Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | LAILA MANDI | -- | 4519 | 2022-04-01 21:12:33 | | | |
| 2 | Conner Chen | Meta information modification | 4519 | 2022-04-06 05:51:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mandi, L.; Mugani, R.; Aba, R.; , .; El Khalloufi, F.; Ouazzani, N.; Campos, A.; Almeida, M.; Carvalho, P.; Vasconcelos, V. Microcystins in Water. Encyclopedia. Available online: https://encyclopedia.pub/entry/21302 (accessed on 28 February 2026).
Mandi L, Mugani R, Aba R, , El Khalloufi F, Ouazzani N, et al. Microcystins in Water. Encyclopedia. Available at: https://encyclopedia.pub/entry/21302. Accessed February 28, 2026.
Mandi, Laila, Richard Mugani, Roseline Aba, , Fatima El Khalloufi, Naaila Ouazzani, Alexandre Campos, Marisa Almeida, Pedro Carvalho, Vitor Vasconcelos. "Microcystins in Water" Encyclopedia, https://encyclopedia.pub/entry/21302 (accessed February 28, 2026).
Mandi, L., Mugani, R., Aba, R., , ., El Khalloufi, F., Ouazzani, N., Campos, A., Almeida, M., Carvalho, P., & Vasconcelos, V. (2022, April 01). Microcystins in Water. In Encyclopedia. https://encyclopedia.pub/entry/21302
Mandi, Laila, et al. "Microcystins in Water." Encyclopedia. Web. 01 April, 2022.
Copy Citation
Eutrophication of surface waters caused by toxic cyanobacteria such as Microcystis aeruginosa leads to the release of secondary metabolites called Microcystins (MCs), which are heptapeptides with adverse effects on soil microbiota, plants, animals, and human health. Therefore, implementing ecotechnologies capable of handling this problem has become necessary.
Toxic cyanobacteria
Harmful algal blooms
ecotechnologies
Remediation
Multi-Soil-Layering
Freshwater
1. Introduction
In recent decades, the phenomenon of freshwater contamination with toxic cyanobacteria, also known as cyanobacterial blooms, has become more widespread worldwide [1]. Cyanobacteria produce diverse cyanotoxins that have deleterious effects on animals and human health [2]. The first report on the deaths of livestock caused by cyanobacteria was published by Francis in 1878 [3]. The author linked the massive death of sheep, horses, dogs, and pigs to the ingestion of cyanobacterial biomass of Nodularia spumigena proliferating in Lake Alexandrina in South Australia.
After Francis’ paper was published, other reports of fatal poisonings of a wide variety of animals—ranging from dogs, cattle and fish to flamingos, bats and bees—have occurred worldwide [4] as a consequence of the consumption of wild waters contaminated by cyanobacteria. Thomas et al. [5] reported the death of three cows and ten calves caused by drinking the toxic bloom water of Cylindrospermopsis raciborskii. To date, the deadliest livestock accident occurred in Australia, where 10,000 animals died as a result of a massive bloom of Anabaena circinalis in the Darling River [6].
Humans, unlike animals, can avoid using water that cyanobacteria have contaminated. They have the option of choosing to use groundwater instead. The main known case of fatal poisoning to date is that in which 76 patients in Caruaru, Brazil, died after hemodialysis by water contaminated with cyanotoxin [7].
Nevertheless, humans can be exposed to poisoning by consuming contaminated products such as fish, which was the case in the study by Chen et al. [8]. The authors found MCs mean concentrations as high as 390 ng/L in the serum of 35 fishermen from Chaohu Lake, and suggested that such chronic exposure may result in hepatocellular damage compared with other experimental studies. They estimated that the average daily consumption of these fishermen was 2.2–3.9 μg MCLR-eq, whereas the World Health Organization sets this dose for a lifetime exposure of 2 μg per person.
Through contact with cyanobacteria-contaminated material, either by skin, inhalation, or ingestion, humans can experience pneumonia, dyspnea, liver damage, and gastrointestinal symptoms including abdominal pain, malaise, nausea, vomiting, and diarrhea. In essence, one should avoid contact with contaminated material. However, in the case of contact and noticeable symptoms, one should consult a doctor because the poisoning is life-threatening [9].
To date, over 279 different MCs with a wide structural variety have been identified [10]. Furthermore, Bouaïcha et al. [10] have reviewed all these variants and elaborated a list of all 279 MCs showing the amino acid substitutions. In addition, the authors have thoroughly documented the lethal doses, LD50 (the amount of toxin that kills 50% of exposed animals), of different variants of MCs ranging from 50 to >1200 μgkg−1 of body weight. They reported that the LD50 value of MCs varies greatly depending on the MC variant involved, the method of toxin purification, and the technique of delivering the toxin to the animal—either orally or intraperitoneally. A single cyanobacterial species can secrete several toxins, and several cyanobacterial species can produce the same toxin. Table 1 presents known microcystin-producing cyanobacteria and the toxins they can release.
Table 1. Overview of the main cyanobacterial species responsible for toxic cyanobacterial blooms with a focus on hepatotoxin-producing species.
| Species | Produced Toxin | Toxin Family | Highest Amount of Toxin Quantified | Range of LD50 | References |
|---|---|---|---|---|---|
| Microcystis aeruginosa | Microcystin | Hepatotoxin | 11,500 μg MC-LR-eq g−1 DW | 50–1200 μgkg−1 mouse body weight | [10][11] |
| Microcystis botrys | Microcystins | Hepatotoxin | 90% of microcystins in analyzed colonies | - | [12] |
| Microcystis flos-aquae | Microcystins | Hepatotoxin | 50% of microcystins in analyzed colonies | - | [12] |
| Microcystis panniformis | Microcystins | Hepatotoxin | 53% of microcystins in analyzed colonies | - | [12] |
| Microcystis wesenbergii | Microcystins | Hepatotoxin | 0% of microcystins in analyzed colonies | - | [12] |
| Microcystis ichthyoblabe | Microcystins | Hepatotoxin | 20% of microcystins in analyzed colonies | - | [12] |
| Microcystis viridis | Microcystin | Hepatotoxin | 17% of microcystins in analyzed colonies | >1200 μgkg−1 | [12] |
| Planktothrix rubescens | Microcystin | Hepatotoxin | 1500 μg MC g−1 DW | [13] | |
| Planktothrix agardhii | Microcystin | Hepatotoxin | 4500 μg MC g−1 DW | - | [14] |
| Woronichinia naegeliana | Microcystin | Hepatotoxin | - | - | [14] |
| Anabaena spiroides | Microcystins | Hepatotoxin | - | - | [15] |
| Nostoc muscorum | Microcystin | Hepatotoxin | 229.4 μg MC g−1 DW | 15–125 mgkg−1 mouse body weight | [16] |
| Dolichospermum flosaquae | Microcystins | Hepatotoxin | - | 160–300 μgkg−1 mouse body weight | [14][17] |
| Nodularia spumigena | Nodularin | Hepatotoxin | 43.6 μg NOD/kg DW sea mullet livers | - | [18] |
| Chrooccocus minutus | Microcystins | Hepatotoxin | 132 MC μg L−1 | - | [19] |
| Oscillatoria limnetica | Microcystins | Hepatotoxin | 877 μg MC-LR-eq μg g−1 DW | - | [20] |
| - | [20] | ||||
| Aphanizomenon ovalisporum | cylindrospermopsin | Hepatotoxin | 8700 µg CYN g−1 DW | - | [21] |
| Cylindrospermopsis raciborskii | cylindrospermopsin | Hepatotoxin | 70.83 µg CYN g−1 DW | [22] | |
| Microcystis sp. | Anatoxin-a | Neurotoxin | 0.12μg ANTX-a g−1 DW | 31 μgkg−1 mouse body weight | [17][23] |
| Aphanizomenon flos-aquae | Anatoxin-a | Neurotoxin | 24.62 μg ANTX-a g−1 DW | - | [23] |
| Anabaena sp. | Anatoxin-a | Neurotoxin | 21.9 μg ANTX-a g−1 DW | - | [23] |
| Cylindrospermopsis raciborskii | saxitoxin | Neurotoxin | 0.20 μgL−1 STXs | 10 μgkg−1 mouse body weight | [17][24] |
| Lyngbya sp. | Debromoaplysiatoxin | Dermatotoxins | 6.31 μg DAT g−1 DW | - | [25] |
The spread of cyanobacterial blooms in freshwater bodies, stimulated by global warming worldwide, leads to the release and dispersal of cyanotoxins in water bodies [25][26]. These blooms impede the development of other aquatic organisms [27][28][29], disrupting the ecosystem [30]. Additionally, continuous loading of nitrogen and phosphorus-rich nutrients into an aquatic environment leads to the invasion and establishment of cyanobacteria [31][32][33]. This phenomenon, known as eutrophication, ultimately leads to the release of cyanotoxins that will pose serious consequences to downstream users of these contaminated waters [29][34][35][36][37].
2. Microcystins in Water: Treatment Methods
In order to guarantee the quality of water intended for consumption, in particular, to eliminate the nuisances and dangers attributable to the presence, in water, of cyanobacteria and their toxins, the WHO (2004) [38] guidelines recommend not to reach above 1.0 µg/L MC-LR in drinking water. Subsequently, conventional water treatment for the production of drinking water uses chemical processes such as ozonation, oxidation or ultraviolet (UV) light [39][40][41], chlorination, or a combination of these processes [42][43]. Yang et al. [44] reported that for 22 days, sunlight PAR (400–700 nm) did not reduce cyanotoxin concentration, whereas exposure to PAR + UV-A (320–400 nm) and PAR + UV-A + UV-B (280–320 nm) induced a reduction in concentration between 20.6% and 27.3%, respectively. In another study, Pelaez et al. [45] stated that Nitrogen-TiO2 photocatalyst calcined at 350 °C exhibited the highest MC-LR degradation efficiency at wavelengths > 420 nm. The efficiency of these techniques has been reported to follow a certain pattern concerning the oxidation rate: O3 > H2O2 > HOCl > ClO2 > KMnO4 > Cl2 [43]. The chlorine dose influences the efficiency of chlorination, since converting one mole of MC-LR stoichiometrically requires 12 moles of chlorine in 30 min, also considering that there may be competition for chlorine by organic matter in the case of natural water treatment [46].
2.1. Ozonation/Oxidation
Given its oxidation-reduction potential of 2.07 eV in acidic conditions, ozone has been recognized as one of the most powerful oxidants used in water treatment [41]. In solution, ozone reacts directly with organic solutes. However, one of its most significant attributes is due to its conversion to OH radicals (H2O2, H2O, OH), with OH being the strongest oxidant in water [47][48][49]. The efficiency of the ozone has been linked to its ability to act on the conjugated double bonds C=C [40]. Several studies have investigated the destruction of microcystin LR molecule (Figure 1) by ozone through the diene of the Adda and the double bonds of the Mdha sites [41][50]. It was also shown that ozone acts on amine groups [51]. In aqueous solutions, even though the ozone undergoes decomposition, leading to OH radicals through electron transfer reactions, molecular ozone still directly attacks microcystins [52].Moreover, its efficacy in oxidizing the microcystin molecules is specifically because of its affinity to diene bonds and amine groups.
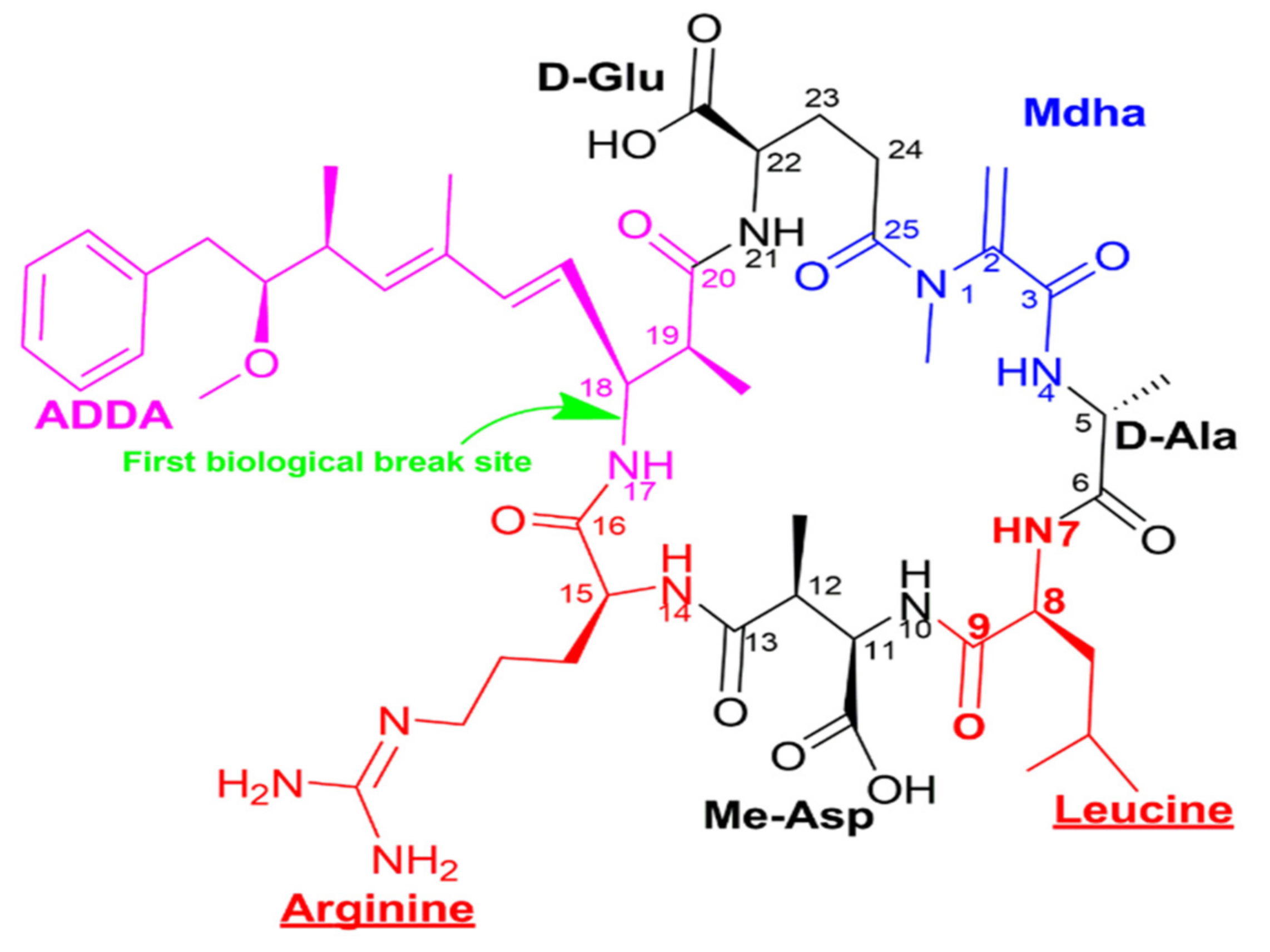
Figure 1. Structural representation of microcystin LR (C49H74N10O12), with different colors indicating the 7 constituent amino acids—ADDA, D-glutamic acid, Mdha, D-alanine, leucine, methyl aspartic acid, arginine - and an indication of the biological opening site of the microcystin circle structure.
However, the OH does not interact specifically; it oxidizes all natural organic matter, hence reducing the efficacy of ozone that it is derived from. This means that OH might be scavenged by organic matter in a sample [51], consequently affecting its efficacy in destroying microcystins. Furthermore, Xie et al., 2021, [53] reported that UV light highly degrades MC- when the experimental medium is based on ultra-pure water. Regardless, Bai et al. [54] supported that 1 mg/L of OH inactivated MC-LR completely within 20 sec in a drinking water treatment process. They hypothesized pathways involved in MC-LR inactivation consisted of breaking the C=C conjugated diene bond and breaking the persistent benzene ring to carboxylic acid m/z 158.0. Similarly, Li et al. [55] observed that 0.88 mg/L OH inactivated 99.3% of Microcystis cells and mineralized 14.4 µg/L of MC-LR to undetectable levels (determined by GC/MS).
Likewise, hydroxide peroxide, another sub-product of ozone decomposition, has been proven to decrease microcystin concentration. To that end, Papadimitriou et al. [56] observed that the addition of H2O2 to natural water samples decreased the MC-LR concentration below the WHO accepted dose in drinking water, 1 µg/L.
Several studies have shown the synergistic effects of combining different oxidation processes to remove cyanobacteria and their toxins. Chiefly, the paired combination of UV, O3, and H2O2 improved the removal of cyanotoxins and cyanobacterial cells compared with the corresponding single-use systems. [40][43][56][57]. Nevertheless, there is evidence that the effectiveness of the various techniques for removing cyanotoxins depends on other parameters such as the dose applied, the pH of the water, the temperature, and the cyanotoxin concentration [58][59].
The concentrations of chemicals used in the treatment must be precise, as deviation from this can lead to the formation of toxic byproducts [11]. It is undisputed that ozonation leads to the formation of new compounds. For example, in a study by Lu et al. [41], six aldehyde-based byproducts were detected as a result of the ozonation of water contaminated with microcystin LR: formaldehyde, acetaldehyde, isovaleraldehyde, glyoxal, methylglyoxal, and an aldehyde with a molecular weight of 160 g. In addition, the biotoxicity test in the same study showed that the treated water was still toxic to Photobacterium (which served as a model for the in vivo test).
Toxicity was thought to be related to some low molecular weight compounds formed during the treatment with ozone. However, given the predominant role of the Adda conjugated diene bond in the toxicity of MCs, Miao et al. [60] observed lower hepatotoxicity of mice in ozonated water and concluded that the toxicity might be due to a small fraction of MCs that could not be degraded by ozonation, because ozone could not withstand room temperature [41], and did not result in byproducts.
Another point to be considered is the mode of inactivation of cyanobacteria. Naturally, cyanotoxins and cyanobacteria almost always exist concomitantly. Accordingly, there are endocyanotoxins and cyanotoxins dissolved in water (exotoxins). For this reason, techniques using cell deactivation without breaking them should be prioritized to avoid endotoxin release. Griffiths and Saker [61] pointed out that the amount of intracellular and extracellular toxin can vary from 19% to 98% of the total amount depending on the stage of bloom maturity, with extracellular toxin portion being low because of environmental conditions and microorganisms that degrade these toxins. Similarly, Serrà et al. [62] noted that some water treatments could even significantly increase the release of intracellular cyanotoxins due to the lysis of cyanobacterial cells depending on the cyanobacteria species and the environmental conditions.
Since the effectiveness of these techniques seems to depend on the concentration of cyanotoxins, strategies that aim to destroy cell membranes, resulting in the release of intracellular toxins into the water, are not environmentally friendly [63] as they can increase toxins in the water. On the other hand, methods such as those using OH are promising, because they contribute to the nonselective mineralization of organic compounds and inactivate Microcystis cells, leaving them intact [50].
The ionizing radiation treatment creates reactive oxidizing and reducing species by radiolysis of the water [64]. Indeed, He et al. [57] and Lin et al. [65] suggested that OH could enter the cell and damage the DNA by breaking double strands or inducing gene mutation. This would alter genomic function and fidelity and hinder protein formation, leading to cell death without destroying the cell membrane. Therefore, Onstad et al. [51] and Lin et al. [54] recommend using a sand filtration process after ozonation, or an OH cell inactivation process to retain dead cells and complete the biodegradation of MCs.
Certainly, these ozonation/oxidation techniques work, but they require qualified personnel to supervise all treatment steps and adequate material resources. However, since most of these treatment processes are energy-dependent, it is worthwhile to thoroughly investigate other treatment processes with lower costs.
2.2. Filtration and Complexation
The water treatment method by filtration is a widely used technique in water production plants. Nevertheless, in some countries, filtration can be used downstream of the purification systems [66], in particular in the production of drinking water. However, filtration can be articulated in various forms. Techniques used for water filtration to remove harmful compounds, such as cyanobacteria and cyanotoxins, include activated carbon filtration, clay-like silica material, adsorption, biologically active sand filtration, and membrane filtration. In most cases, these techniques are preceded by coagulation–flocculation processes [67][68][69][70][71][72].
Filtration always leads to retention either through physical or chemical interaction [66][68]. Thus, filtration can relate to solid particles or chemical molecules, neutral or electrically charged. The adsorption of MCs on the activated carbon occurs according to the electrostatic or hydrophobic interactions [63][73][74][75]. While electrostatic interactions occur between the charged functional groups of cyanotoxin and the ionic functional groups on the activated carbon–oxygen-containing groups [11][66], the hydrophobic properties are due to van der Waals interactions between the cyanotoxin molecules and the nonpolar activated carbon surface [66][75]. Similarly, Huang et al. [73] postulated that weak ionic interactions might participate in the adsorption of MC-LR to activated carbon surfaces due to the association of the positively charged arginine side chain of the toxin with the negatively charged carbon surface.
Another feature that makes activated carbon the first-choice material for water treatment involving adsorption is its porous state [76]. Indeed, the inner surface of the porous adsorbent, such as activated carbon, can have 400 to 1500 m2/g of coal and a pore volume ranging from 0.1 to 0.8 cm3/g [66]. These properties confer a very high adsorption capacity estimated at 0.2 g of adsorbate per gram of adsorbent.
For large molecules such as microcystin- LR with a molecular weight (MW) of 995.189 g/mol and size between 1 and 3 nm [72], studies have shown that the best MC-LR adsorption, and thus removal, is achieved when the adsorbent material consists mainly of mesopores and macropores because of the intraparticle diffusion rate [17][73]. Conventionally, the International Union of Pure and Applied Chemistry (IUPAC) defines three types of porosity, namely: micropores: dp < 2 nm; mesopores: 2 nm < dp < 50 nm; and macropores: d > 50 nm [76][77]. Still, microporesare best suited for the adsorption of small molecules [72]. Similarly, the nature of functional groups on the carbon surface is determinant in the capacity of trapping MCs [73]. For instance, it was reported that there is strong proton adsorption in the case of the presence of several hydroxyl or phenolic groups under certain pH conditions [73][75][78]. Activated carbon may derive from different materials, namely wood, coal, coconut shells, or peat [52][70][79][80][81]. Notwithstanding, Albuquerque et al. [80] related the existence of MC-LR adsorption differences even among types of woods from which charcoal can be made; therefore, a wise choice of the sample of wood to be used has been made to maximize adsorption.
As water contains many other organics and suspended solids, before filtration, operations involving coagulation and flocculation are carried out to avoid clogging and to ensure the durability of downstream facilities. Moreover, Zhao et al. [82] and Xu et al. [83] showed that chemicals, such as aluminum sulfate (Al2 (SO4)3), polyaluminium chloride (PACl), iron chloride (FeCl3), polymeric ferric sulfate (PFS), and titanium tetrachloride (TiCl4) are the most utilized in the flocculation–coagulation process for water sanitation. As a result, several researchers have asserted that complexation reactions between positive charges and the functional groups with negative charges are primarily involved [84][85][86][87]. Indeed, in aqueous solution and at a certain pH, due to the zeta potential of metal ions such as iron, aluminum, copper, or magnesium, either flocculation is promoted by coagulation or flocculation is controlled by adsorption.
Overall, Zhao et al. [82] discussed the fact that flocculation could effectively remove the unsaturated organic compounds in water and hypothesized that flocculation could be achieved by charge-neutralization and a bridge-formation mechanism. At a higher pH, flocculation is due to adsorption [82]. For example, at a controlled pH, El Bouaidi et al. [88] examined the coagulation–flocculation processes to eliminate Microcystis aeruginosa cells, using Vicia faba and Opuntia ficus indica while harnessing the power of the phenol and flavonoid groups contained in these plants. The authors indicated that the richness in these compounds increases the size of the flocs, and consequently allows for better suppression of Microcystis aeruginosa cells.
Microcystis cells have specialized gas vesicles responsible for the buoyancy of the mucilaginous bloom cells. The buoyancy of Microcystis colonies is thought to contribute to the formation of blooms and the success of this genus in freshwater [29]. Colonial life confers many ecological advantages to Microcystis, including adaptation to light variation, sustained growth with low nutrient supply, protection from chemical stressors, and protection from grazing [30]. However, Microcystis colony formation comes at the cost of a lower specific growth rate compared to a unicellular lifestyle [27]. A large colony size allows Microcystis to attain rapid floating velocities compared with small colonies, and the larger the colony, the faster the colony ascent speed [89][90].
Piezer et al. [91] argue that with high turbidity occurring because of algal contamination, KMnO4 can be used as an effective flocculant with a dose as low as (2 mg/L). Alternatively, Zhao et al. [82] and Xu et al. [92] showed that with a size flocculation trend of TiCl4 (800.9 µm) > FeCl3 (603.9 µm) > PFS (513.4 µm) > Al2(SO4)3 (404.8 µm) > PACl (331.9 µm), both organic matter and fluorescent substances were removed. Moreover, the TiCl4 could remove up to 85% of MCs.
Furthermore, many studies have investigated the involvement of extracellular polymeric substances (EPS) in colony formation by Microcystis species with charge neutralization. Indeed, Omori et al. [93] and Sakurai et al. [94] highlighted the fact that the colony formation process in M. aeruginosa was sped up by EPS and bi-cations such as Ca2+ and Mg2+,concluding therefore that cationic ions neutralize the surface charge of M. aeruginosa. In addition, they demonstrated EPS powder previously prepared from M. aeruginosa contained carboxyl groups, which are negatively charged, and observed that metallic ions could neutralize the negative charge. Many investigations that sought to remove organic matter and MCs from water by neutralization or complexation agreed that the reaction occurrence between carboxyl, carbonyl, and amino groups was due to a negative charge and positively charged ions [95][96][97][98][99][100][101].
Microcystin Immobilization onto Soil Particles
Geochemical properties condition the fate of newly introduced chemicals in soil. Infiltration–percolation technology has mainly been used for water sanitation. However, in most cases, the goal of this technique is to reduce dissolved natural organic matter pollutants in biological oxygen demand, chemical oxygen demand, suspended solids, and pathogenic microorganisms [102][103]. It has been shown that the possibility of MCs sorption onto soil particles highly depends upon soil characteristics [31][102]. Furthermore, for MC sorption onto soil constituents, researchers agreed on the fact that rich content in clay leads to high MC immobilization [31][104][105][106][107]. Moreover, Babica et al. [107] and Chen et al. [108] stated that the MC structure guides adsorption. For example, Tsuji et al. [109] and Wu et al. [79] reported that there wasa significantly higher adsorption of MC-RR than that of MC-LR on natural sediments and the clay mineral montmorillonite.
Nonetheless, De Maagd et al. [110] highlighted that MC-LR is negatively charged within a pH range of 2.19 and 12.48, which makes electrostatic interactions with clay-bearing negative charges limited [104][111]. In contrast, other studies have strengthened the existence of MCs’ clay sorption phenomena; therefore, they have proposed that the property should be exploited for water decontamination [68][112][113][114]. Considering that the mechanism involved in adsorption requires the positively charged guanidinium in the Arginine residues of Arginine-containing MCs to interact with the negatively charged mineral surfaces [79][99][109], MC-RR would be much more adsorbed by the clay-rich soil than the other MC variants [115]. Aspartic acid from MC adsorbs onto Ca-montmorillonite via hydrogen bonding, while NH3 interacts with the oxygen atom on the siloxane surface [116]. Moreover, other parameters such as pH, organic matter, sediment texture, and type may impact the adsorption of MC-LR [115][117].
Overall, Wu et al. [79], Thirumavalavan et al. [117], and Munusamy et al. [118] showed that silty and clay textures of the samples containing a significant amount of organic matter potentiated MCs’ adsorption. Moreover, the sorption of MCs onto the complex clay–organic matter may facilitate the anoxic and aerobic biodegradation of MCs [119].
3. Fate of Immobilized Microcystins: Biological Activity
While several studies have reported the risk of MCs contaminating groundwater by leaching from the soil [104][120][121], other researchers have demonstrated that MC-LR could be biologically degraded. Corbel et al. [104] observed the mineralization of 11% of the input labeled 14C after 28 days, as opposed to Cousins et al. [120], whose laboratory experiment results showed MC-LR rapid mineralization to non-detectable levels within only six days by indigenous mixed bacterial populations. However, Corbel et al. [104] reported the involvement of MCs in regulating some physiological processes, such as the dephosphorylation of regulatory proteins. Therefore, at specific doses [31], MCs disturb soil microbiota functioning and possibly lead to microbiota decline [122][123][124]. In a study conducted by Lemes et al. [125], the authors observed that many bacterial species that they had isolated from bloomed water and sediments could not grow in flasks containing solely [D-Leu1] microcystin-LR media, except one species: Pseudomonas aeruginosa.
Indeed, when cultured on a (D-Leu1) microcystin-LR-like media, the inoculum of P. aeruginosa developed from 71 × 105 bacteria mL−1 to 117 × 105 bacteria mL−1 in only 12 days and reduced the initial concentration of MC-LR by 40% during the first 15 days, and the remaining 60% of MC-LR in only 5 days from the 15th to the 20th day. Similarly, Chen et al. [126] showed that the microbial richness of the sediment confers a high microcystin degradation potential compared to the MC degradation that takes place in the water column. Moreover, they stated that the sediment recirculates the microbial degraders in the water through water mixing. Additionally, in another study, Terin and Sabogal-Paz [127] investigated the role of household slow sand filters operating in continuous and intermittent flows to remove M. aeruginosa and MC-LR. The authors found that the two systems could reach 2.39 log10 units and 2.01 log10 units of M. aeruginosa inactivation, respectively.
Furthermore, the two systems could reduce MC-LR from 5.55 μg L−1 to under 0.1 μg L−1. Moreover, the processes involved in these removals were linked to the retention of cyanobacteria in the filter medium and the biodegradation of MC-LR. Today, bacteria belonging to specific phyla, namely Proteobacteria (α-, β-, and γ-Proteobacteria), Actinobacteria, and Bacteroidetes, have been documented to degrade MCs: Sphingomonas, Sphingopoxyis, Rhodococous, Arthrobacter, Brevibacterium, Pseudomonas, Sphingobiu, and Methylobacillus [128][92][125][127][129][130][131][132][133][134][135]. Albeit, in most study cases, degrading bacteria required a particular time to acclimate to the MCs’ presence to mineralize them [133] fully. Bioaugmentation could be an option to shorten the latent phase and speed up the biodegradation [136][137][138].
Although some microorganisms mineralizing MC do so without the mlr genes, the enzymatic method of degradation described by Bourne et al. [133] remains the most documented. This pathway was first reported in Sphingomonas sp., where three enzymes operate sequentially to decompose MC-LR. The mlrA gene encoding microcystinase, by hydrolysis, cleaves the Arg-Adda peptide bond of the toxin and converts the cyclic microcystin-LR to a linear form [139][140], which then becomes 160 times less toxic. A second enzyme, encoded by mlrB, hydrolyzes the Ala-Leu bond, converting the linearized microcystin-LR into a tetra-peptide. Finally, an enzyme encoded by mlrC breaks the tetra-peptide into smaller peptides and amino acids that are not toxic [132][133].Therefore, microbial degradation may be an effective means of degrading microcystins, and implementing this through, for example, biotechnological tools, deserves further investigation.
4. Some Eco-Technologies for the Elimination of Cyanobacterial Pollution
Although the concept of eco-technology is emerging in the fight against cyanobacterial pollution, there is still work to be done. Some studies in the last half-decade have opened the door to the idea of using microorganisms’ biological activity to eliminate cyanobacteria and cyanotoxins (Table 2). These microbial activities are facilitated by choosing local, available and efficient materials that first allow the adsorption and trapping of cyanotoxins in the systems and then provide a suitable environment for the essential microorganisms to develop into biofilms to improve the purification process [141][131][139][142][143]. Interestingly, Westrick et al. [144] reported that the biofilm handled 95% dissolved microcystin degradation. In contrast, this degradation capacity was reduced to 65% in fall, which was attributed to the drop in temperature [144].
Table 2. Example of eco-technologies for the removal of cyanobacteria or cyanotoxins with their accomplished removal performances.
| Eco-Technology Name | Processing Basis: System Strength | Highest Initial MC Concentration Used (µg/L) | Highest MC Removal Rate Obtained (%) | Lowest MC Concentration Reached (µg/L) | Presence of Cyanobacterial Cells (cells/ml) * or Chl a (µg/L) ** | Highest Cyanobacterial Cell Abatement (log10 unit) a or (% Chl a Removal) b | References |
|---|---|---|---|---|---|---|---|
| Microbial bioaugmented constructed wetlands | Constructed wetlands material and biological activity | 16.07 | 90 | na | 179.3 | 90 | [136] |
| A household slow sand filter (C-HSSF) | HSSF material and biological activity | 5 | na | <1 | 1 × 105 * | 2.39 ± 0.34 a | [127] |
| A household slow sand filter (I-HSSF) | HSSF material and biological activity | 5 | na | <1 | 1 × 105 * | 2.01 ± 0.43 a | [127] |
| Constructed Wetlands | Constructed wetlands material and biological activity | 50 | 99.9 | na | 1 × 106 * | 94 | [145] |
| Multi-soil-layering system (MSL) | MSL material and biological activity | 10 | 99.35 | na | na | na | [146] |
| Repurposed Osmotic membrane | Discarded Osmotic membrane and bioaugmented MC degrading bacterial strain | 836 | 90 | <0.2 | na | na | [147] |
| Constructed wetlands | Constructed wetlands material and biological activity | 14.41 | 80 | <0.5 | na | na | [148] |
* (cells/ml), ** µg/L, a log10 unit, b Chl a removal, na: not applicable.
Eco-technological purification systems for the removal of cyanobacterial contaminants include constructed wetlands (CWs) using various types of plants [145][149][148], depending on whether these plants have purifying effects on the cyanotoxins in question or household slow sand filters [145][149][148]. For instance, a study with CWs [148] found MC-LR reductions from 5.55 g L−1 to less than 0.1 g L−1, as well as a 2.39 log10 unit reduction in Microcystis aeruginosa, while Bavithra et al. [145] showed 94–99% cyanobacteria and microcystins (MC-LR) removal from lake water in CWmicrocosms.
Moreover, in these eco-technologies, some propose the development of biofilm-based reactors by recycling reverse osmosis (RO) membranes used in desalination plants that have reached the end of their working life. The bioaugmentation of these reverse osmosis membranes by a single strain or a bacterial consortium seems to be an ideal solution [147][150], as the removal performances reported are very high (Table 2). In fact, for initial MCs with a concentration ranging from 0.836 to 400 mg/L, removal rates reached 90%. Nevertheless, there are some discussions as to whether this solution can be applied on a large scale given the basic material (RO membrane), as even ancient ones are not available everywhere.
Considering the preliminary results of cyanotoxin removal by the MSL system in the laboratory [146], this technology may be the most promising in this field.
The best eco-technology should be conceivable on a large scale, with the material widely available to offer the best treatment to all who need it. The multi-soil-layering (MSL) system could meet these requirements to treat cyanotoxin-contaminated waters.
The main reasons are that the system requires a small footprint and only simple maintenance, and can handle high hydraulic loads without frequent clogging [102][151][152]. It is easy to set up, operate, and run with little or no energy [150][151]. The MSL system has a continuous operational life of 20 years [140] by treating domestic wastewater without the need to replace or disassemble components to rebuild it. The system is adapted for use in remote places, mainly rural villages, to filter cyanobacteria- and cyanotoxin-contaminated water for reuse in sustainable agriculture. However, the mechanisms involved in removing cyanobacterial contamination in these systems are unknown. Therefore, further studies are needed to understand and improve the MSL system’s performance.
References
- Aguilera, A.; Haakonsson, S.; Martin, M.V.; Salerno, G.L.; Echenique, R.O. Bloom-forming cyanobacteria and cyanotoxins in Argentina: A growing health and environmental concern. Limnologica 2018, 69, 103–114.
- Wood, R. Acute animal and human poisonings from cyanotoxin exposure—A review of the literature. Environ. Int. 2016, 91, 276–282.
- Francis, G. Poisonous Australian lake. Nature 1878, 18, 11–12.
- Stewart, I.; Seawright, A.A.; Shaw, G.R. Cyanobacterial Poisoning in Livestock, Wild Mammals and Birds—An overview. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Advances in Experimental Medicine and Biology; Hudnell, H.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 619, pp. 613–637.
- Thomas, A.D.; Saker, M.L.; Norton, J.H.; Olsen, R.D. Cyanobacterium Cylindrospermopsis raciborskii as a probable cause of death in cattle in northern Queensland. Aust. Vet. J. 1998, 76, 592–594.
- Falconer, I. Algal Toxins and Human Health. In The Handbook of Environmental Chemistry; Hrubec, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 53–82.
- Carmichael, W.W.; Azevedo, S.M.; An, J.S.; Molica, R.J.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668.
- Chen, J.; Xie, P.; Li, L.; Xu, J. First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage. Toxicol. Sci. 2009, 108, 81–89.
- Kubickova, B.; Babica, P.; Hilscherová, K.; ŠIndlerová, L. Effects of cyanobacterial toxins on the human gastrointestinal tract and the mucosal innate immune system. Environ. Sci. Eur. 2019, 31, 1–27.
- Bouaïcha, N.; Miles, C.; Beach, D.; Labidi, Z.; Djabri, A.; Benayache, N.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714.
- ElKhalloufi, F.; Oufdou, K.; Lahrouni, M.; Faghire, M.; Peix, A.; Ramírez-Bahena, M.H.; Vasconcelos, V.; Oudra, B. Physiological and antioxidant responses of Medicago sativa-rhizobia symbiosis to cyanobacterial toxins (Microcystins) exposure. Toxicon 2013, 76, 167–177.
- Via-Ordorika, L.; Fastner, J.; Kurmayer, R.; Hisbergues, M.; Dittmann, E.; Komarek, J.; Erhard, M.; Chorus, I. Distribution of Microcystin-Producing and Non-Microcystin-Producing Microcystis sp. in European Freshwater Bodies: Detection of Microcystins and Microcystin Genes in Individual Colonies. Syst. Appl. Microbiol. 2004, 27, 592–602.
- Brient, L.; ben Gamra, N.; Periot, M.; Roumagnac, M.; Zeller, P.; Bormans, M.; Méjean, A.; Ploux, O.; Biegala, I.C. Rapid Characterization of Microcystin-Producing Cyanobacteria in Freshwater Lakes by TSA-FISH (Tyramid Signal Amplification-Fluorescent in Situ Hybridization). Front. Environ. Sci. 2017, 5, 43.
- Overlingė, D.; Toruńska-Sitarz, A.; Kataržytė, M.; Pilkaitytė, R.; Gyraitė, G.; Mazur-Marzec, H. Characterization and Diversity of Microcystins Produced by Cyanobacteria from the Curonian Lagoon (SE Baltic Sea). Toxins 2021, 13, 838.
- Fortin, N.; Aranda-Rodriguez, R.; Jing, H.; Pick, F.; Bird, D.; Greer, C.W. Detection of Microcystin-Producing Cyanobacteria in Missisquoi Bay, Quebec, Canada, Using Quantitative PCR. Appl. Environ. Microbiol. 2010, 76, 5105–5112.
- Oudra, B.; Dadi-El Andaloussi, M.; Vasconcelos, V.M. Identification and quantification of microcystins from a Nostoc muscorum bloom occurring in Oukaïmeden River (High-Atlas mountains of Marrakech, Morocco). Environ. Monit. Assess. 2008, 149, 437–444.
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; CRC Press: London, UK, 2021.
- Stewart, I.; Eaglesham, G.K.; McGregor, G.B.; Chong, R.; Seawright, A.A.; Wickramasinghe, W.A.; Sadler, R.; Hunt, L.; Graham, G. First Report of a Toxic Nodularia spumigena (Nostocales/ Cyanobacteria) Bloom in Sub-Tropical Australia. II. Bioaccumulation of Nodularin in Isolated Populations of Mullet (Mugilidae). Int. J. Environ. Res. Public Health 2012, 9, 2396–2411.
- Mohamed, Z.; Bakr, A.; Campos, A.; Vasconcelos, V.; Nasr SA, M. Growth inhibition and microcystin accumulation in bush bean (Phaseolus vulgaris L.) plant irrigated with water containing toxic Chrooccocus minutus. Agric. Water Manag. 2022, 261, 107381.
- Mohamed, Z.A. Breakthrough of Oscillatoria limnetica and microcystin toxins into drinking water treatment plants—Examples from the Nile River, Egypt. Water SA 2016, 42, 161.
- Guzmán-Guillén, R.; Prieto, A.I.; Moreno, I.; Vasconcelos, V.M.; Moyano, R.; Blanco, A.; Cameán Fernandez, A.M. Cyanobacterium producing cylindrospermopsin cause histopathological changes at environmentally relevant concentrations in subchronically exposed tilapia (Oreochromis niloticus). Environ. Toxicol. 2013, 30, 261–277.
- Nguyen TT, L.; Hoang, T.H.; Nguyen, T.K.; Duong, T.T. The occurrence of toxic cyanobacterium Cylindrospermopsis raciborskii and its toxin cylindrospermopsin in the Huong River, Thua Thien Hue province, Vietnam. Environ. Monit. Assess. 2017, 189, 490.
- Osswald, J.; Rellán, S.; Gago-Martinez, A.; Vasconcelos, V. Production of anatoxin-a by cyanobacterial strains isolated from Portuguese fresh water systems. Ecotoxicology 2009, 18, 1110–1115.
- Casali, S.P.; Dos Santos, A.C.A.; de Falco, P.B.; Calijuri, M.D.C. Influence of environmental variables on saxitoxin yields by Cylindrospermopsis raciborskii in a mesotrophic subtropical reservoir. J. Water Health 2017, 15, 509–518.
- Harr, K.E.; Szabo, N.J.; Cichra, M.; Phlips, E.J. Debromoaplysiatoxin in Lyngbya-dominated mats on manatees (Trichechus manatus latirostris) in the Florida King’s Bay ecosystem. Toxicon 2008, 52, 385–388.
- Walls, J.T.; Wyatt, K.H.; Doll, J.C.; Rubenstein, E.M.; Rober, A.R. Hot and toxic: Temperature regulates microcystin release from cyanobacteria. Sci. Total Environ. 2018, 610, 786–795.
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725.
- Figueroa-Sanchez, M.A.; Nandini, S.; Sarma, S.S.S. Zooplankton community structure in the presence of low levels of cyanotoxins: A case study in a high altitude tropical reservoir (Valle de Bravo, Mexico). J. Limnol. 2014, 73, 157–166.
- Duan, Z.; Tan, X.; Parajuli, K.; Upadhyay, S.; Zhang, D.; Shu, X.; Liu, Q. Colony formation in two Microcystis morphotypes: Effects of temperature and nutrient availability. Harmful Algae 2018, 72, 14–24.
- Rollwagen-bollens, G.; Lee, T.; Rose, V.; Bollens, S.M. Beyond Eutrophication: Vancouver Lake, WA, USA as a Model System for Assessing Multiple, Interacting Biotic and Abiotic Drivers of Harmful Cyanobacterial Blooms. Water 2018, 10, 757.
- Corbel, S.; Mougin, C.; Martin-laurent, F.; Crouzet, O.; Bru, D.; Nélieu, S.; Bouaïcha, N. Evaluation of phytotoxicity and ecotoxicity potentials of a cyanobacterial extract containing microcystins under realistic environmental concentrations and in a soil-plant system. Chemosphere 2015, 128, 332–340.
- Jankowiak, J.; Hattenrath-Lehmann, T.; Kramer, B.J.; Ladds, M.; Gobler, C.J. Deciphering the effects of nitrogen, phosphorus, and temperature on cyanobacterial bloom intensification, diversity, and toxicity in western Lake Erie. Limnol. Oceanogr. 2019, 64, 1347–1370.
- Davis, T.W.; Harke, M.J.; Marcoval, M.A.; Goleski, J.; Orano-dawson, C.; Berry, D.L.; Gobler, C.J. Effects of nitrogenous compounds and phosphorus on the growth of toxic and non-toxic strains of Microcystis during cyanobacterial blooms. Aquat Microb. Ecol. 2010, 61, 149–162.
- Carpenter, S.R. Eutrophication of aquatic ecosystems: Bistability and soil phosphorus. Proc. Natl. Acad. Sci. USA 2005, 102, 10002–10005.
- Yang, X.; Wu, X.; Hao, H.; He, Z. Mechanisms and assessment of water eutrophication. J. Zhejiang Univ. Sci. B 2008, 9, 197–209.
- Chaffin, J.D.; Bridgeman, T.B. Organic and inorganic nitrogen utilization by nitrogen-stressed cyanobacteria during bloom conditions. J. Appl. 2014, 26, 299–309.
- Lu, J.; Zhu, B.; Struewing, I.; Xu, N.; Duan, S. Nitrogen-phosphorus- associated metabolic activities during the development of a cyanobacterial bloom revealed by metatranscriptomics. Sci. Rep. 2019, 9, 2480.
- WHO. Guidelines for Drinking-Water Quality; WORLD Health Organization: Geneva, Switzerland, 2004.
- Von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467.
- Chang, J.; Chen, Z.; Wang, Z.; Shen, J.; Chen, Q. Ozonation degradation of microcystin-LR in aqueous solution: Intermediates, byproducts, and pathways. Water Res. 2014, 63, 52–61.
- Lu, S.Y.; Wang, N.Y.; Wang, C. Oxidation and biotoxicity assessment of microcystin-LR using different AOPs based on UV, O3, and H2O2. Front. Environ. Sci. Eng. 2018, 12.
- Von Gunten, U. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodide, or chlorine. Water Res. 2003, 37, 1469–1487.
- Sharma, V.K.; Triantis, T.M.; Antoniou, M.G.; He, X.; Pelaez, M.; Han, C.; Song, W.; O’Shea, K.E.; Armah, A.; Kaloudis, T.; et al. Destruction of microcystins by conventional and advanced oxidation processes: A review. Sep. Purif. Technol. 2012, 91, 3–17.
- Yang, Y.; Yu, G.; Chen, Y.; Jia, N.; Li, R. Four decades of progress in cylindrospermopsin research: The ins and outs of a potent cyanotoxin. J. Hazard. Mater. 2021, 406, 124653.
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349.
- Merel, S.; Clément, M.; Thomas, O. State of the art on cyanotoxins in water and their behaviour towards chlorine. Toxicon 2010, 55, 677–691.
- Staehelld, J.; Jurg, H.; Cited, L.; Staehelld, J.; Jurg, H. Decomposition of Ozone in Water in the Presence of Organic Solutes Acting as Promoters and Inhibitors of Radical Chain Reactions. Environ. Sci. Technol. 1985, 19, 1206–1213.
- Ershov, B.G.; Morozov, P.A. The Kinetics of Ozone Decomposition in Water, the Influence of pH and Temperature. Chem. Kinet. Catal. 2009, 83, 1295–1299.
- Yershov, B.G.; Morozov, P.A.; Gordeev, A.V.; Seliverstov, A.F. Kinetic Regularities of Ozone Decomposition in Water. Phys. Chem. Water Treat. Processes 2009, 31, 381–388.
- Chang, J.; Chen, Z.; Wang, Z.; Kang, J.; Chen, Q.; Yuan, L.; Shen, J. Oxidation of microcystin-LR in water by ozone combined with UV radiation: The removal and degradation pathway. Chem. Eng. J. 2015, 276, 97–105.
- Onstad, G.D.; Strauch, S.; Meriluoto, J.; Codd, G.A.; Von Gunten, U. Selective oxidation of key functional groups in cyanotoxins during drinking water ozonation. Environ. Sci. Technol. 2007, 41, 4397–4404.
- Hitzfeld, B.C.; Höger, S.J.; Dietrich, D.R.; Hoger, S.J.; Dietrich, D.R. Cyanobacterial Toxins: Removal during Drinking Water Treatment, and Human Risk Assessment Cyanobacteria. Environ. Health Perspect. 2000, 108, 113–122.
- Xie, G.; Hu, X.; Du, Y.; Jin, Q.; Liu, Y.; Tang, C.; Hu, X.; Li, G.; Chen, Z.; Zhou, D.; et al. Light-driven breakdown of microcystin-LR in water: A critical review. Chem. Eng. J. 2021, 417, 129244.
- Bai, M.; Zheng, Q.; Zheng, W.; Li, H.; Lin, S.; Huang, L. OH, Inactivation of Cyanobacterial Blooms and Degradation of Toxins in Drinking Water Treatment System. Water Res. 2019, 154, 144–152.
- Li, H.; Bai, M.; Yang, X.; Zhong, Z.; Gao, M.; Tian, Y. OH, pre-treatment of algae blooms and degradation of microcystin-LR in a drinking water system of 480 m3/day: Comparison with ClO2. Chem. Eng. J. 2019, 367, 189–197.
- Papadimitriou, T.; Kormas, K.; Dionysiou, D.D. Using H2O2 treatments for the degradation of cyanobacteria and microcystins in a shallow hypertrophic reservoir. Environ. Sci. Pollut. Res. 2016, 23, 21523–21535.
- He, X.; de la Cruz, A.A.; Hiskia, A.; Kaloudis, T.; O’Shea, K.; Dionysiou, D.D. Destruction of microcystins (cyanotoxins) by UV-254nm-based direct photolysis and advanced oxidation processes (AOPs): Influence of variable amino acids on the degradation kinetics and reaction mechanisms. Water Res. 2015, 74, 227–238.
- Jasim, S.Y.; Saththasivam, J. Advanced oxidation processes to remove cyanotoxins in water. Desalination 2017, 406, 83–87.
- Kumar, P.; Dayana, H.; Rubio, P.; Hegde, K.; Kaur, S.; Cledon, M.; Kermanshahi-pour, A.; Sauvé, S. Agro-industrial residues as unique support in a sand filter to enhance the bioactivity to remove microcystin-Leucine arginine and organics. Sci. Total Environ. 2019, 670, 971–981.
- Miao, H.; Qin, F.; Tao, G.; Tao, W.; Ruan, W. Detoxification and degradation of microcystin-LR and -RR by ozonation. Chemosphere 2010, 79, 355–361.
- Griffiths, D.J.; Saker, M.L. The Palm Island mystery disease 20 years on a review of research on the cyanotoxin cylindrospermopsin. Environ. Toxicol. 2003, 18, 78–93.
- Serrà, A.; Philippe, L.; Perreault, F.; Garcia-Segura, S. Photocatalytic treatment of natural waters. Reality or hype ? The case of cyanotoxins remediation. Water Res. 2021, 188, 116543.
- Kibuye, F.A.; Zamyadi, A.; Wert, E.C. A critical review on operation and performance of source water control strategies for cyanobacterial blooms: Part I-chemical control methods. Harmful Algae 2021, 109, 102099.
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60.
- Lin, Y.; Chen, A.; Peng, L.; Luo, S.; Zeng, Q.; Shao, J. Physiological characteristics and toxin production of Microcystis aeruginosa (Cyanobacterium) in response to DOM in anaerobic digestion effluent. Sci. Total Environ. 2019, 685, 902–910.
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. Coagulation and Flocculation, MWH’s Water Treatment: Principles and Design, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 541–639.
- Gągała, I.; Mankiewicz-boczek, J. The Natural Degradation of Microcystins (Cyanobacterial Hepatotoxins) in Fresh Water—The Future of Modern Treatment Systems and Water Quality Improvement. Pol. J. Environ. Stud. 2012, 21, 1125–1139.
- Romero, L.G.; Mondardo, R.I.; Sens, M.L.; Grischek, T. Removal of cyanobacteria and cyanotoxins during lake bank filtration at Lagoa do Peri, Brazil. Clean Technol. Environ Policy 2014, 16, 1133–1143.
- Şengül, A.B.; Ersan, G.; Tüfekçi, N. Removal of intra- and extracellular microcystin by submerged ultrafiltration (UF) membrane combined with coagulation/flocculation and powdered activated carbon (PAC) adsorption. J. Hazard. Mater. 2018, 343, 29–35.
- Frišták, V.; Laughinghouse, H.D.; Bell, S.M. The Use of Biochar and Pyrolysed Materials to Improve Water Quality through Microcystin Sorption Separation. Water 2020, 12, 2871.
- Chaffin, J.D.; Fox, E.L.; Nauman, C.A.; Slodysko, K.N. The ability of household pitcher-style water purifiers to remove microcystins depends on filtration rate and activated carbon source. Water Science and Technology: Water Supply 2019, 19, 336–345.
- Abbas, T.; Kajjumba, G.W.; Ejjada, M.; Masrura, S.U.; Marti, E.J.; Khan, E.; Jones-Lepp, T.L. Recent Advancements in the Removal of Cyanotoxins from Water Using Conventional and Modified Adsorbents—A Contemporary Review. Water 2020, 12, 2756.
- Huang, W.; Cheng, B.; Cheng, Y. Adsorption of microcystin- LR by three types of activated carbon. J. Hazard. Mater. 2007, 141, 115–122.
- Villars, K.; Huang, Y.; Lenhart, J.J. Drinking Water Using Granular Activated Carbon. Environ. Eng. Sci. 2020, 37, 585–595.
- Pendleton, P.; Schumann, R.; Wong, S.H. Microcystin-LR adsorption by activated carbon. J. Colloid Interface Sci. 2001, 240, 1–8.
- Robert, L.; Burwell, J.R. Manualof symbols and terminology for physicochemical quantities and units—Appendix ii. Pure Appl. Chem. 1976, 46, 71–90.
- Zdravkov, B.D.; Čermák, J.J.; Šefara, M.; Janků, J.; Sefara, M.; Zdravkov, B.D.; Čermák, J.J.; Šefara, M.; Janků, J.; Sefara, M. Pore classification in the characterization of porous materials: A perspective. Cent. Eur. J. Chem. 2007, 5, 385–395.
- Araújo, L.S.; Coutinho, A.D.; Alvarez-Mendez, M.O.; Moruzzi, R.B.; Calijuri, M.D.; Cunha, D.G. Caracterização e avaliação de fatores que determinam a remoção de microcistin-LR em carvão ativado granular produzido a partir de diferentes matérias-primas. Eng. Sanit. E Ambient. 2018, 23, 1131–1142.
- Wu, X.; Xiao, B.; Li, R.; Wang, C.; Huang, J.; Wang, Z. Mechanisms and Factors Affecting Sorption of Microcystins onto Natural Sediments. Environ. Sci. Technol. 2011, 45, 2641–2647.
- Albuquerque Júnior, E.C.; Méndez, M.O.; Coutinho, A.D.; Franco, T.T. Removal of Cyanobacteria Toxins from Drinking Water by Adsorption on Activated Carbon Fibers. Mater. Res. 2008, 11, 371–380.
- Bajracharya, A.; Liu, Y.L.; Lenhart, J.J. The influence of natural organic matter on the adsorption of microcystin-LR by powdered activated carbon. Environ. Sci. Water Res. Technol. 2019, 5, 582.
- Zhao, Y.X.; Gao, B.Y.; Shon, H.K.; Cao, B.C.; Kim, J.H. Coagulation characteristics of titanium (Ti) salt coagulant compared with aluminum (Al) and iron (Fe) salts. J. Hazard. Mater. 2011, 185, 1536–1542.
- Xu, J.; Zhao, Y.; Gao, B.; Zhao, Q. Enhanced algae removal by Ti-based coagulant: Comparison with conventional Al- and Fe-based coagulants. Environ. Sci. Pollut. Res. 2018, 25, 13147–13158.
- Fuentes, M.; Olaetxea, M.; Baigorri, R.; Zamarreño, A.M.; Etienne, P.; Laîné, P.; Ourry, A.; Yvin, J.; Garcia-mina, J.M. Main binding sites involved in Fe (III) and Cu (II) complexation in humic-based structures. J. Geochem. Explor. J. 2013, 129, 14–17.
- Klein, A.R.; Baldwin, D.S.; Silvester, E. Proton and iron-binding by the cyanobacterial toxin microcystin-LR. Environ. Sci. Technol. 2013, 47, 5178–5184.
- Pochodylo, A.L.; Klein, A.R.; Aristilde, L. Metal-binding selectivity and coordination dynamics for cyanobacterial microcystins with Zn, Cu, Fe, Mg, and Ca. Environ. Chem. Lett. 2017, 15, 695–701.
- Dai, G.; Gan, N.; Song, L.; Fang, S.; Peng, N. Fast adsorption of microcystin-LR by Fe (III)-modified powdered activated carbon. J. Oceanol. Limnol. 2018, 36, 1103–1111.
- El Bouaidi, W.; Essalhi, S.; Douma, M.; Tazart, Z.; Ounas, A.; Enaime, G.; Yaacoubi, A. Evaluation of the potentiality of Vicia faba and Opuntia ficus indica as eco-friendly coagulants to mitigate Microcystis aeruginosa blooms. Desalination Water Treat. 2020, 195, 198–213.
- Nakamura, T.; Adachi, Y.; Suzuki, M. Flotation and sedimentation of a single Microcystis floc collected from surface bloom. Water Res. 1993, 27, 979–983.
- Zhu, W.; Li, M.; Luo, Y.; Dai, X.; Guo, L.; Xiao, M.; Huang, J.; Tan, X. Vertical distribution of Microcystis colony size in Lake Taihu: Its role in algal blooms. J. Great Lakes Res. 2014, 40, 949–955.
- Piezer, K.; Li, L.; Jeon, Y.; Kadudula, A.; Seo, Y. The Application of Potassium Permanganate to Treat Cyanobacteria-Laden Water: A Review. Process Saf. Environ. Prot. 2021, 148, 400–414.
- Xu, Z.; Woodhouse, J.N.; Te, S.H.; Yew-Hoong Gin, K.; He, Y.; Xu, C.; Chen, L.; Gin, K.Y.; He, Y. Seasonal variation in the bacterial community composition of a large estuarine reservoir and response to cyanobacterial proliferation. Chemosphere 2018, 202, 576–585.
- Omori, K.; Sato, M.; Amano, Y.; Machida, M. Induction of colony formation of Microcystis aeruginosa by controlling extracellular polysaccharides and metal cation concentrations. J. Chem. Eng. Jpn. 2018, 51, 289–297.
- Sakurai, S.; Omori, K.; Amano, Y.; Machida, M. Removal of Microcystis blooms using enhanced colony formation and buoyancy by controlling extracellular polysaccharides and cation concentrations. Int. J. Environ. Sci. Technol. 2019, 16, 4793–4802.
- Hadjoudja, S.; Deluchat, V.; Baudu, M. Cell surface characterisation of Microcystis aeruginosa and Chlorella vulgaris. J. Colloid Interface Sci. 2010, 342, 293–299.
- Takaara, T.; Sano, D.; Masago, Y.; Omura, T. Surface-retained organic matter of Microcystis aeruginosa inhibiting coagulation with polyaluminum chloride in drinking water treatment. Water Res. 2010, 44, 3781–3786.
- Amano, Y.; Hosoi, T.; Machida, M.; Imazeki, F. Effects of extracellular polymeric substances (EPS) and iron on colony formation of unicellular Microcystis aeruginosa. J. Jpn. Soc. Civ. Eng. 2013, 69, 39–44.
- Xu, H.; Yu, G.; Jiang, H. Investigation on extracellular polymeric substances from mucilaginous cyanobacterial blooms in eutrophic freshwater lakes. Chemosphere 2013, 93, 75–81.
- Pochodylo, A.L.; Aoki, T.G.; Aristilde, L. Adsorption mechanisms of microcystin variant conformations at water–mineral interfaces: A molecular modeling investigation. J. Colloid Interface Sci. 2016, 480, 166–174.
- Sato, M.; Datta, T.; Amano, Y.; Omori, K. Influence of Extracellular Polysaccharides and Calcium Ion on Colony Formation of Unicellular Microcystis aeruginosa. Environ. Eng. Sci. 2017, 34, 149–157.
- Sato, M.; Amano, Y.; Machida, M.; Imazeki, F. Colony formation of highly dispersed Microcystis aeruginosa by controlling extracellular polysaccharides and calcium ion concentrations in aquatic solution. Limnology 2017, 18, 111–119.
- Bali, M.; Gueddari, M. Removal of phosphorus from secondary effluents using infiltration–percolation process. Appl. Water Sci. 2019, 9, 54.
- Latrach, L.; Ouazzani, N.; Masunaga, T.; Hejjaj, A.; Bouhoum, K.; Mahi, M.; Mandi, L. Domestic wastewater disinfection by combined treatment using multi-soil-layering system and sand filters (MSL-SF): A laboratory pilot study. Ecol. Eng. 2016, 91, 294–301.
- Corbel, S.; Bouaïcha, N.; Mougin, C. Dynamics of the toxic cyanobacterial microcystin-leucine-arginine peptide in agricultural soil. Environ. Chem. Lett. 2014, 12, 535–541.
- Holst, T.; Jørgensen, N.O.; Jørgensen, C.; Johansen, A. Degradation of microcystin in sediments at oxic and anoxic, denitrifying conditions. Water Res. 2003, 37, 4748–4760.
- Babica, P.; Kohoutek, J.; Bláha, L.; Adamovský, O.; Maršálek, B.; Per, O.L.P.A. Evaluation of extraction approaches linked to ELISA and HPLC for analyses of microcystin-LR, -RR, and -YR in freshwater sediments with different organic material contents. Anal. Bioanal. Chem. 2006, 385, 1545–1551.
- Chen, W.; Song, L.; Gan, N.; Li, L. Sorption, degradation, and mobility of microcystins in Chinese agriculture soils: Risk assessment for groundwater protection. Environ. Pollut. 2006, 144, 752–758.
- Chen, W.; Li, L.; Gan, N.; Song, L. Optimization of an effective extraction procedure for the analysis of microcystins in soils and lake sediments. Environ. Pollut. 2006, 143, 241–246.
- Tsuji, K.; Masui, H.; Uemura, H.; Mori, Y.; Harada, K. Analysis of microcystins in sediments using MMPB method. Toxicon 2021, 39, 687–692.
- De Maagd, P.G.; Hendriks, A.J.; Seinen, W.; Sijm, D.T. pH-dependent hydrophobicity of the cyanobacteria toxin microcystin-LR. Water Res. 1999, 33, 677–680.
- Miller, M.J.; Critchley, M.M.; Hutson, J.; Fallowfield, H.J. The adsorption of cyanobacterial hepatotoxins from water onto soil during batch experiments. Water Res. 2001, 35, 1461–1468.
- Chénard, C.; Kolundžija, S.; Lauro, F.M.; Kolund, S.; Lauro, F.M. Complete genome sequence of the cyanophage S-PRM1 isolated from Singapore coastal waters. Mar. Genom. 2018, 43, 58–60.
- Kang, S.; Xing, B. Adsorption of dicarboxylic acids by clay minerals as examined by in situ ATR-FTIR and ex situ DRIFT. Langmuir 2007, 23, 7024–7031.
- Morris, R.J.; Williams, D.E.; Luu, H.A.; Holmes, C.F.; Andersen, R.J.; Calvert, S.E. The adsorption of microcystin-LR by natural clay particles. Toxicon 2000, 38, 303–308.
- Liu, Y.L.; Walker, H.W.; Lenhart, J.J. Adsorption of microcystin-LR onto kaolinite, illite, and montmorillonite. Chemosphere 2019, 220, 696–705.
- Naidja, A.; Huang, P.M. Aspartic acid interaction with Ca-montmorillonite: Adsorption, desorption, and thermal stability. Appl. Clay Sci. 1994, 9, 265–281.
- Thirumavalavan, M.; Hu, Y.; Lee, J. Effects of humic acid and suspended soils on adsorption and photo-degradation of microcystin-LR onto samples from Taiwan reservoirs and rivers. J. Hazard. Mater. 2012, 217, 323–329.
- Munusamy, T.; Hu, Y.; Lee, J. Adsorption and photodegradation of microcystin-LR onto sediments collected from reservoirs and rivers in Taiwan: A laboratory study to investigate the fate, transfer, and degradation of microcystin-LR. Environ. Sci. Pollut. Res. 2012, 19, 2390–2399.
- Santos, A.; Rachid, C.; Pachecoc, A.B.; Magalhães, V. Biotic and abiotic factors affect microcystin-LR concentrations in water/ sediment interface. Microbiol. Res. 2020, 236, 126452.
- Corbel, S.; Mougin, C.; Bouaïcha, N. Cyanobacterial toxins: Modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 2014, 96, 1–15.
- Mohamed, Z.A.; Al Shehri, A.M. Microcystins in groundwater wells and their accumulation in vegetable plants irrigated with contaminated waters in Saudi Arabia. J. Hazard. Mater. 2009, 172, 310–315.
- Cousins, I.T.; Bealing, D.J.; James, H.A.; Sutton, A.; Vol, W.R. Biodegradation of microcystin-LR by indigenous mixed bacterial populations. Water Res. 1996, 30, 481–485.
- El Khalloufi, F.; Oufdou, K.; Bertrand, M.; Lahrouni, M.; Oudra, B.; Ortet, P.; Barakat, M.; Heulin, T.; Achouak, W. Microbiote shift in the Medicago sativa rhizosphere in response to cyanotoxins extract exposure. Sci. Total Environ. 2015, 539, 135–142.
- Redouane, E.M.; El Amrani Zerrifi, S.; El Khalloufi, F.; Oufdou, K.; Oudra, B.; Lahrouni, M.; Campos, A.; Vasconcelos, V. Mode of action and faith of microcystins in the complex soil-plant ecosystems. Chemosphere 2019, 225, 270–281.
- Lemes GA, F.; Kist, L.W.; Bogo, M.R.; Yunes, J.S. Biodegradation of microcystin-LR by a bacterium isolated from sediment of Patos Lagoon estuary, Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 1–8.
- Chen, W.; Song, L.; Peng, L.; Wan, N.; Zhang, X.; Gan, N. Reduction in microcystin concentrations in large and shallow lakes: Water and sediment-interface contributions. Water Res. 2007, 42, 763–773.
- Terin, U.C.; Sabogal-Paz, L.P. Microcystis aeruginosa and microcystin-LR removal by household slow sand filters operating in continuous and intermittent flows. Water Res. 2019, 150, 29–39.
- Su, X.; Steinman, A.D.; Tang, X.; Xue, Q.; Zhao, Y.; Xie, L. Response of bacterial communities to cyanobacterial harmful algal blooms in Lake Taihu, China. Harmful Algae 2017, 68, 168–177.
- Li, H.; Xing, P.; Chen, M.; Bian, Y.Y.; Wu, Q.L. Short-term bacterial community composition dynamics in response to accumulation and breakdown of Microcystis blooms. Water Res. 2011, 45, 1702–1710.
- Lezcano, M.Á.; Velázquez, D.; Quesada, A.; El-shehawy, R. Diversity and temporal shifts of the bacterial community associated with a toxic cyanobacterial bloom: An interplay between microcystin producers and degraders. Water Res. 2017, 125, 52–61.
- Kumar, P.; Hegde, K.; Kaur, S.; Cledon, M.; Brar, S.K.; Cledon, M.; Kermanshahi-pour, A. Potential of biological approaches for cyanotoxin removal from drinking water: A review. Ecotoxicol. Environ. Saf. 2019, 172, 488–503.
- Kormas, K.A.; Lymperopoulou, D.S. Cyanobacterial Toxin Degrading Bacteria: Who Are They? BioMed Res. Int. 2013, 2013, 463894.
- Bourne, D.G.; Blakeley, R.L.; Riddles, P.; Jones, G.J. Biodegradation of the cyanobacterial toxin microcystin LR in natural water and biologically active slow sand filters. Water Res. 2006, 40, 1294–1302.
- Li, J.; Li, J.; Shi, G.; Mei, Z.; Wang, R.; Li, D. Discerning biodegradation and adsorption of microcystin-LR in a shallow semi-enclosed bay and bacterial community shifts in response to associated process. Ecotoxicol. Environ. Saf. 2016, 132, 123–131.
- Tsao, S.; Wei, D.J.; Chang, Y.T.; Lee, J.F. Aerobic biodegradation of microcystin-LR by an indigenous bacterial mixed culture isolated in Taiwan. Int. Biodeterior. Biodegrad. 2017, 124, 101–108.
- Wang, R.; Tai, Y.; Wan, X.; Ruan, W.; Man, Y.; Wang, J.; Yang, Y.; Yang, Y. Enhanced removal of Microcystis bloom and microcystin-LR using microcosm constructed wetlands with bioaugmentation of degrading bacteria. Chemosphere 2018, 210, 29–37.
- Eleuterio, L.; Batista, J.R. Biodegradation studies and sequencing of microcystin-LR degrading bacteria isolated from a drinking water biofilter and a fresh water lake. Toxicon 2010, 55, 1434–1442.
- Schmidt, J.R.; Wilhelm, S.W.; Boyer, G.L. The fate of microcystins in the environment and challenges for monitoring. Toxins 2014, 6, 3354–3387.
- Gurbuz, F.; Codd, G.A. Microcystin Removal by a Naturally-Occurring Substance: Pumice. Bull. Environ. Contam. Toxicol. 2008, 81, 323–327.
- Chen, X.; Yang, X.; Yang, L.; Xiao, B.; Wu, X.; Wang, J.; Wan, H. An effective pathway for the removal of microcystin LR via anoxic biodegradation in lake sediments. Water Res. 2010, 44, 1884–1892.
- Kumar, P.; Hegde, K.; Brar, S.K.; Kermanshahi-pour, A.; Roy-lachapelle, A.; Galvez-cloutier, R. Co-culturing of Native Bacteria from Drinking Water Treatment Plant with Known—Biorefining and Remediation Laboratory, Department of Process Engineering and Applied. Chem. Eng. J. 2019, 383, 123090.
- Kumar, P.; Hegde, K.; Brar, S.K.; Cledon, M.; Kermanshahi-pour, A.; Roy-Lachapelle, A.; Galvez-Cloutier, R. Novel fluidized-bed biofilm reactor for concomitant removal of microcystin-LR and organics. Chem. Eng. J. 2019, 359, 99–111.
- Jeon, Y.; Li, L.; Calvillo, J.; Ryu, H.; Santo Domingo, J.W.; Choi, O.; Brown, J.; Seo, Y. Impact of algal organic matter on the performance, cyanotoxin removal, and biofilms of biologically-active filtration systems. Water Res. 2020, 184, 116120.
- Westrick, J.A.; Szlag, D.C.; Southwell, B.J.; Sinclair, J.A.; Szlag, D.C.; Southwell, B.J.; Sinclair, J. A Review of Cyanobacteria and Cyanotoxins Removal/Inactivation in Drinking Water Treatment. In Analytical and Bioanalytical Chemistry; Springer Science and Business: Berlin/Heidelberg, Germany, 2010; Volume 397, pp. 1705–1714.
- Bavithra, G.; Azevedo, J.; Oliveira, F.; Morais, J.; Pinto, E.; Ferreira, I.M.; Vasconcelos, V.; Campos, A.; Almeida, C.M. Assessment of Constructed Wetlands’ Potential for the Removal of Cyanobacteria and Microcystins (MC-LR). Water 2020, 12, 10.
- Aba, R.P.; Mugani, R.; Hejjaj, A.; Brugerolle de Fraissinette, N.; Oudra, B.; Ouazzani, N.; Campos, A.; Vasconcelos, V.; Carvalho, P.N.; Mandi, L. First Report on Cyanotoxin (MC-LR) Removal from Surface Water by Multi-Soil-Layering (MSL) Eco-Technology: Preliminary Results. Water 2021, 13, 1403.
- Morón-López, J.; Nieto-Reyes, L.; Molina, S.; Lezcano, M.Á. Exploring microcystin-degrading bacteria thriving on recycled membranes during a cyanobacterial bloom. Sci. Total Environ. 2020, 736, 139672.
- Cheng, R.; Zhu, H.; Shutes, B.; Yan, B. Treatment of microcystin (MC-LR) and nutrients in eutrophic water by constructed wetlands: Performance and microbial community. Chemosphere 2021, 263, 128139.
- Kochi, L.Y.; Freitas, P.L.; Maranho, L.T.; Juneau, P.; Gomes, M.P. Aquatic macrophytes in constructed wetlands: A fight against water pollution. Sustainability 2020, 12, 9202.
- Morón-López, J.; Molina, S. Optimization of Recycled-Membrane Biofilm Reactor (R-MBfR) as a sustainable biological treatment for microcystins removal. Biochem. Eng. J. 2020, 153, 107422.
- Wei, C.; Wu, W. Performance of single-pass and by-pass multi-step multi-soil-layering systems for low- (C/N) -ratio polluted river water treatment. Chemosphere 2018, 206, 579–586.
- Song, P.; Huang, G.; An, C.; Shen, J.J.; Zhang, P.; Chen, X.; Shen, J.J.; Yao, Y. Treatment of rural domestic wastewater using multi-soil-layering systems: Performance evaluation, factorial analysis, and numerical modeling. Sci. Total Environ. 2018, 644, 536–546.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
06 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





