
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nicoleta Sorina Nemeş | -- | 3842 | 2022-04-01 14:34:46 | | | |
| 2 | Rita Xu | + 1 word(s) | 3843 | 2022-04-02 03:53:19 | | |
Video Upload Options
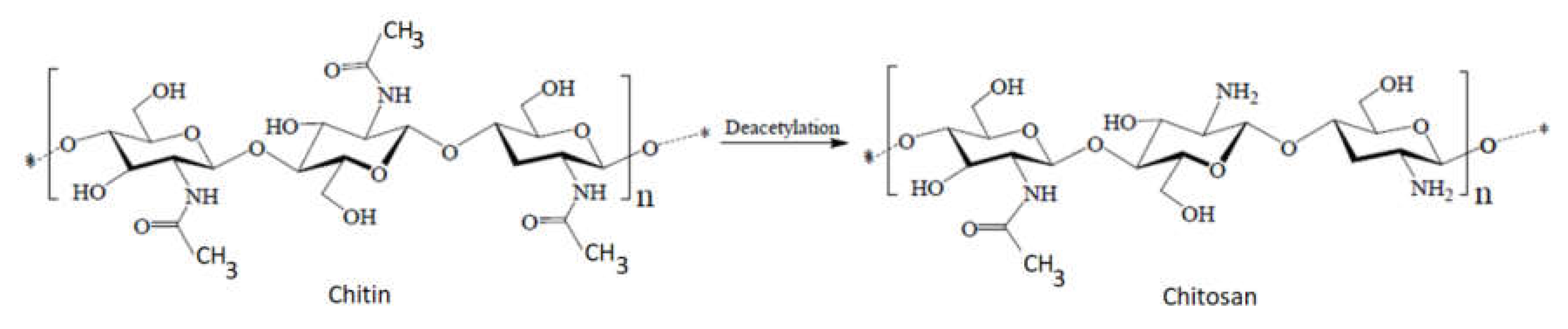
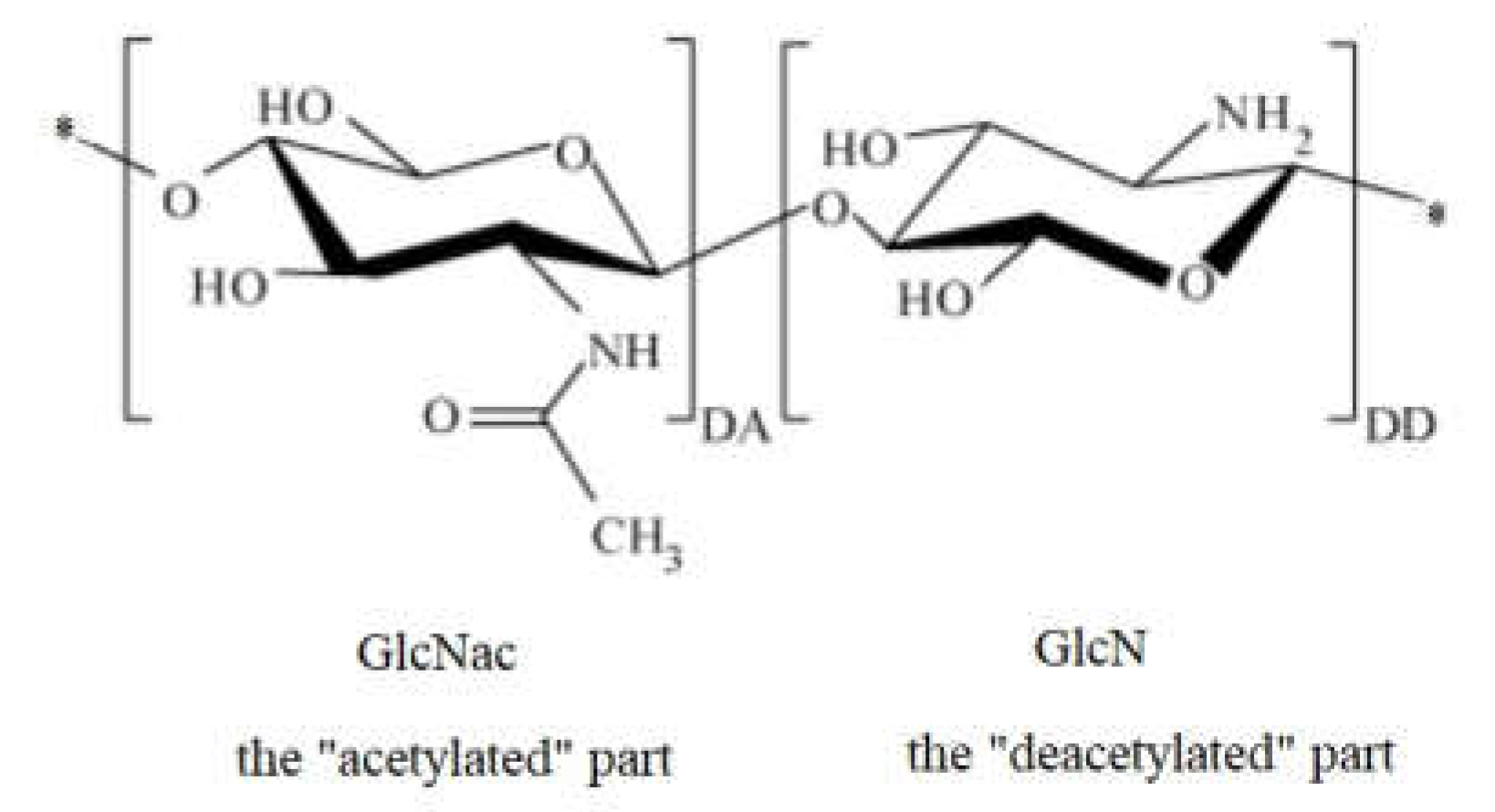
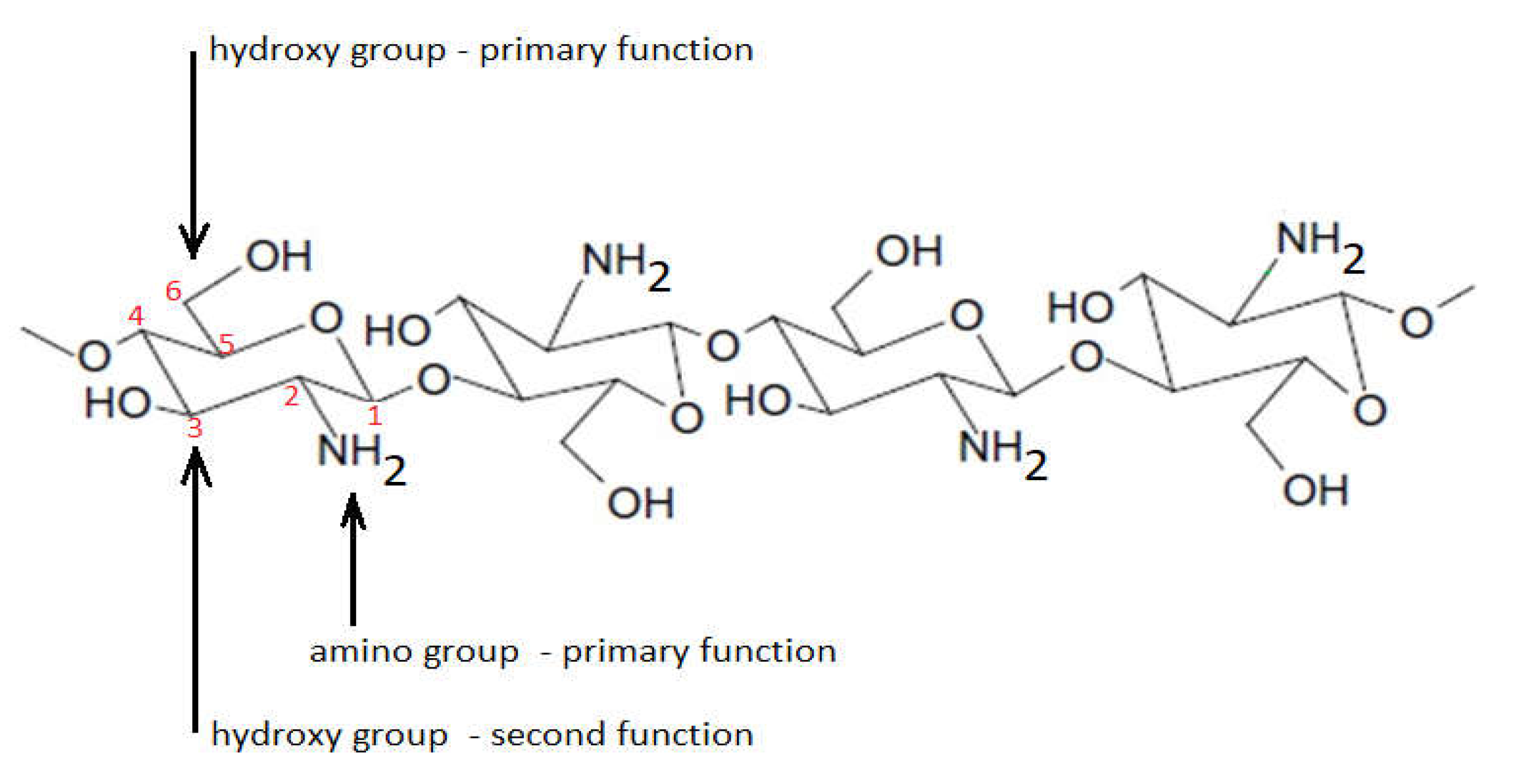
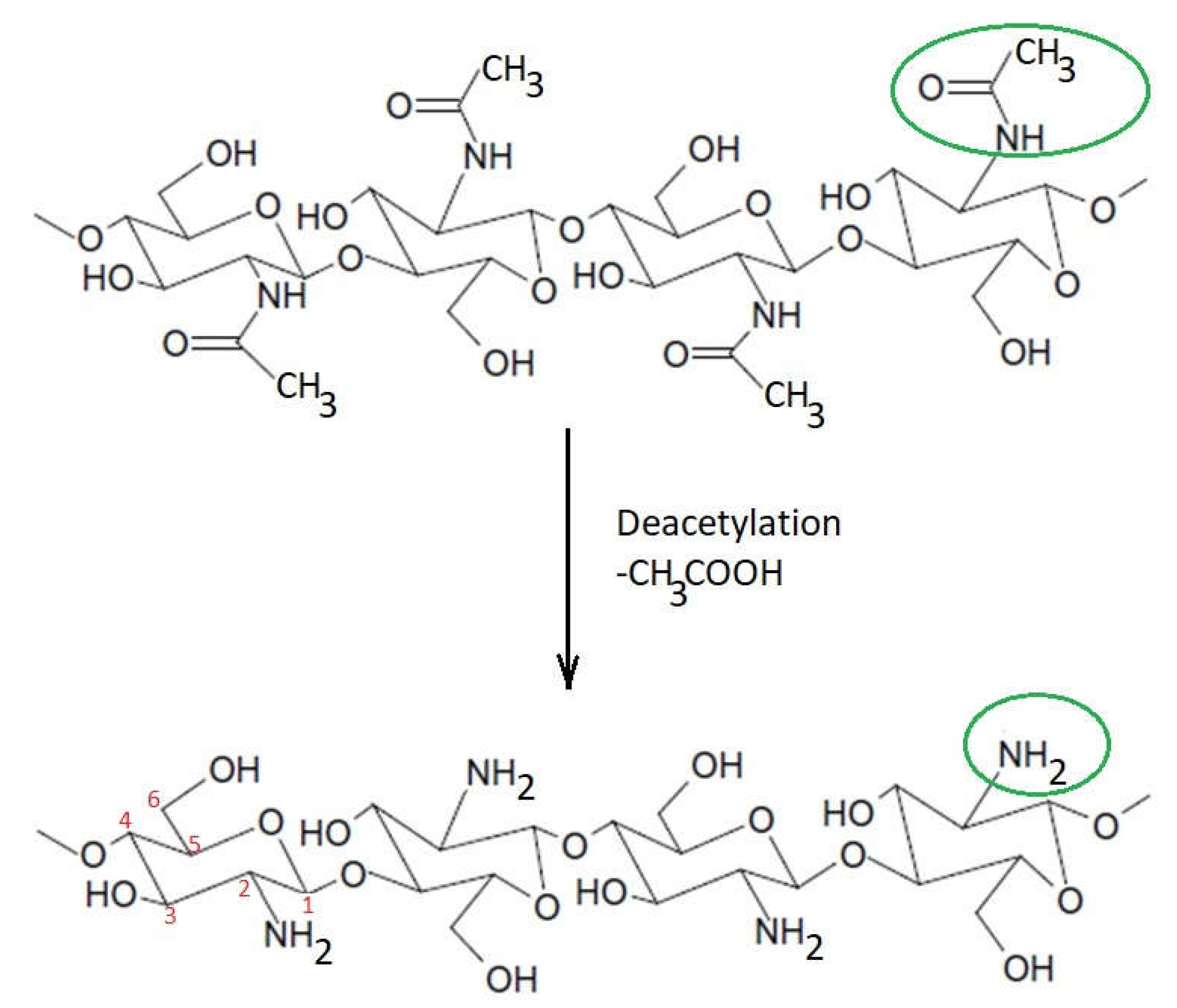
The biomedical and therapeutic importance of chitosan and chitosan derivatives is the subject of interdisciplinary research. In this entry, researchers intended to consolidate some of the recent discoveries regarding the potential of chitosan and its derivatives to be used for biomedical and other purposes. Why chitosan? Because chitosan is a natural biopolymer that can be obtained from one of the most abundant polysaccharides in nature, which is chitin. Compared to other biopolymers, chitosan presents some advantages, such as accessibility, biocompatibility, biodegradability, and no toxicity, expressing significant antibacterial potential. In addition, through chemical processes, a high number of chitosan derivatives can be obtained with many possibilities for use.
1. Introduction
- −
-
low molecular weight chitosan, with a mass lower than 100 kDa;
- −
-
medium molecular weight chitosan, with a mass between 100 and 1000 kDa;
- −
-
high molecular weight chitosan, with a mass higher than 1000 kDa.
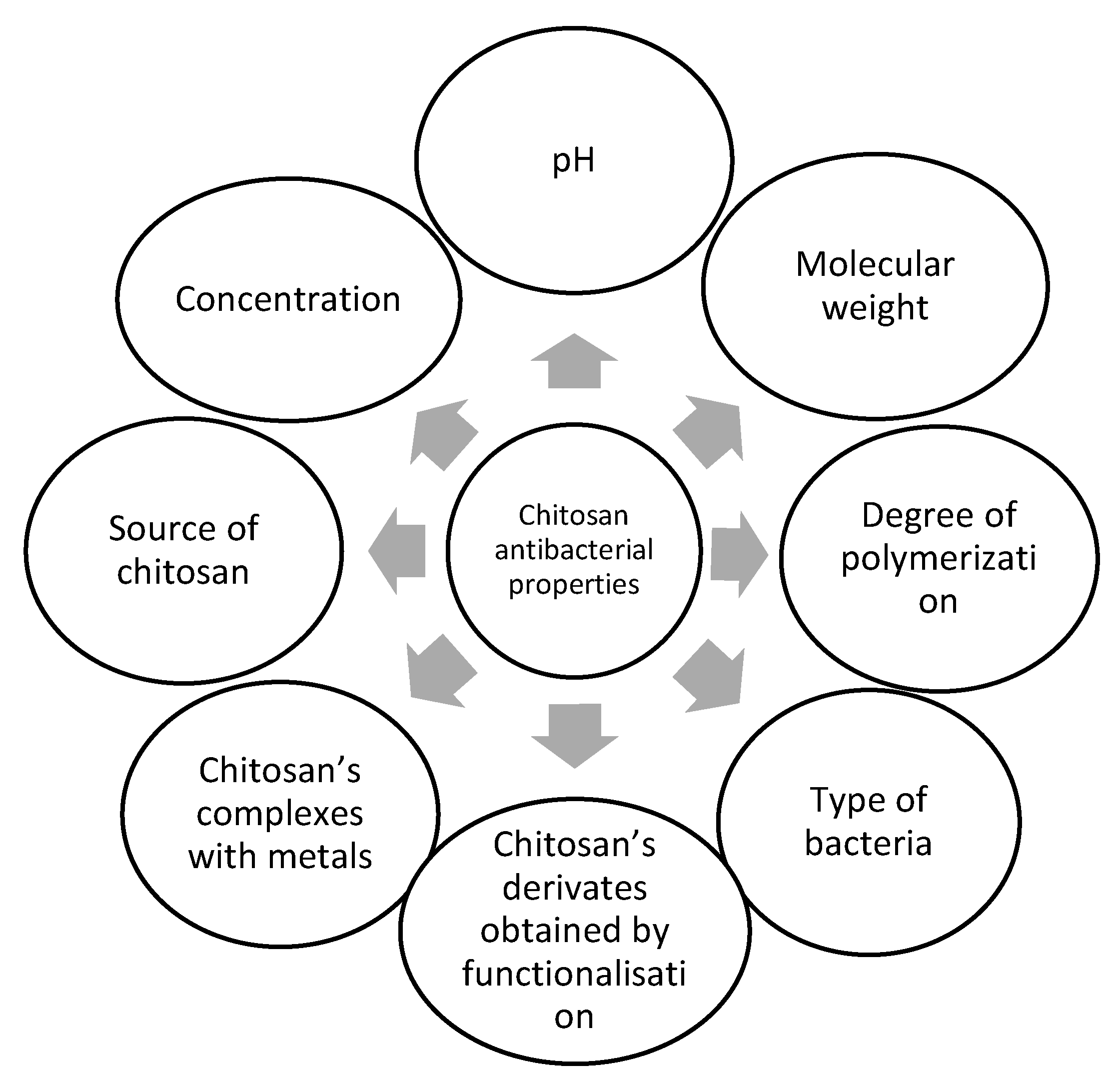
2. Chitosan Sources and Their Contribution to the Antibacterial Activity of Chitosan
3. Influence of Chitosan Concentration on the Antibacterial Effect
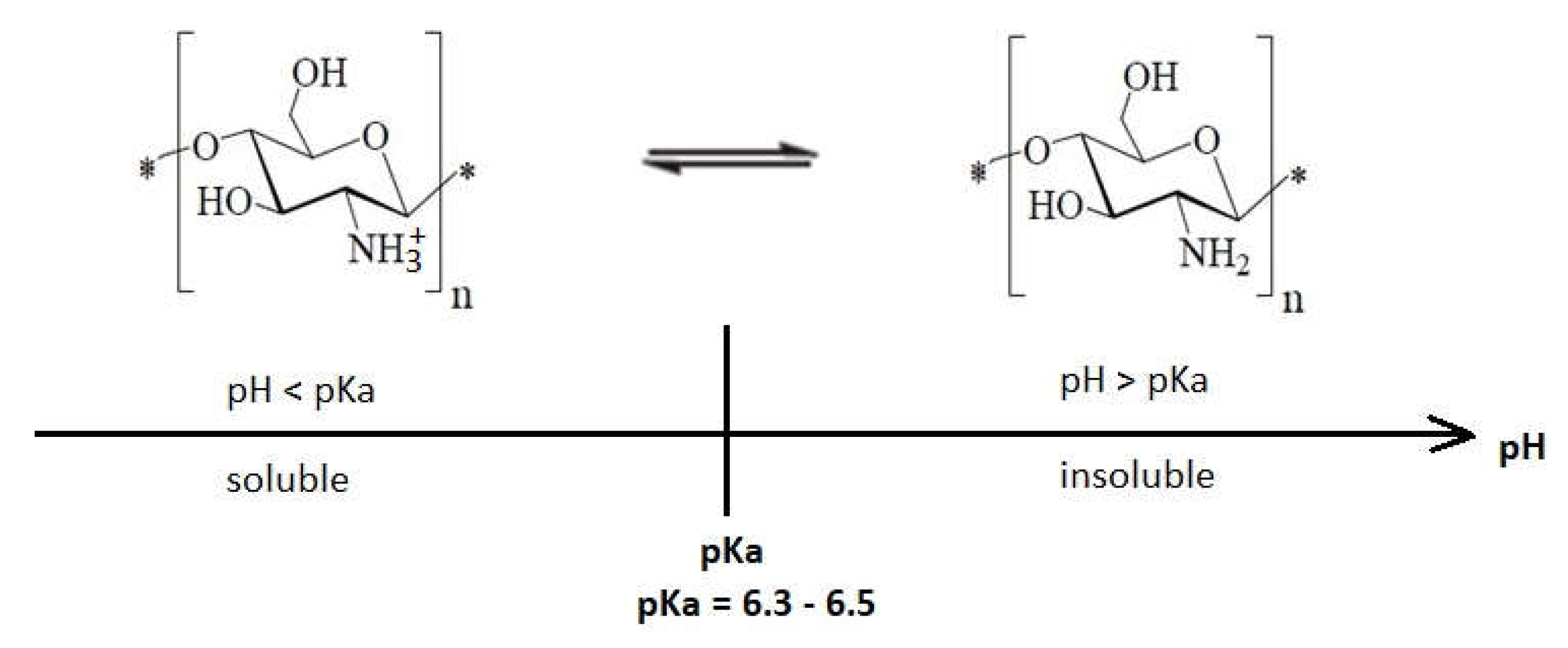
4. Environment pH Influence on the Antibacterial Effect of Chitosan
5. The Molecular Weight Contribution to the Antibacterial Effect of Chitosan
- ✓
-
low molecular weight (LMw) chitosan, named also “oligo-chitosan” or “short chain chitosan” (molecular weight < 50 kDa);
- ✓
-
medium molecular weight (MMw) chitosan, with molecular weight between 50 kDa and 250 kDa;
- ✓
6. The Contribution of the Type of Bacteria to the Antibacterial Effect of Chitosan
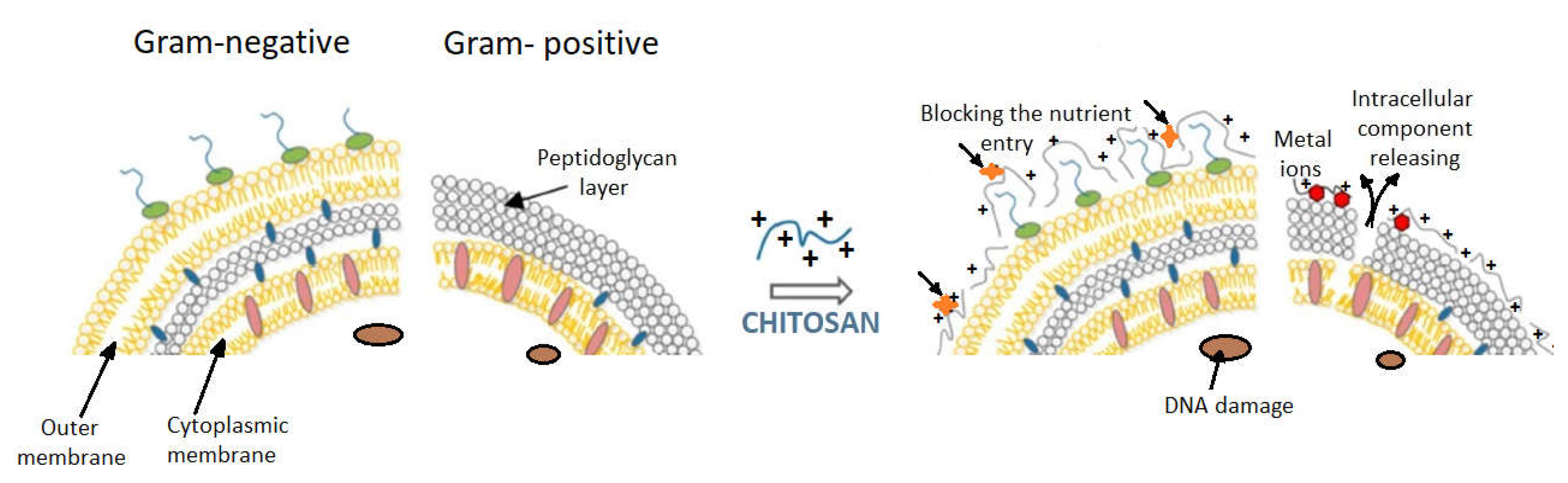
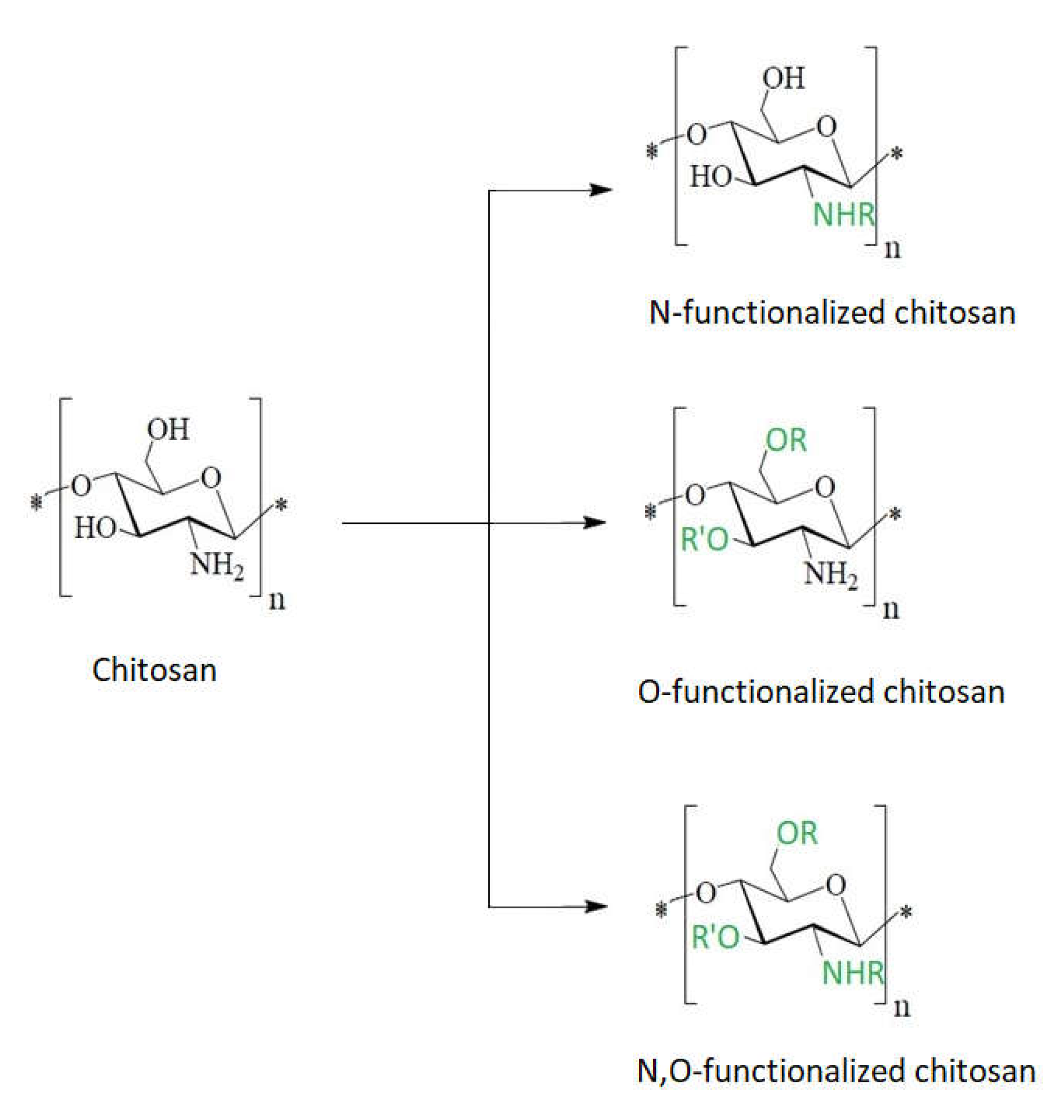
7. The Chitosan Derivatives’ Contributions to the Antibacterial Effect
References
- Grégorio, C. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643.
- Dimzon, I.K.D.; Ebert, J.; Knepper, T.P. The interaction of chitosan and olive oil: Effects of degree of deacetylation and degree of polymerization. Carbohydr. Polym. 2013, 92, 564–570.
- Hadwiger, L.A.; Chinag, C.C.; Victory, S.; Horovitz, D. Chitin and Chitosan; Elsevier: London, UK, 1989; p. 138.
- Se-Kwon, K. (Ed.) Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activities and Applications; CRC Press: Boca Raton, FL, USA, 2011.
- Prashanth, K.V.H.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application—an overview. Trends Food Sci. Technol. 2007, 18, 117–131.
- De Farias, B.S.; Grundmann, D.D.R.; Rizzi, F.Z.; Martins, N.S.S.; Sant’Anna Cadaval, T.R., Jr.; de Almeida Pinto, L.A. Production of low molecular weight chitosan by acid and oxidative pathways: Effect on physicochemical properties. Food Res. Int. 2019, 123, 88–94.
- Alvarenga, E. Characterization and Properties of Chitosan. In Biotechnology of Biopolymers; InTech: Rijeka, Croatia, 2011; Volume 24.
- Barbosa, H.F.G.; Attjioui, M.; Leitao, A.; Moerschbacher, B.M.; Cavalheiro, E.T.G. Characterization, solubility and biological activity of amphihilic biopolymeric Schiff bases synthesized using chitosans. Carbohydr. Polym. 2019, 220, 1–11.
- Anan, N.A.; Hassan, S.M.; Saad, E.M.; Butler, I.S.; Mostafa, S.I. Preparation, characterization and pH-metric measurements of 4-hydroxysalicylidenechitosan Schiff-base complexes of Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Ru(III), Rh(III), Pd(II) and Au(III). Carbohydr. Res. 2011, 346, 775–793.
- Vadivel, T.; Dhamodaran, M. Synthesis, characterization and antibacterial studies of ruthenium(III) complexes derived from chitosan schiff base. Int. J. Biol. Macromol. 2016, 90, 44–52.
- Hafdani, F.; Sadeghinia, N. A review on application of chitosan as a natural antimicrobial. World Acad. Sci. Eng. Technol. 2011, 74, 257–261.
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018.
- Shankar, S.; Rhim, J.W. Preparation of sulfur nanoparticle-incorporated antimicrobial chitosan films. Food Hydrocoll. 2018, 82, 116–123.
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283.
- Salama, H.E.; Saad, G.R.; Sabaa, M.W. Synthesis, characterization and biological activity of Schiff bases based on chitosan and arylpyrazole moiety. Int. J. Biol. Macromol. 2015, 79, 996–1003.
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244.
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21.
- Belbekhouche, S.; Bousserrhine, N.; Alphonse, V.; Le Floch, F.; Mechiche, Y.C.; Menidjel, I.; Carbonnier, B. Chitosan based self-assembled nanocapsules as antibacterial agent. Colloid Surf. B Biointerfaces 2019, 181, 158–165.
- Periayah, M.; Halim, A.; Mat Saad, A. Biotechnology & Biomaterials Chitosan: A Promising Marine Polysaccharide for Biomedical Research. Biotechnol. Biomater. 2014, 4, 4.
- Bhaskara Reddy, M.V.; Arul, J.; Angers, P.; Couture, L. Chitosan Treatment of Wheat Seeds Induces Resistance to Fusarium graminearum and Improves Seed Quality. J. Agric. Food Chem. 1999, 47, 1208–1216.
- Bhaskara Reddy, M.V.; Arul, J.; Essaid, A.B.; Angers, P.; Richard, C.; Castaigne, F. Effect of chitosan on growth and toxin production by Alternaria alternata f. sp. lycopersici. Biocontrol Sci. Technol. 1998, 8, 43.
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987.
- Rendina, N.; Nuzzaci, M.; Scopa, A.; Cuypers, A.; Sofo, A. Chitosan-elicited defense responses in Cucumber mosaic virus (CMV)-infected tomato plants. J. Plant Physiol. 2019, 234–235, 9–17.
- Beuchat, L.R. Natural Antimicrobial Sytstems and Food Preservation; CAB International: Wallingford, UK, 1994; p. 179.
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186.
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2018, 263, 131–194.
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.J.; Venkatraman, S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar. Drugs 2019, 17, 381.
- Liu, X.; Howard, K.A.; Dong, M.; Andersen, M.Ø.; Rahbek, U.L.; Johnsen, M.G.; Hansen, O.C.; Besenbacher, F.; Kjems, J. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials 2007, 28, 1280–1288.
- Byun, S.M.; No, H.K.; Hong, J.-H.; Lee, S.I.; Prinyawiwatkul, W. Comparison of physicochemical, binding, antioxidant and antibacterial properties of chitosans prepared from ground and entire crab leg shells. Int. J. Food Sci. Technol. 2013, 48, 136–142.
- Ai, H.; Wang, F.; Xia, Y.; Chen, X.; Lei, C. Antioxidant, antifungal and antiviral activities of chitosan from the larvae of housefly, Musca domestica L. Food Chem. 2012, 132, 493–498.
- Batista, A.C.d.L.; Souza Neto, F.E.d.; Paiva, W.d.S. Review of fungal chitosan: Past, present and perspectives in Brazil. Polímeros Ciência Tecnol. 2018, 28, 275–283.
- Liu, N.; Chen, X.-G.; Park, H.-J.; Liu, C.-G.; Liu, C.-S.; Meng, X.-H.; Yu, L.-J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65.
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475.
- Vishu Kumar, A.B.; Varadaraj, M.C.; Lalitha, R.G.; Tharanathan, R.N. Low molecular weight chitosans: Preparation with the aid of papain and characterization. Biochim. Biophys. Acta 2004, 1670, 137–146.
- Kulikov, S.N.; Chirkov, S.N.; Il’ina, A.V.; Lopatin, S.A.; Varlamov, V.P. Effect of the molecular weight of chitosan on its antiviral activity in plants. Appl. Biochem. Microbiol. 2006, 42, 200–203.
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63.
- Cabrera, J.; Cutsem, P.V. Preparation of chitooligosaccharides with degree of polymerization higher than 6 by acid or enzymatic degradation of chitosan. Biochem. Eng. J. 2005, 25, 165–172.
- Meng, X.; Xing, R.; Liu, S.; Yu, H.; Li, K.; Qin, Y.; Li, P. Molecular weight and pH effects of aminoethyl modified chitosan on antibacterial activity in vitro. Int. J. Biol. Macromol. 2012, 50, 918–924.
- Jarmila, V.; Eva, V. Chitosan Derivatives with Antimicrobial, Antitumour and Antioxidant Activities—A Review. Curr. Pharm. Des. 2011, 17, 3596–3607.
- Chung, Y.C.; Su, Y.P.; Chen, C.C.; Jia, G.; Wang, H.I.; Wu, J.C.G.; Lin, J.G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936.
- Leuba, J.L.; Stossel, P. Chitin in Nature and Technology; Springer: Berlin/Heidelberg, Germany, 1986; pp. 215–222.
- Chien, R.C.; Yen, M.T.; Mau, J.L. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 2016, 138, 259–264.
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465.
- Errington, N.; Harding, S.E.; Varum, K.M.; Illum, L. Hydrodynamic characterization of chitosans varying in degree of acetylation. Int. J. Biol. Macromol. 1993, 15, 117.
- Lim, S.H.; Hudson, S.M. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr. Res. 2004, 339, 313–319.
- Varlamov, V.P.; Il’ina, A.V.; Shagdarova, B.T.; Lunkov, A.P.; Mysyakina, I.S. Chitin/Chitosan and Its Derivatives: Fundamental Problems and Practical Approaches. Biochemistry 2020, 85 (Suppl. 1), S154–S176.
- Ristic, T.; Hribernik, S.; Fras-Zemljic, L. Electrokinetic properties of fibres functionalised by chitosan and chitosan nanoparticles. Cellulose 2015, 22, 3811–3823.
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374.
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and Applications of Chitosan. Sustain. Agric. Rev. 2019, 35, 49–123.
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084.
- Côté, F.; Roberts, K.A.; Hahn, M.G. Identification of high-affinity binding sites for the hepta-β-glucoside elicitor in membranes of the model legumes Medicago truncatula and Lotus japonicus. Planta 2000, 211, 596–605.
- Yang, T.; Zall, R.R. Chitosan membranes for reverse osmosis application. J. Food Sci. 1984, 49, 91–93.
- Amro, N.A.; Kotra, L.P.; Wadu-Mesthrige, K.; Bulychev, A.; Mobashery, S.; Liu, G.Y. High-resolution atomic force microscopy studies of the Escherichia coli outer membrane: Structural basis for permeability. Langmuir 2000, 16, 2789–2796.
- Honarkar, H.; Barikani, M. Applications of biopolymers I: Chitosan. Mon. Chem. 2009, 140, 1403–1420.
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551.
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules 2019, 24, 1960.
- Ezati, P.; Rhim, J.W. pH-responsive chitosan-based film incorporated with alizarin for intelligent packaging applications. Food Hydrocoll. 2020, 102, 10.
- Kulikov, S.N.; Tikhonov, V.E.; Bezrodnykh, E.A.; Lopatin, S.A.; Varlamov, V.P. Comparative evaluation of antimicrobial activity of oligochitosans against Klebsiella pneumoniae. Russ. J. Bioorg. Chem. 2015, 41, 57–62.
- Daly, W.H.; Manuszak-Guerrini, M.A. Biocidal Chitosan Derivatives for Cosmetics and Pharmaceuticals. U.S. Patent 6,306,835, 23 October 2001.
- Baba, Y.; Noma, H.; Nakayama, R.; Matsushita, Y. Preparation of chitosan derivatives containing methylthiocarbamoyl and phenylthiocarbamoyl groups and their selective adsorption of copper(II) over iron(III). Anal. Sci. 2002, 18, 359–361.
- Martins, F.A.; Facchi, P.S.; Follmann, D.M.H.; Pereira, G.B.A.; Rubira, F.A.; Edvani, M.C. Antimicrobial Activity of Chitosan Derivatives Containing N-Quaternized Moieties in Its Backbone: A Review. Int. J. Mol. Sci. 2014, 15, 20800–20832.
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868.
- Riaz Rajoka, M.S.; Mehwish, H.M.; Wu, Y.; Zhao, L.; Arfat, Y.; Majeed, K.; Anwaar, S. Chitin/chitosan derivatives and their interactions with microorganisms: A comprehensive review and future perspectives. Crit. Rev. Biotechnol. 2020, 40, 365–379.
- Kaplan, S.; Aslan, S.; Ulusoy, S.; Oral, A. Natural-based polymers for antibacterial treatment of absorbent materials. J. Appl. Polym. Sci. 2020, 137, 12.
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. Funct. Chitosan 2020, 457–489.
- Fernandez-Saiz, P.; Lagarón, J.M.; Ocio, M.J. Optimization of the film-forming and storage conditions of chitosan as an antimicrobial agent. J. Agric. Food Chem. 2009, 57, 3298–3307.
- Peter, M.G. Applications and Environmental Aspects of Chitin and Chitosan. J. Macromol. Sci. Part A 1995, 32, 629–640.
- Chirkov, S.N. The Antiviral Activity of Chitosan (Review). Appl. Biochem. Microbiol. 2002, 38, 1–8.
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63.
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201.
- Vishu Kumar, A.B.; Varadaraj, M.C.; Gowda, L.R.; Tharanathan, R.N. Characterization of chito-oligosaccharides prepared by chitosanolysis with the aid of papain and Pronase, and their bactericidal action against Bacillus cereus and Escherichia coli. Biochem. J. 2005, 391 Pt 2, 167–175.
- Allan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979, 3, 287.
- Hassan, M.A.; Omer, A.M.; Abbas, E.; Baset, W.M.A.; Tamer, T.M. Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Sci. Rep. 2018, 8, 11416.
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889.
- Pavinatto, A.; Souza, A.L.; Delezuk, J.A.M.; Pavinatto, F.J.; Campana-Filho, S.P.; Oliveira, O.N. Interaction of O-acylated chitosans with biomembrane models: Probing the effects from hydrophobic interactions and hydrogen bonding. Colloids Surf. B Biointerfaces 2014, 114, 53–59.
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polim. Cienc. Tecnol. 2009, 19, 241–247.
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Xavier Malcata, F. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134.
- Li, Z.; Yang, F.; Yang, R. Synthesis and characterization of chitosan derivatives with dual-antibacterial functional groups. Int. J. Biol. Macromol. 2015, 75, 378–387.
- Severino, R.; Ferrari, G.; Vu, K.D.; Donsì, F.; Salmieri, S.; Lacroix, M. Antimicrobial effects of modified chitosan based coating containing nanoemulsion of essential oils, modified atmosphere packaging and gamma irradiation against Escherichia coli O157:H7 and Salmonella Typhimurium on green beans. Food Control 2015, 50, 215–222.
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74.
- Muzzarelli, R.A.A.; Weckx, M.; Filippini, O. Removal of trace metal ions from industrial waters, nuclear effluents and drinking water, with the aid of cross-linked N-carboxymethyl chitosan. Carbohydr. Polym. 1989, 11, 306.
- Krishnapriya, K.R.; Kandaswamy, M. Synthesis and characterization of a crosslinked chitosan derivative with a complexing agent and its adsorption studies toward metal(II) ions. Carbohydr. Res. 2009, 344, 1632–1638.
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N.A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 57.
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82.
- Elshaarawy, R.F.M.; Refaee, A.A.; El-Sawi, E.A. Pharmacological performance of novel poly-(ionic liquid)-grafted chitosan-N-salicylidene Schiff bases and their complexes. Carbohydr. Polym. 2016, 146, 376–387.
- Garoufis, A.; Hadjikakou, S.K.; Hadjiliadis, N. Palladium coordination compounds as anti-viral, anti-fungal, anti-microbial and anti-tumor agents. Coord. Chem. Rev. 2009, 253, 1384–1397.
- Kurita, K.; Ikeda, H.; Yoshida, Y.; Shimojoh, M.; Harata, M. Chemoselective protection of the amino groups of chitosan by controlled phthaloylation: Facile preparation of a precursor useful for chemical modifications. Biomacromolecules 2002, 3, 1–4.
- Sahariah, P.; Gaware, V.S.; Lieder, R.; Jónsdóttir, S.; Hjálmarsdóttir, M.Á.; Sigurjonsson, O.E.; Másson, M. The effect of substituent, degree of acetylation and positioning of the cationic charge on the antibacterial activity of quaternary chitosan derivatives. Mar. Drugs 2014, 12, 4635–4658.
- Kurita, K.; Hirakawa, M.; Kikuchi, S.; Yamanaka, H.; Yang, J. Trimethylsilylation of chitosan and some properties of the product. Carbohydr. Polym. 2004, 56, 333–337.
- Rúnarsson, O.V.; Malainer, C.; Holappa, J.; Sigurdsson, S.T.; Másson, M. tert-Butyldimethylsilyl O-protected chitosan and chitooligosaccharides: Useful precursors for N-modifications in common organic solvents. Carbohydr. Res. 2008, 343, 2576–2582.





