
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kester Bull-Hereñu | -- | 1061 | 2022-04-01 21:07:16 | | | |
| 2 | Beatrix Zheng | + 244 word(s) | 1305 | 2022-04-02 03:32:09 | | | | |
| 3 | Kester Bull-Hereñu | -249 word(s) | 1056 | 2022-04-04 09:47:35 | | | | |
| 4 | Kester Bull-Hereñu | + 152 word(s) | 1208 | 2022-04-06 09:20:57 | | | | |
| 5 | Beatrix Zheng | Meta information modification | 1208 | 2022-04-06 09:52:08 | | | | |
| 6 | Beatrix Zheng | Meta information modification | 1208 | 2022-04-06 09:52:39 | | | | |
| 7 | Beatrix Zheng | -1 word(s) | 1207 | 2022-04-06 10:10:23 | | |
Video Upload Options
Floral organs develop within a bud enclosed by previously formed organs, leaves, and axes. The tight junction among structures and the observation of contact margins and associated shapes in the bud suggest the influence of forces on the flower configuration. The rationale here is that the sections of the floral bud that are under higher pressure are delayed in the inception and growth of an organ or prevented from initiating, while areas with less pressure would have a more rapid organ initiation and growth.
The occurrence and reach of these forces can be categorized as: 1. Effects of bracts and inflorescence axis pressing against the flower meristem; 2. Effects of involucra in flowers, floral units, and inflorescences; 3. Within-flower organ interaction.
1. Bracts and Inflorescence Axis Pressing against the Flower Meristem
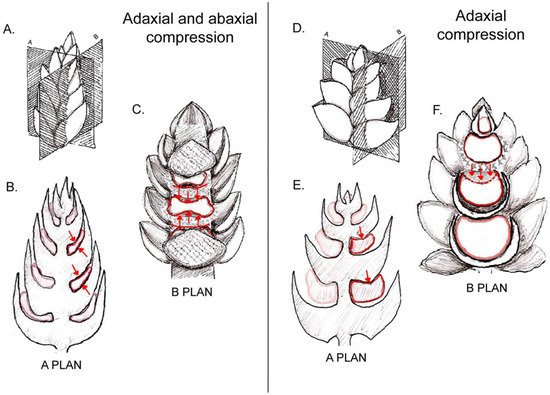
2. Involucra in Flowers, Floral units, and Inflorescences
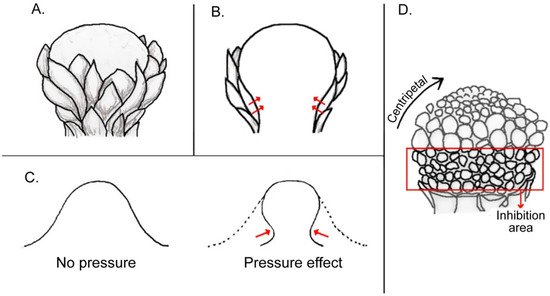
3. Within-Flower Organ Interaction
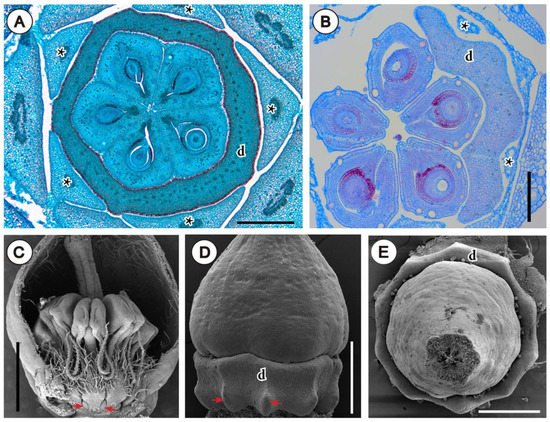
4. Conclusions and Outcomes
References
- Claßen-Bockhoff, R.; Bull-Hereñu, K. Towards an ontogenetic understanding of inflorescence diversity. Ann. Bot. 2013, 112, 1523–1542.
- Sun, J.; Gong, Y.; Renner, S.S.; Huang, S. Multifunctional Bracts in the Dove Tree Davidia involucrata (Nyssaceae: Cornales): Rain protection and pollinator attraction. Am. Nat. 2008, 171, 119–124.
- Yang, Y.; Sun, H. The Bracts of Saussurea velutina (Asteraceae) Protect inflorescences from fluctuating weather at high elevations of the Hengduan mountains, Southwestern China. Arc. Antarct. Alp. Res. 2009, 41, 515–521.
- Gagliardi, K.B.; Cordeiro, I.; Demarco, D. Protection and attraction: Bracts and secretory structures in reduced inflorescences of Malpighiales. Flora 2016, 220, 52–62.
- Camazine, S.; Niklas, K.J. Aerobiology of Symplocarpus foetidus: Interactions between the spathe and spadix. Am. J. Bot. 1984, 71, 843–850.
- Poisson, G.; Barabé, D. Arcitecture de l’appareil végetatif et organisation florale du Dracontium polyphyllum L. (Araceae). Adansonia 1998, 20, 195–210.
- Barabé, D.; Lacroix, C. Developmental morphology of the flower of Anaphyllopsis amricana and its relevance to our understanding of basal Araceae. Botany 2008, 6, 1467–1473.
- González, F.; Bello, M.A. Intra-individual variation of flowers in Gunnera subgenus Panke (Gunneraceae) and proposed apomorphies for Gunnerales. Bot. J. Linn. Soc. 2009, 160, 262–283.
- Leins, P.; Erbar, C. Studien zur Blütenentwicklung an Compositen. Bot. Jahrb. Syst. 1987, 108, 381–401.
- Harris, E.M.; Tucker, S.C.; Urbatsch, L.E. Floral initiation and early development in Erigeron philadelphicus (Asteraceae). Am. J. Bot. 1991, 78, 108–121.
- Dadpour, M.R.; Naghiloo, S.; Gohari, G. Inflorescence and floral ontogeny in Osteospermum ecklonis (Asteraceae). Botany 2011, 89, 605–614.
- Oraei, M.; Gohari, G.; Esmaillou, Z.; Naghiloo, S. Comparative ontogeny of perfect and pistillate florets in Senecio vernalis (Asteraceae). Flora 2013, 208, 285–292.
- Jerominek, M.; Bull-Hereñu, K.; Arndt, M.; Claßen-Bockhoff, R. Live imaging of developmental processes in a living meristem of Davidia involucrata (Nyssaceae). Front. Plant Sci. 2014, 5, 613.
- Heß, D. Die Blüte. Struktur, Funktion, Ökologie, Evolution; Ulmer: Stuttgart, Germany, 1983.
- Ramp, E. Struktur, Funktion und Systematische Bedeutung des Gynoeciums bei den Rutaceae und Simaroubaceae. Ph.D. Thesis, University of Zurich, Zurich, Switzerland, 1998.




