Floral organs develop within a bud enclosed by previously formed organs, leaves, and axes. The tight junction among structures and the observation of contact margins and associated shapes in the bud suggest the influence of forces on the flower configuration. The rationale here is that the sections of the floral bud that are under higher pressure are delayed in the inception and growth of an organ or prevented from initiating, while areas with less pressure would have a more rapid organ initiation and growth. https://www.mdpi.com/2223-7747/11/5/661
The occurrence and reach of these forces can be categorized as:
1. Effects of bracts and inflorescence axis pressing against the flower meristem
2. Effects of involucra in flowers, floral units, and inflorescences
3. Within-flower organ interaction
1. Bracts and Inflorescence Axis Pressing against the Flower Meristem
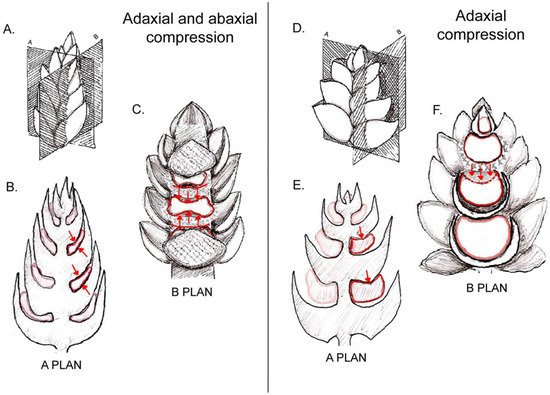
Lateral flowers are born from lateral meristems that develop in the axils of bracts. When the bract is of a rigid configuration, rapidly developing and tightly pressed against the axis, it reduces the available space for the axillary meristem and compresses the young developing flower against the axis. When the pressure of the bract is extensive, the floral primordium becomes squeezed and even flattened (Figure 1A–C). When the effect is weaker, the inhibition is restricted to one of the sides of the floral primordium, either abaxial (to the side of the bract) or adaxial (towards the inflorescence axis, Figure 1D–F).
Figure 1. Examples of possible compression forces on floral primordia derived from subtending bracts and inflorescence axis: (A) scheme of an inflorescence bud and spatial orientation of sections shown in diagrams in (B,C). (B) Longitudinal radial section (corresponding to plan A shown in (A)) showing squeezed floral primordia as a consequence of a “sandwich” effect caused by compressing bracts. (C) Longitudinal tangential section (corresponding to plan B shown in (A)) revealing the elongated shape of floral primordia due to both ad- and abaxial pressure from bracts. (D) Scheme of an inflorescence bud and spatial orientation of sections of it shown in diagrams in (E,F). (E) Longitudinal radial section of inflorescence bud (corresponding to plan A shown in (D)) showing deformation on adaxial side of a floral primordium caused by its overlying bract. (F) Longitudinal section of inflorescence bud through plan B shown in (D). Note that the oppressed adaxial zone of the primordium is not capable of producing floral organs.
2. Involucra in Flowers, Floral units, and Inflorescences
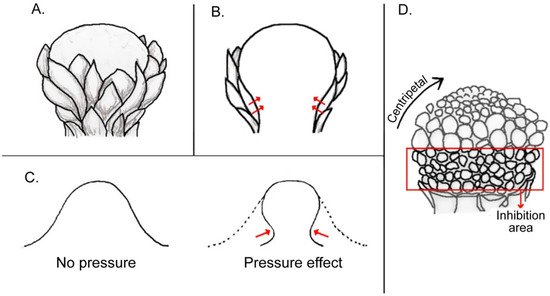
Specialized foliar organs such as an involucrum and spathe may surround flower-like inflorescences, which are known as ‘floral units’ [
41] (such as heads of Asteraceae, umbels of Apiaceae, spadices of Araceae, etc.) and play an important role in the early development of flowers (
Figure 2). While these structures are linked to the protection of the developing reproductive organs and attracting pollinators (e.g., [
42,
43,
44]), in many cases they may also play a decisive developmental role by exerting a compressing force on the enclosed developing young flowers (
Figure 2). As the major pressure of involucra and spathes occurs at the base of the floral unit close to the attachment point, the consequence is a retardation of the initiation and/or growth of the basal-most flowers. This was documented in the development of the spadices of Araceae [
45,
46,
47] and
Gunnera [
48], as well as in the heads (capitula) of Asteraceae [
49,
50,
51,
52] and of
Davidia [
53]. Interestingly, the retardation and inhibition of basal florets in heads of Asteraceae coincides with their identity as ray florets (
Figure 2) [
50]. In this regard, it could be hypothesized that the pressure of involucral bracts on the basal florets of the asteracean head is a key ontogenetic trigger for their configuration as ray florets (
Figure 2), probably even triggering the corresponding genetic cascade that leads to their differentiation. This pressure may also affect sex differentiation, since ray florets are sterile in some taxa (e.g.,
Helianthus annuus L. [
54]). Conversely, it could be also inferred that capitula lacking ray florets would develop from buds lacking a tight involucrum, and consequently, a lack of pressure in the basal-most part of the capitulum primordium.
Figure 2. Illustration of compression forces of an involucrum on a central meristem: (A) figure of an involucrum formed by several bracts surrounding a central reproductive meristem. (B) Longitudinal section of (A), showing pressure exerted by involucrum at the base of the meristem (red arrows). (C) Diagram of a typical FUM, i.e., a head meristem, showing centripetal progression of florets in the upper part and delayed floret growth at its base in area of inhibition covered by involucrum. (D) Silhouette of a terminal meristem without (left) and with an involucrum (right). Red arrows denote deformation of the meristem by an involucrum.
3. Within-Flower Organ Interaction
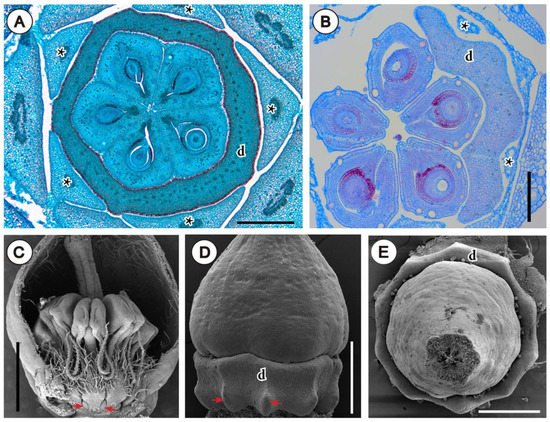
A good example for this is the nectary shape that can be molded by the pressure exerted by previously formed organs during development. This can be observed in buds of Rutaceae, where space availability is restricted at the moment of nectary growth, which occurs usually late in floral development [
103]. Such “imprint” in the outline of the annular intrastaminal nectaries can be observed in some Galipeinae of Rutaceae, mostly due to the pressing of filaments and petals in its outer side, and to carpels in its inner side (
Figure 3A,B). Similarly, in
Arctostaphylos pungens Kunth (Ericaceae), this is also clear in the basal protuberances of the nectary, formed only between stamen filaments, where more space is available (
Figure 3C,D). Also, the decagonal shape of the nectary (from above,
Figure 3E), is created due to the pressing of the 10 filaments (El Ottra and Nogueira, unpublished data).
Figure 3. Inferred effect of spatial interaction of organs within young flower. (A,B) Photomicrographs of microtome transections of floral buds of Rutaceae, close to the floral base. (A) Detail of Conchocarpus macrocarpus (Engl.) Kallunki & Pirani, note polygonal shape of nectary and five protuberances between filaments. (B) Detail of Ertela bahiensis (Engl.) Kuntze, at mid-level of ovary; note outline of unilateral disc. (C–E) SEM micrographs of flowers of Arctostaphylos pungens (Ericaceae). (C) Bud longitudinally opened; arrows indicate basal protuberant part of nectary formed between filaments. (D) Lateral view of ovary and nectary (stamens removed), with arrows indicating same region as in (C). (E) Same as in (D), frontal view, showing decagonal outline of nectary disc. Abbreviations: asterisk, filaments; d, nectary disc. Scale bar in (A,D,E) = 500 μm, in (B) = 200 μm, in (C) = 1 mm.
This entry is adapted from the peer-reviewed paper 10.3390/plants11050661