydroxyl functional groups. Reprinted with permission from [23].
Figure 4. Schematic representation of a cyanobacterial cell encapsulated in silica gel (not to scale). The gel encloses the cell completely within a microporous bulk. The mesopores are large enough to allow diffusion of minerals and nutrients but small enough to contain the encapsulated cell. With alkoxide or aqueous precursors, the surface of the gel is likely composed of hydrophilic condensed silica with some uncondensed hydroxyl functional groups. Reprinted with permission from [23].Cells were immobilized in a sol-gel framework based on tetraethoxysilane (TEOS) under acid catalysis. Glycerol was used as an organic additive. As a result, cyanobacteria were encased in a porous organosilicon capsule, which, on the one hand, protects each cell from mechanical damage and, on the other hand, does not prevent the rapid diffusion of low molecular weight substances through the pores of the material [23].
A silica-based adsorbent biogel was created by incorporating the bacteria Pseudomonas sp. NCIB 9816-4 that degrade a wide range of aromatic contaminants. The adsorbent matrix was synthesized using the silica precursors methyltrimethoxysilane (MTMS) and tetramethoxysilane (TMOS) (Figure 5).
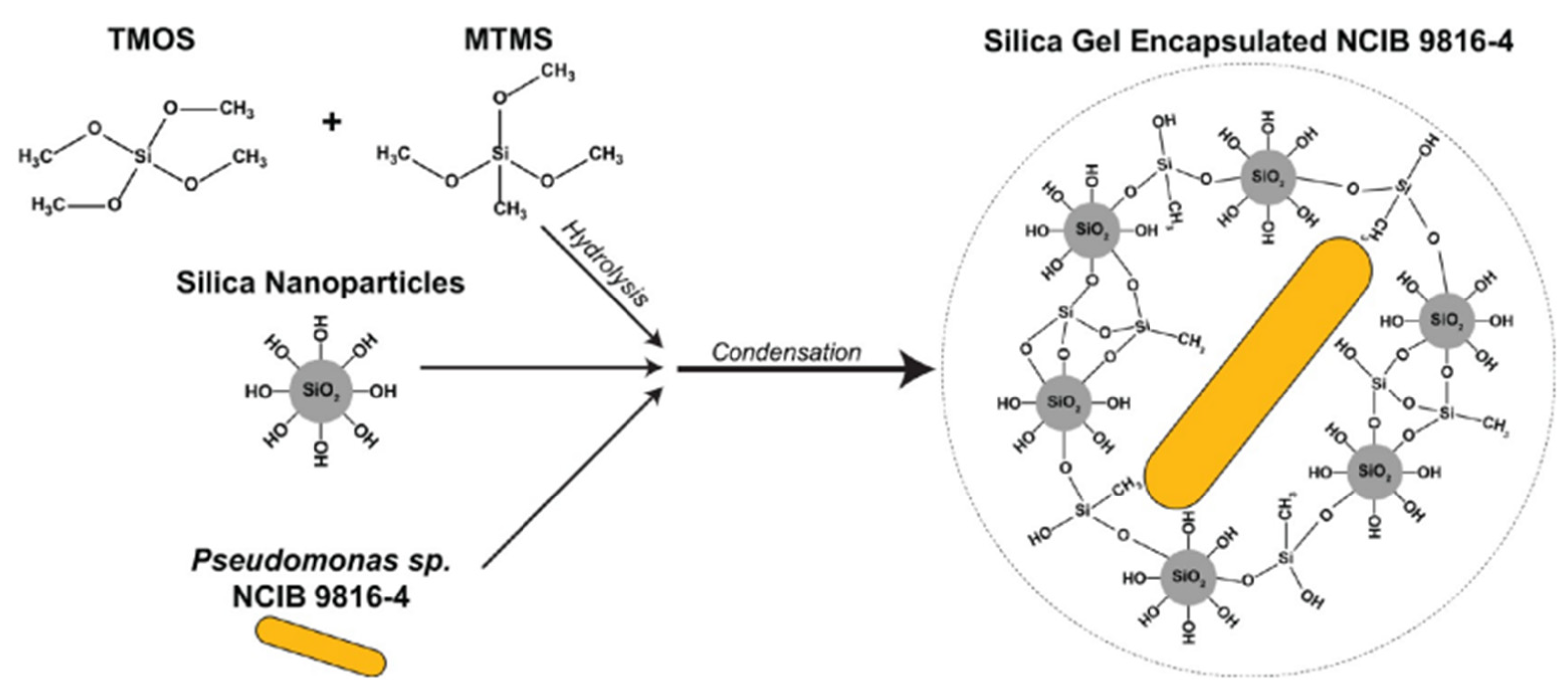 Figure 5. Synthesis process of the silica biogel through hydrolysis of TMOS and MTMS and condensation by mixing the hydrolyzed monomers with colloidal SNPs and Pseudomonas sp. NCIB 9816-4. Reprinted with permission from [42].
Figure 5. Synthesis process of the silica biogel through hydrolysis of TMOS and MTMS and condensation by mixing the hydrolyzed monomers with colloidal SNPs and Pseudomonas sp. NCIB 9816-4. Reprinted with permission from [42].The encapsulated bacteria increase the rate of removal of the aromatic chemical mixture. Immobilized Pseudomonas bacteria have been successfully used to decolorize Remazol black, methylene orange, and benzyl orange, which are azo dyes commonly used in industrial processes [41] (Figure 6). The immobilized cells produced more than seven extracellular enzymes involved in the biodegradation of azo dyes. The reusability of immobilized bacteria has been evaluated through multiple experiments [41][42].
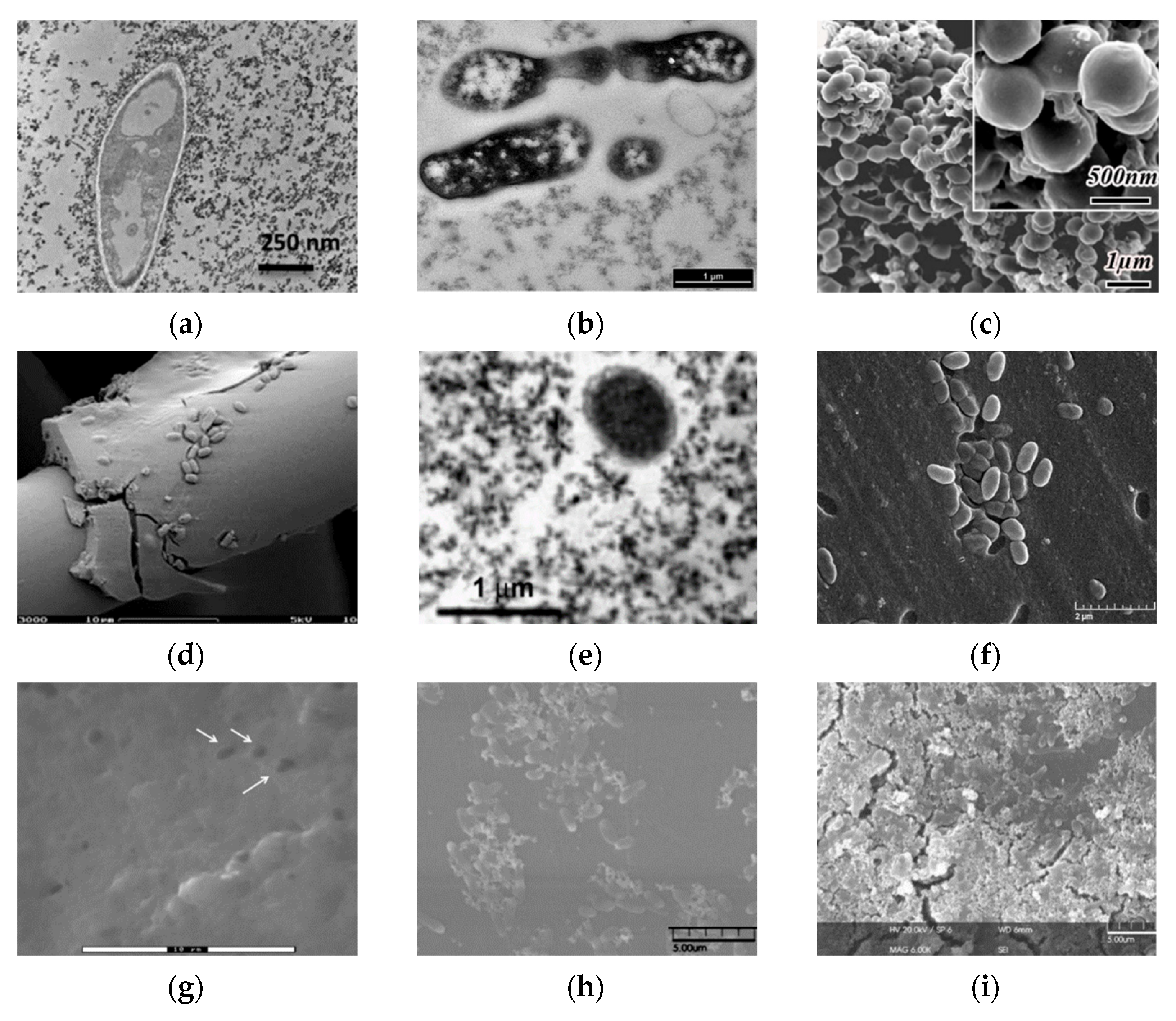
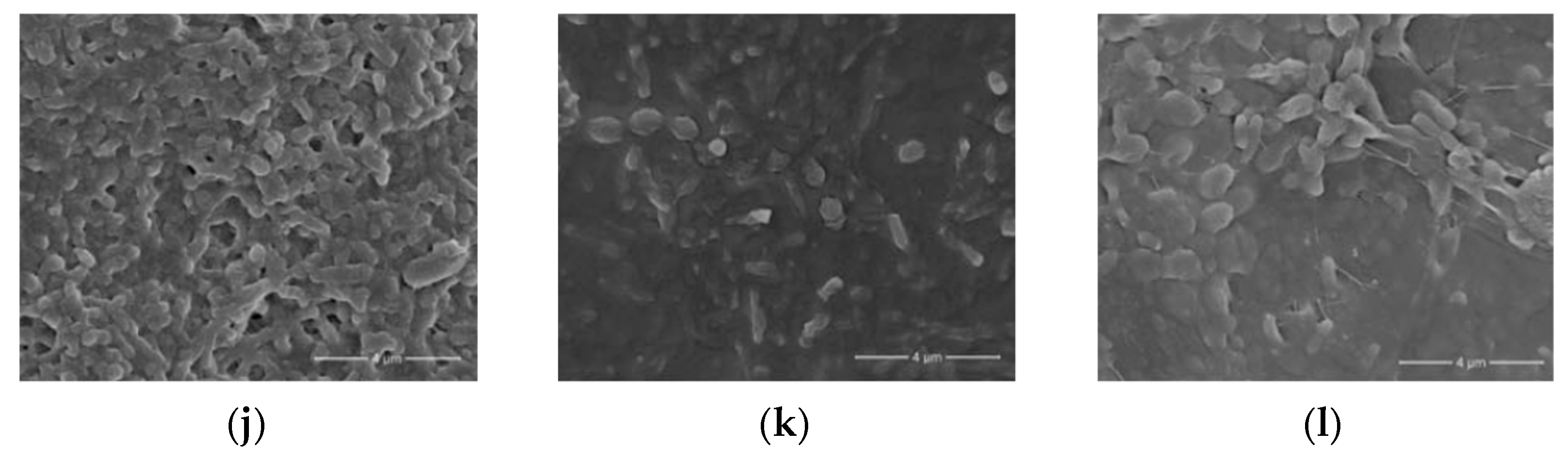 Figure 6. Micrographs of bacterial cells immobilized in various sol-gel matrices. (a) Transmission electron microscopy (TEM) image of E. coli bacteria entrapped in a ferrihydrite gel [27]; (b) transmission electron microscopy. Thin-cross-section TEM images of E. coli cells entrapped within an SS matrix after 24 h [29]; (c) field emission scanning electron microscopy images of bacteria/TiO2 gel hybrid spheres using Str. Theromophilus as templates, with the inset of a magnified image. The surface sol-gel deposition was repeated five times [51]; (d) different types of biocer-microstructure (scanning electron micrographs) carbon felt coated with a silica–B. sphaericus layer [52]; (e) transmission electron microscopy of the E. coli B 54,125 cell within an aqueous silica gel, SiO2–glycerol 10%, aged for one day [24]; (f) scanning electron microscopy (SEM) photos of mold silica gel-entrapped P. aeruginosa MR01 immediately after gel immobilization [40]; (g) SEM image of silica matrices with immobilized bacteria [41]; (h) SEM micrographs of Sphingomonas sp. cells [53]; (i) biohybrid of Sphingomonas sp.-(f) Si NP immobilized on microplate [53]; (j) SEM images of biofilm surface. Silica layer present after encapsulation. Representative electron microscopy images of N. europaea biofilm 30 min after encapsulation [54]; (k) 30 days after encapsulation [54]; (l) 90 days after encapsulation. Scale bars represent 4 mm [54]. Micrographs reprinted with permissions from the given references.
Figure 6. Micrographs of bacterial cells immobilized in various sol-gel matrices. (a) Transmission electron microscopy (TEM) image of E. coli bacteria entrapped in a ferrihydrite gel [27]; (b) transmission electron microscopy. Thin-cross-section TEM images of E. coli cells entrapped within an SS matrix after 24 h [29]; (c) field emission scanning electron microscopy images of bacteria/TiO2 gel hybrid spheres using Str. Theromophilus as templates, with the inset of a magnified image. The surface sol-gel deposition was repeated five times [51]; (d) different types of biocer-microstructure (scanning electron micrographs) carbon felt coated with a silica–B. sphaericus layer [52]; (e) transmission electron microscopy of the E. coli B 54,125 cell within an aqueous silica gel, SiO2–glycerol 10%, aged for one day [24]; (f) scanning electron microscopy (SEM) photos of mold silica gel-entrapped P. aeruginosa MR01 immediately after gel immobilization [40]; (g) SEM image of silica matrices with immobilized bacteria [41]; (h) SEM micrographs of Sphingomonas sp. cells [53]; (i) biohybrid of Sphingomonas sp.-(f) Si NP immobilized on microplate [53]; (j) SEM images of biofilm surface. Silica layer present after encapsulation. Representative electron microscopy images of N. europaea biofilm 30 min after encapsulation [54]; (k) 30 days after encapsulation [54]; (l) 90 days after encapsulation. Scale bars represent 4 mm [54]. Micrographs reprinted with permissions from the given references.Immobilization of the methanotrophic bacterium Methylomonas sp. GYJ3 by the sol-gel technique enhances the activity of microorganisms at higher pH and temperature. However, the cells encaged by the sol-gel matrix based on MTMS had a lower activity compared to the activity of free cells [55]. At the same time, sol-gel matrices with immobilized Gram-positive Rhodococcus ruber bacteria demonstrated unchanged mechanical strength and good activity of immobilized cells when stored for several months at 4 °C [21]. The created ceramic composites can be reused for 12 months without loss of biological activity for bioremediation processes, as Rhodococcus spp. decompose a large number of pollutants that are difficult to oxidize, such as petroleum hydrocarbons, chlorinated, nitrogen-containing and other complex organic substances.
2.3. Immobilization of Yeast Cells by the Sol-Gel Method
The immobilization of yeast cells in a sol-gel matrix attracts much attention, as the alcohol released during the sol-gel reactions is not as harmful to yeast as to bacteria. In addition, yeast cells are often used as templates for the formation of porous inorganic structures [35].
The possibility of obtaining channel-like meso/macroporous TiO2, a potential anode material for lithium-ion batteries, has been described [28]. For this, a sol-gel process based on titanium tetraisopropoxide using yeast cells of Saccharomyces cerevisiae was utilized.
The first work on the immobilization of the Saccharomyces cerevisiae whole cells in sol-gel was published in 1989 [56][57]. Since then, some S. cerevisiae cells have been used as models for studying yeast viability after encapsulation in a sol-gel matrix based on tetraethoxysilane, tetramethoxysilane, and diethoxymethylsilane [52][58][59][60] (Figure 7). Yeast Saccharomyces cerevisiae cells genetically engineered to produce yellow fluorescent protein in response to galactose were encapsulated in polyglycerol silicate matrices. The matrix consisted of glycerol, TEOS, and titanium isopropoxide [31]. A biohybrid of nanosilica and the model organism Saccharomyces cerevisiae was synthesized to remove mercury from an aqueous solution [61]. The efficiency of biosorption of heavy metals by microbial biomass is mainly related to the structure of the microorganism’s cell wall. Therefore, the structure and properties of the cell surface determine the nature of the interaction between the microorganism and the metal cation. The walls of yeast cells are negatively charged due to the presence of functional groups such as amino groups and phosphate and hydroxyl groups, which are involved in the binding of heavy metals. It is well known that among the various reactive compounds associated with cell walls, extracellular polymeric substances such as exopolysaccharides have a great ability to form complexes with heavy metals. The biohybrid has been shown to exhibit high Hg(II) adsorption capacity, demonstrating a rapid removal of more than 98 ± 2% of this contaminant in 30 min. The synthesized biohybrid material can be easily regenerated, and the efficiency of Hg(II) removal can be maintained when reused. In addition, the encapsulation of Yarrowia lipolytica in silicon matrices based on TEOS enables the development of a heterogeneous biomaterial that not only has the ability to remove Cr(III) and Cr(VI) pollutants from water without special pretreatment and with high efficiency but also to dispose of hydrocarbons in aqueous conditions. This process is possible due to Yarrowia lipolytica’s ability to produce various enzymes (proteases, lipases, and esterases), emulsifiers, and surfactants. The resulting biohybrid material has the advantages of a hydrophobic and porous structure and is able to achieve almost 100% removal efficiency of chromium and n-hexadecane ions in an aqueous medium [62].
It was found that in a hybrid material formed by silicon dioxide, polyvinyl alcohol, and 4-vinylpyridine with immobilized cells of the yeast Trichosporon cutaneum, a biocompatible microenvironment is formed, which contributes to the preservation of the viability of encapsulated cells [63]. Arthroconidia that have formed in the extracellular material play an important role in maintaining the long-term viability of microorganisms, which may be related to their ability to withstand environmental stresses. A biosensor based on the encapsulated yeast Trichosporon cutaneum was used to analyze the biochemical oxygen consumption in contaminated effluents.
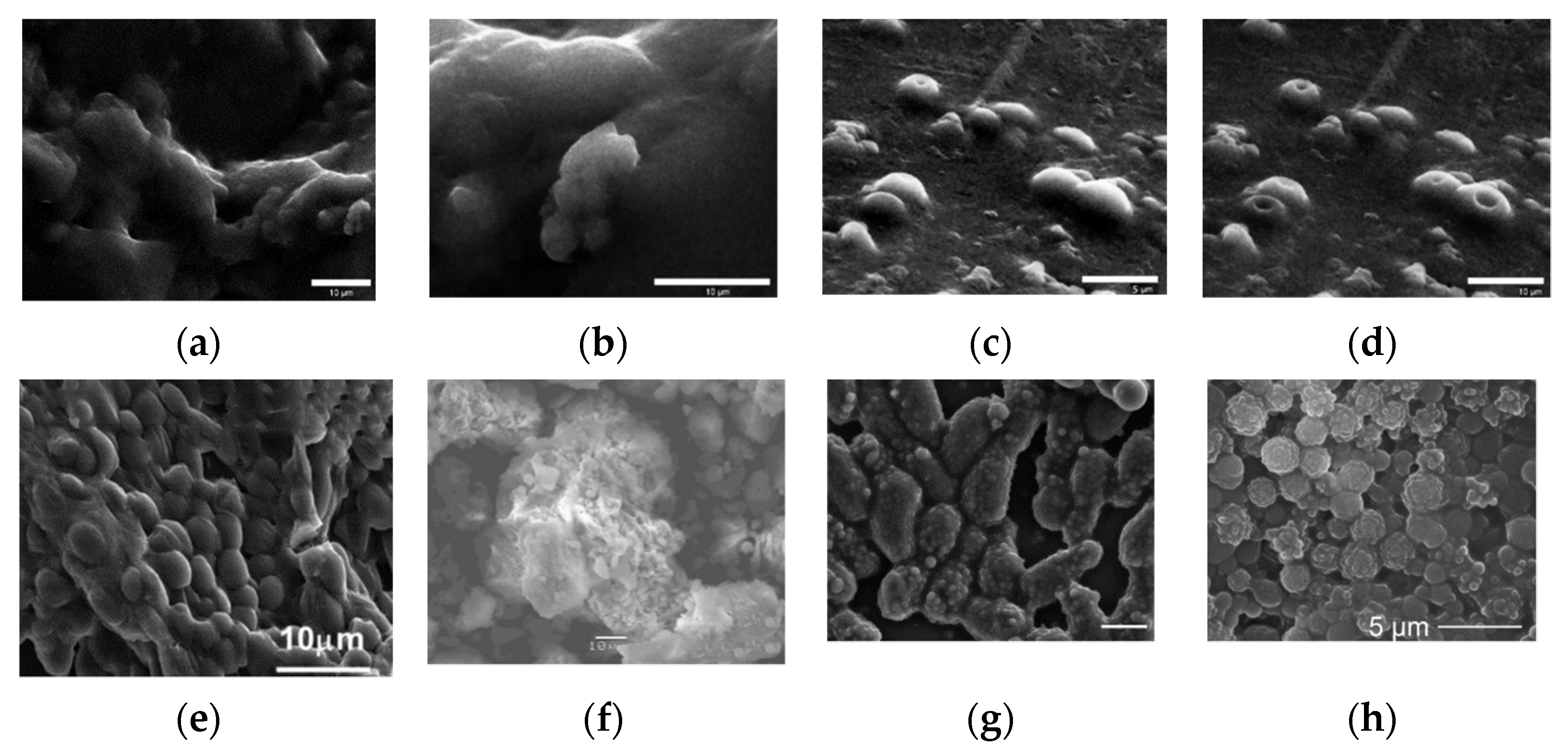 Figure 7. Micrographics of immobilized yeast cells in various sol-gel matrices. (a) SEM images of PGS-derived silica gels containing S. cerevisiae cells. (b) Typical long-range morphology with some shallowly encapsulated cells visible. Image (d) was collected several minutes after image (c), showing the development of depressions during imaging [31]. FE-SEM images of baker’s yeast encapsulated in sol–gel silica: (e) [60]. (f) Freshly harvested Lodderomyceselongisporus cells immobilized in a second generation (supported with hollow-silica microspheres) sol-gel system [64]. (g) Cryptococcus curvatus encapsulation in silica sol-gel. SEM micrographs showing the formation of 3D sol-gel biomatrix architecture when the ratio between the silane precursors (TEOS and MTES) (vol%) was 85:15. Scale bar, 5 µm [65]. (h) SEM micrograph showing the formation of a 3D structure hybrid material based on Ogataea polymorpha VKM Y-2559 cells encapsulated in an organosilica hydrogel MTES:TEOS 85:15 vol. % and PVA [22]. Micrographs reprinted with permissions from the given references.
Figure 7. Micrographics of immobilized yeast cells in various sol-gel matrices. (a) SEM images of PGS-derived silica gels containing S. cerevisiae cells. (b) Typical long-range morphology with some shallowly encapsulated cells visible. Image (d) was collected several minutes after image (c), showing the development of depressions during imaging [31]. FE-SEM images of baker’s yeast encapsulated in sol–gel silica: (e) [60]. (f) Freshly harvested Lodderomyceselongisporus cells immobilized in a second generation (supported with hollow-silica microspheres) sol-gel system [64]. (g) Cryptococcus curvatus encapsulation in silica sol-gel. SEM micrographs showing the formation of 3D sol-gel biomatrix architecture when the ratio between the silane precursors (TEOS and MTES) (vol%) was 85:15. Scale bar, 5 µm [65]. (h) SEM micrograph showing the formation of a 3D structure hybrid material based on Ogataea polymorpha VKM Y-2559 cells encapsulated in an organosilica hydrogel MTES:TEOS 85:15 vol. % and PVA [22]. Micrographs reprinted with permissions from the given references.Therefore, materials synthesis technology by the sol-gel method can be effectively used for the immobilization of a wide range of microorganisms, including Gram-positive and Gram-negative bacteria, as well as different types of yeast. At the same time, the sol-gel approach provides a biocompatible environment that protects cells from external influences, regardless of the microorganism type.
References
- Ananikov, V.P. Organic–Inorganic Hybrid Nanomaterials. Nanomaterials 2019, 9, 1197.
- Máková, V.; Holubová, B.; Krabicová, I.; Kulhánková, J.; Řezanka, M. Hybrid Organosilane Fibrous Materials and Their Contribution to Modern Science. Polymer 2021, 228, 123862.
- Romashov, L.V.; Ananikov, V.P. Self-Assembled Selenium Monolayers: From Nanotechnology to Materials Science and Adaptive Catalysis. Chem. (Weinh. Bergstr. Ger.) 2013, 19, 17640–17660.
- Júnior, A.A.d.T.; Ladeira, Y.F.X.; França, A.d.S.; Souza, R.O.M.A.d.; Moraes, A.H.; Wojcieszak, R.; Itabaiana, I., Jr.; Miranda, A.S.d. Multicatalytic Hybrid Materials for Biocatalytic and Chemoenzymatic Cascades—Strategies for Multicatalyst (Enzyme) Co-Immobilization. Catalysts 2021, 11, 936.
- Cavallini, C.; Vitiello, G.; Adinolfi, B.; Silvestri, B.; Armanetti, P.; Manini, P.; Pezzella, A.; d’Ischia, M.; Luciani, G.; Menichetti, L. Melanin and Melanin-Like Hybrid Materials in Regenerative Medicine. Nanomaterials 2020, 10, 1518.
- Prosa, M.; Bolognesi, M.; Fornasari, L.; Grasso, G.; Lopez-Sanchez, L.; Marabelli, F.; Toffanin, S. Nanostructured Organic/Hybrid Materials and Components in Miniaturized Optical and Chemical Sensors. Nanomaterials 2020, 10, 480.
- Hayta, E.N.; Ertelt, M.J.; Kretschmer, M.; Lieleg, O. Bacterial Materials: Applications of Natural and Modified Biofilms. Adv. Mater. Interfaces 2021, 8, 2101024.
- Yoon, J.; Shin, M.; Lim, J.; Kim, D.; Lee, T.; Choi, J.-W. Nanobiohybrid Material-Based Bioelectronic Devices. Biotechnol. J. 2020, 15, 1900347.
- Palomo, J.M. Nanobiohybrids: A New Concept for Metal Nanoparticles Synthesis. Chem. Commun. 2019, 55, 9583–9589.
- Guo, Z.; Richardson, J.J.; Kong, B.; Liang, K. Nanobiohybrids: Materials Approaches for Bioaugmentation. Sci. Adv. 2020, 6, eaaz0330.
- Deshmukh, K.; Kovářík, T.; Křenek, T.; Docheva, D.; Stich, T.; Pola, J. Recent Advances and Future Perspectives of Sol–Gel Derived Porous Bioactive Glasses: A Review. RSC Adv. 2020, 10, 33782–33835.
- Prasad, T.; Halder, S.; Dhar, S.S. Process Parameter Effects on Particle Size Reduction of Sol-Gel Synthesized Silica Nanoparticles. Mater. Today Proc. 2020, 22, 1669–1675.
- Benson, J.J.; Sakkos, J.K.; Radian, A.; Wackett, L.P.; Aksan, A. Enhanced Biodegradation of Atrazine by Bacteria Encapsulated inorganically Modified Silica Gels. J. Colloid Interface Sci. 2018, 510, 57–68.
- Ismail, W.N.W. Sol--Gel Technology for Innovative Fabric Finishing—A Review. J. Sol-Gel Sci. Technol. 2016, 78, 698–707.
- Braun, S.; Bhattacharyya, S.; Ducheyne, P. 5.10 Encapsulation of Cells (Cellular Delivery) Using Sol–Gel Silica. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; pp. 175–186. ISBN 978-0-08-100692-4.
- Grandi, S.; Tomasi, C.; Mustarelli, P.; Clemente, F.; Carbonaro, C.M. Characterisation of a New Sol-Gel Precursor for a SiO2-Rhodamine 6G Hybrid Class II Material. J. Sol-Gel Sci. Technol. 2007, 41, 57–63.
- Palmqvist, N.G.M.; Nedelec, J.-M.; Seisenbaeva, G.A.; Kessler, V.G. Controlling Nucleation and Growth of Nano-CaCO3 via CO2 Sequestration by a Calcium Alkoxide Solution to Produce Nanocomposites for Drug Delivery Applications. Acta Biomater. 2017, 57, 426–434.
- Chouler, J.; Di Lorenzo, M. Water Quality Monitoring in Developing Countries; Can Microbial Fuel Cells Be the Answer? Biosensors 2015, 5, 450–470.
- Chen, J.-P.; Lin, Y.-S. Sol–Gel-Immobilized Recombinant E. coli for Biosorption of Cd2+. J. Chin. Inst. Chem. Eng. 2007, 38, 235–243.
- Ciriminna, R.; Ilharco, L.M.; Fidalgo, A.; Campestrini, S.; Pagliaro, M. The Structural Origins of Superior Performance in Sol–Gel Catalysts. Soft Matter 2005, 1, 231–237.
- Pannier, A.; Mkandawire, M.; Soltmann, U.; Pompe, W.; Böttcher, H. Biological Activity and Mechanical Stability of Sol–Gel-Based Biofilters Using the Freeze-Gelation Technique for Immobilization of Rhodococcus ruber. Appl. Microbiol. Biotechnol. 2012, 93, 1755–1767.
- Lavrova, D.G.; Kamanina, O.A.; Alferov, V.A.; Rybochkin, P.V.; Machulin, A.V.; Sidorov, A.I.; Ponamoreva, O.N. Impact of Hydrophilic Polymers in Organosilica Matrices on Structure, Stability, and Biocatalytic Activity of Immobilized Methylotrophic Yeast Used as Biofilter Bed. Enzym. Microb. Technol. 2021, 150, 109879.
- Dickson, D.; Ely, R. Silica Sol-Gel Encapsulation of Cyanobacteria: Lessons for Academic and Applied Research. Appl. Microbiol. Biotechnol. 2013, 97, 1809–1819.
- Ferrer, M.L.; Garcia-Carvajal, Z.Y.; Yuste, L.; Rojo, F.; del Monte, F. Bacteria Viability in Sol−Gel Materials Revisited: Cryo-SEM as a Suitable Tool To Study the Structural Integrity of Encapsulated Bacteria. Chem. Mater. 2006, 18, 1458–1463.
- Nguyen-Ngoc, H.; Tran-Minh, C. Sol–Gel Process for Vegetal Cell Encapsulation. Mater. Sci. Eng. C 2007, 27, 607–611.
- Soltmann, U.; Böttcher, H. Utilization of Sol–Gel Ceramics for the Immobilization of Living Microorganisms. J. Sol-Gel Sci. Technol. 2008, 48, 66–72.
- Amoura, M.; Brayner, R.; Perullini, M.; Sicard, C.; Roux, C.; Livage, J.; Coradin, T. Bacteria Encapsulation in a Magnetic Sol–Gel Matrix. J. Mater. Chem. 2009, 19, 1241–1244.
- Chang, Y.-C.; Lee, C.-Y.; Chiu, H.-T. Porous Inorganic Materials from Living Porogens: Channel-like TiO2 from Yeast-Assisted Sol–Gel Process. ACS Appl. Mater. Interfaces 2014, 6, 31–35.
- Eleftheriou, N.M.; Ge, X.; Kolesnik, J.; Falconer, S.B.; Harris, R.J.; Khursigara, C.; Brown, E.D.; Brennan, J.D. Entrapment of Living Bacterial Cells in Low-Concentration Silica Materials Preserves Cell Division and Promoter Regulation. Chem. Mater. 2013, 25, 4798–4805.
- Yulizar, Y.; Juliyanto, S.; Sudirman; Apriandanu, D.O.B.; Surya, R.M. Novel Sol-Gel Synthesis of CeO2 Nanoparticles Using Morinda Citrifolia L. Fruit Extracts: Structural and Optical Analysis. J. Mol. Struct. 2021, 1231, 129904.
- Harper, J.C.; Lopez, D.M.; Larkin, E.C.; Economides, M.K.; McIntyre, S.K.; Alam, T.M.; Tartis, M.S.; Werner-Washburne, M.; Brinker, C.J.; Brozik, S.M.; et al. Encapsulation of S. cerevisiae in Poly(Glycerol) Silicate Derived Matrices: Effect of Matrix Additives and Cell Metabolic Phase on Long-Term Viability and Rate of Gene Expression. Chem. Mater. 2011, 23, 2555–2564.
- Alhaji, M.H.; Sanaullah, K.; Khan, A.; Hamza, A.; Muhammad, A.; Ishola, M.S.; Rigit, A.R.H.; Bhawani, S.A. Recent Developments in Immobilizing Titanium Dioxide on Supports for Degradation of Organic Pollutants in Wastewater—A Review. Int. J. Environ. Sci. Technol. 2017, 14, 2039–2052.
- Amoura, M.; Nassif, N.; Roux, C.; Livage, J.; Coradin, T. Sol–Gel Encapsulation of Cells Is Not Limited to Silica: Long-Term Viability of Bacteria in Alumina Matrices. Chem. Commun. 2007, 39, 4015–4017.
- Kessler, V.G.; Seisenbaeva, G.A.; Unell, M.; Håkansson, S. Chemically Triggered Biodelivery Using Metal-Organic Sol-Gel Synthesis. Angew. Chem. (Int. Ed. Engl.) 2008, 47, 8506–8509.
- Li, Y.-N.; Su, J.; Lv, X.-Y.; Long, Y.-F.; Wen, Y.-X. Yeast Bio-Template Synthesis of Porous Anatase TiO2 and Potential Application as an Anode for Sodium-Ion Batteries. Electrochim. Acta 2015, 182, 596–603.
- Sicard, C.; Perullini, M.; Spedalieri, C.; Coradin, T.; Brayner, R.; Livage, J.; Jobbagy, M.; Bilmes, S.A. CeO2 Nanoparticles for the Protection of Photosynthetic Organisms in Silica Gels. Chem. Mater. 2011, 23, 1374–1378.
- Samuneva, B.; Kabaivanova, L.; Chernev, G.; Djambaski, P.; Kashchieva, E.; Emanuilova, E.; Salvado, I.M.M.; Fernandes, M.H.V.; Wu, A.; Miranda Salvado, I.M.; et al. Sol–Gel Synthesis and Structure of Silica Hybrid Materials. J. Sol-Gel Sci. Technol. 2008, 48, 73–79.
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of Immobilized Bacteria for Environmental Bioremediation: A Review. J. Environ. Chem. Eng. 2021, 9, 105920.
- Kamanina, O.; Arlyapov, V.; Rybochkin, P.; Lavrova, D.; Podsevalova, E.; Ponamoreva, O. Application of Organosilicate Matrix Based on Methyltriethoxysilane, PVA and Bacteria Paracoccus Yeei to Create a Highly Sensitive BOD. 3 Biotech 2021, 11, 331.
- Bagheri Lotfabad, T.; Ebadipour, N.; Roostaazad, R.; Partovi, M.; Bahmaei, M. Two Schemes for Production of Biosurfactant from Pseudomonas Aeruginosa MR01: Applying Residues from Soybean Oil Industry and Silica Sol–Gel Immobilized Cells. Colloids Surf. B Biointerfaces 2017, 152, 159–168.
- Tuttolomondo, M.V.; Alvarez, G.S.; Desimone, M.F.; Diaz, L.E. Removal of Azo Dyes from Water by Sol–Gel Immobilized Pseudomonas Sp. J. Environ. Chem. Eng. 2014, 2, 131–136.
- Sakkos, J.K.; Mutlu, B.R.; Wackett, L.P.; Aksan, A. Adsorption and Biodegradation of Aromatic Chemicals by Bacteria Encapsulated in a Hydrophobic Silica Gel. ACS Appl. Mater. Interfaces 2017, 9, 26848–26858.
- Mukundan, S.; Melo, J.S.; Sen, D.; Bahadur, J. Enhancement in β-Galactosidase Activity of Streptococcus Lactis Cells by Entrapping in Microcapsules Comprising of Correlated Silica Nanoparticles. Colloids Surf. B Biointerfaces 2020, 195, 111245.
- Rathnayake, I.V.N.; Megharaj, M.; Naidu, R. Green Fluorescent Protein Based Whole Cell Bacterial Biosensor for the Detection of Bioavailable Heavy Metals in Soil Environment. Environ. Technol. Innov. 2021, 23, 101785.
- Nassif, N.; Bouvet, O.; Noelle Rager, M.; Roux, C.; Coradin, T.; Livage, J. Living Bacteria in Silica Gels. Nat. Mater. 2002, 1, 42–44.
- Nassif, N.; Roux, C.; Coradin, T.; Rager, M.-N.; Bouvet, O.M.M.; Livage, J. A Sol-Gel Matrix to Preserve the Viability of Encapsulated Bacteria. J. Mater. Chem. 2003, 13, 203–208.
- Kim, K.O.; Lim, S.Y.; Hahn, G.H.; Lee, S.H.; Park, C.B.; Kim, D.M. Cell-Free Synthesis of Functional Proteins Using Transcription/Translation Machinery Entrapped in Silica Sol-Gel Matrix. Biotechnol. Bioeng. 2009, 102, 303–307.
- Molnar, Z.; Farkas, E.; Lako, A.; Erdelyi, B.; Kroutil, W.; Vertessy, B.G.; Paizs, C.; Poppe, L. Immobilized Whole-Cell Transaminase Biocatalysts for Continuous-Flow Resolution of Amines. Catalysts 2019, 9, 438.
- Ferrer, M.L.; Yuste, L.; Rojo, F.; del Monte, F. Biocompatible Sol−Gel Route for Encapsulation of Living Bacteria in Organically Modified Silica Matrixes. Chem. Mater. 2003, 15, 3614–3618.
- Alvarez, G.S.; Pieckenstain, F.L.; Desimone, M.F.; Estrella, M.J.; Ruiz, O.A.; Díaz, L.E. Evaluation of Sol-Gel Silica Matrices as Inoculant Carriers for Mesorhizobium Spp. Cells. In Current Reseach, Technology and Education Topics in Applied Microbiology and Microbioal Biotechnology; Mendez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2010; pp. 160–166.
- Zhou, H.; Fan, T.; Ding, J.; Zhang, D.; Guo, Q. Bacteria-Directed Construction of Hollow TiO2 Micro/Nanostructures with Enhanced Photocatalytic Hydrogen Evolution Activity. Opt. Express 2012, 20 (Suppl. 2), A340–A350.
- Boettcher; Soltmann, U.; Mertig, M. Biocers: Ceramics with Incorporated Microorganisms for Biocatalytic, Biosorptive and Functional Materials Development. Mater. Chem. 2004, 14, 2176–2188.
- Mishra, A.; Kumar, J.; Melo, J.S. An Optical Microplate Biosensor for the Detection of Methyl Parathion Pesticide Using a Biohybrid of Sphingomonas Sp. Cells-Silica Nanoparticles. Biosens. Bioelectron. 2017, 87, 332–338.
- Jaroch, D.; McLamore, E.; Zhang, W.; Shi, J.; Garland, J.; Banks, M.K.; Porterfield, D.M.; Rickus, J.L. Cell-Mediated Deposition of Porous Silica on Bacterial Biofilms. Biotechnol. Bioeng. 2011, 108, 2249–2260.
- Chen, J.; Xu, Y.; Xin, J.; Li, S.; Xia, C.; Cui, J. Efficient Immobilization of Whole Cells of Methylomonas Sp. Strain GYJ3 by Sol–Gel Entrapment. J. Mol. Catal. B Enzym. 2004, 30, 167–172.
- Carturan, G.; Campostrini, R.; Dire, S.; Scardi, V.; De Alteriis, E. Inorganic Gels for Immobilization of Biocatalysts: Inclusion of Invertase-Active Whole Cells of Yeast (Saccharomyces cerevisiae) into Thin Layers of Silica Gel Deposited on Glass Sheets. J. Mol. Catal. 1989, 57, 6–13.
- Inama, L.; Diré, S.; Carturan, G.; Cavazza, A. Entrapment of Viable Microorganisms by SiO2 Sol-Gel Layers on Glass Surfaces: Trapping, Catalytic Performance and Immobilization Durability of Saccharomyces cerevisiae. J. Biotechnol. 1989, 30, 197–200.
- Podrazky, O.; Ripp, S.; Trogl, J.; Sayler, G.; Demnerova, K.; Vankova, R. Monitoring the Viability of Cells Immobilized by the Sol-Gel Process. J. Sol-Gel. Sci. Technol. 2004, 31, 335–342.
- Avnir, D.; Coradin, T.; Lev, O.; Livage, J. Recent Bio-Applications of Sol-Gel Materials. J. Mater. Chem. 2006, 16, 1013–1030.
- Kato, K.; Nakamura, H.; Nakanishi, K. Asymmetric Bioreduction of Acetophenones by Baker’s Yeast and Its-Free Extract Encapsulated in Sol-Gel Silica Materials. Appl. Surf. Sci. 2014, 293, 312–317.
- Shukla, P.; Mishra, A.; Manivannan, S.; Melo, J.S.; Mandal, D. Parametric Optimization for Adsorption of Mercury (II) Using Self Assembled Bio-Hybrid. J. Environ. Chem. Eng. 2020, 8, 103725.
- Wang, H.; Wang, Z.; Liu, G.; Cheng, X.; Chi, Z.; Madzak, C.; Liu, C.; Chi, Z. Genetical Surface Display of Silicatein on Yarrowia Lipolytica Confers Living and Renewable Biosilica-Yeast Hybrid Materials. ACS Omega 2020, 5, 7555–7566.
- Liu, L.; Shang, L.; Guo, S.; Li, D.; Liu, C.; Qi, L.; Dong, S. Organic-Inorganic Hybrid Material for the Cells Immobilization: Long-Term Viability Mechanism and Application in BOD Sensors. Biosens. Bioelectron. 2009, 25, 523–526.
- Nagy-Gyor, L.; Farkas, E.; Lacatus, M.; Toth Gergoand Incze, D.; Hornyanszky, G.; Bodai, V.; Paizs, C.; Poppe, L.; Balogh-Weiser, D. Conservation of the Biocatalytic Activity of Whole Yeast Cells by Supported Sol—Gel Entrapment for Efficient Acyloin Condensation. Period. Polytech. Chem. Eng. 2020, 64, 153–161.
- Fiedler, D.; Hager, U.; Franke, H.; Soltmann, U.; Bottcher, H. Algae Biocers: Astaxanthin Formation in Sol-Gel Immobilised Living Microalgae. J. Mater. Chem. 2007, 17, 261–266.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Entry Collection:
Chemical Bond
Revisions:
3 times
(View History)
Update Date:
06 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No


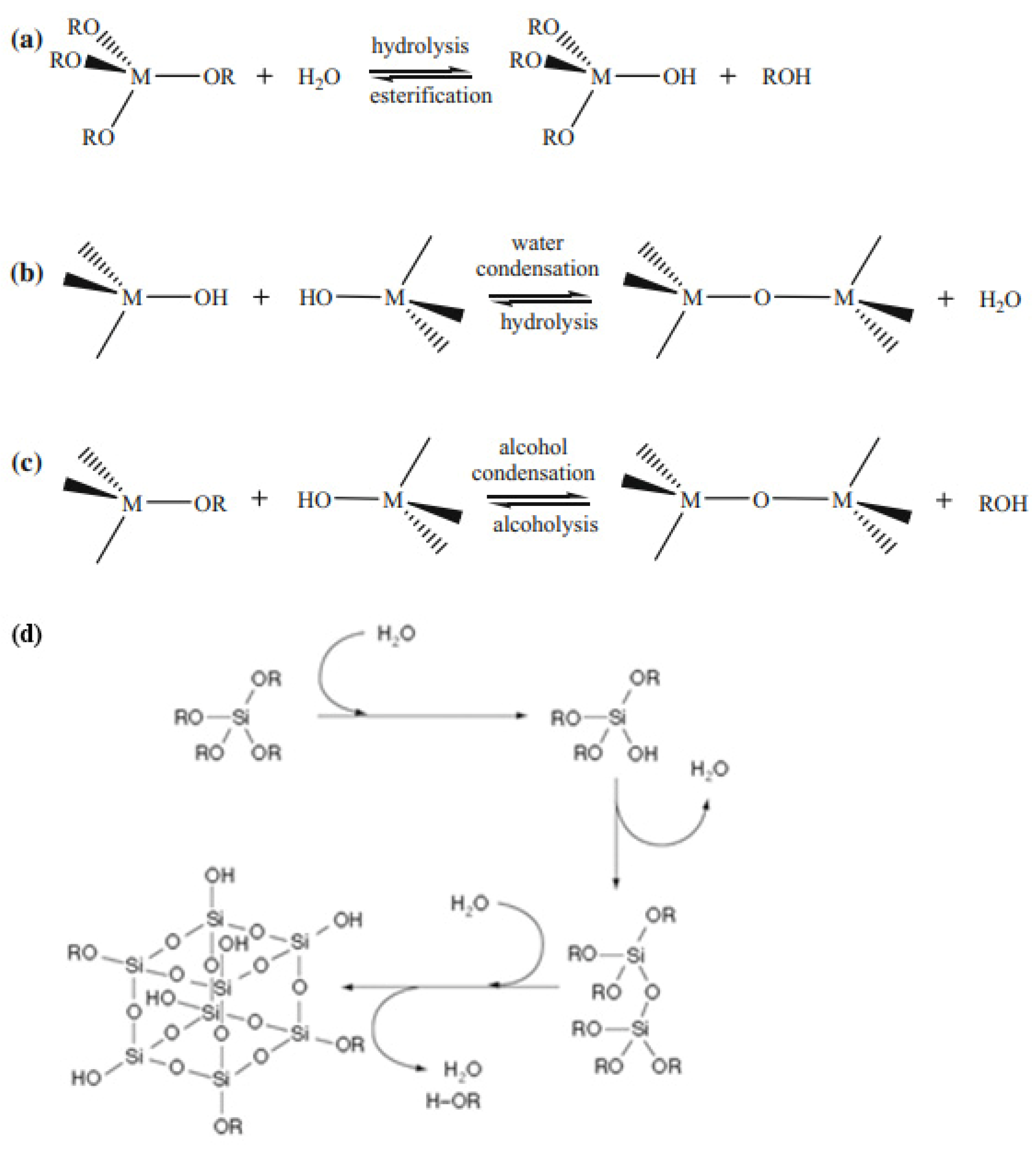 Figure 1. Hydrolysis (a) and condensation (b,c) of metal alkoxides. Reprinted with permission from [
Figure 1. Hydrolysis (a) and condensation (b,c) of metal alkoxides. Reprinted with permission from [


