The biomedical and therapeutic importance of chitosan and chitosan derivatives is the subject of interdisciplinary research. In this entry, researchers intended to consolidate some of the recent discoveries regarding the potential of chitosan and its derivatives to be used for biomedical and other purposes. Why chitosan? Because chitosan is a natural biopolymer that can be obtained from one of the most abundant polysaccharides in nature, which is chitin. Compared to other biopolymers, chitosan presents some advantages, such as accessibility, biocompatibility, biodegradability, and no toxicity, expressing significant antibacterial potential. In addition, through chemical processes, a high number of chitosan derivatives can be obtained with many possibilities for use.
1. Introduction
In 1859, Rouget discovered that by heating chitin in alkaline environment, a soluble material in organic acids can be obtained. The name of this material, “chitosan”, was given by Hoppe-Seyler in 1894, but the chemical structure was unknown until 1950
[1].
Chitosan can be obtained by partial or total deacetylation of chitin, acetyl groups in the molecular chain of chitin being removed to form amino groups in chitosan. For this reason, chitosan can be classified as a copolymer composed mainly of 2-amino-2-deoxy-β-
d-glucopyranose (glucosamine) and 2-acetamide-2-deoxy-β-
d-glucopyranose units (N-acetylglucosamine) linked by glycosidic β(1→4) bonds
[2,3,4,5,6][2][3][4][5][6] (
Figure 1).
Figure 1. Partial deacetylation of chitin to chitosan according to
[7].
The structure of chitosan includes an “acetylated” part and a “deacetylated” part; the molecular structure of the monomeric units that are repeated in the chitosan molecule is shown in Figure 2. If the left side is 2-amino-2-deoxy-β-(1-4)-d-glucopyranose representing the degree of acetylation (DA) of chitosan, the right side is the deacetylated result of the left side, representing the degree of deacetylation of chitosan (DD).
Figure 2. Molecular structure of the chitosan monomer repeated unit.
Monomer units are distributed randomly or as blocks around chitosan polymer
[8,9,10][8][9][10]. This mode of distribution gives chitosan a rigid and uneven structure. The presence of hydroxyl groups located at C6 (hydroxyl group with main activity) and C3 (hydroxyl group with secondary activity) and amino groups (located on C2 of the molecule), which are highly reactive, with a concomitant tendency of intramolecular and intermolecular hydrogen bonds, results in the formation of the linear structure of the chitosan molecule (
Figure 3).
Figure 3. Linear structure of chitosan.
The major difference between chitin and chitosan consists of the amino groups (
Figure 4). Thus, their physicochemical properties, which are correlated with their biological functions and their chemical behavior, are different. Chitosan has a nitrogen content of 6.80% or higher. Generally, when the content of N-acetyl groups is higher than 50%, chitin is considered, while for lower values, chitosan is considered
[7].
Figure 4. Difference between the structures of chitin and chitosan.
By taking into account its molecular mass, chitosan can be split up in three different categories:
- −
-
low molecular weight chitosan, with a mass lower than 100 kDa;
-
- −
-
medium molecular weight chitosan, with a mass between 100 and 1000 kDa;
-
- −
-
high molecular weight chitosan, with a mass higher than 1000 kDa.
-
The molecular mass of chitosan affects its properties, so it is important to know it in order to make a strong correlation between chitosan type and any further application prior to chitosan modification
[11,12][11][12].
The most important property of chitosan is its antibacterial activity. This feature reaches a new dimension in the context of the need to find new materials that have bactericidal effects on bacteria resistant to existing antibiotics. It is known that chitosan is a bactericide/bacteriostatic agent acting upon a various number of common bacteria, both Gram-positive and Gram-negative
[5,13,14,15,16,17][5][13][14][15][16][17].
Chitosan has several advantages over other types of antimicrobial agents because it shows a higher antibacterial activity, a wider spectrum of antibacterial activity, and a lower toxicity towards mammalian cells
[8,15,18][8][15][18]. The use of chitosan in medicine is very important. Over time it has acquired various uses, which are spectacular as areas of interest. In terms of the extremely diverse “bio” field, chitosan has been used in biotechnology
[19,20,21,22[19][20][21][22][23],
23], food preservation
[13,24][13][24], drugs and pharmaceuticals
[5[5][25][26],
25,26], and gene therapy
[27,28][27][28].
In theory, chitosan has a high biological activity with very wide applicability, but its poor aqueous solubility limits this theoretical advantage, including its antimicrobial behavior
[8]. The antimicrobial action of chitosan is influenced by numerous intrinsic factors, such as its sources (crustaceans
[29], insect shells
[30], fungi
[31]), concentration
[32], the molecular weight that generates its type
[33[33][34][35][36],
34,35,36], polymerization degree
[2,37][2][37], but also by external factors, including pH of the environment
[33[33][38],
38], the type and sensitivity of targeted microorganisms
[14,16,33[14][16][33][39][40],
39,40], the chemical composition of the substrate, etc. Many factors change the behavior of chitosan. The pathway to obtain chitosan derivatives is directly linked to their practical applicability, so different properties of these derivatives will determine their (re)activity.
Figure 5 shows the most important intrinsic and external factors influencing the antibacterial effects of chitosan.
Figure 5.
Intrinsic and external factors contributing to chitosan’s antibacterial activity.
It is difficult to rank these factors, taking in account their influence on the antibacterial activity of chitosan. WResearchers will discuss some of these factors further on. Therefore, as many factors as possible that may influence the antibacterial activity of chitosan must be considered.
2. Chitosan Sources and Their Contribution to the Antibacterial Activity of Chitosan
Chitin is the most abundant polysaccharide on earth, after cellulose; it is the main structural polymer found in the fungal cell wall
[1]. It is also present in the exoskeletons of arthropods
[41] and insects
[30]. Chitosan, the main derivative of chitin, can therefore come from fungi and the exoskeleton of crustaceans or insects.
Some studies have mentioned that growing certain mushrooms could provide an effective source of chitosan for industrial applications
[30]. Many researchers have concluded that chitosan can be extracted not only from
Zygomycete fungi, but also from non-
Zygomycete fungi
[31].
Chien et al. reported that crude chitin from crab shells did not show any antimicrobial activity, but chitin from mushroom exhibited an inhibitory effect on bacterial growth, compared with chitin from crab shells
[42].
Their results showed that the antimicrobial activity of fungal chitosan was lower than that of chitosan obtained from crustacean shells. However, fungal chitosan, similar to crustacean chitosan, exhibited better inhibitory effects against Gram-positive bacteria compared with Gram-negative bacteria
[33].
It has been demonstrated by Byun et al. that chitosan prepared from the entire crab shell and the shell of the crab leg shows major differences in terms of physical–chemical and functional characteristics. For example, chitosan prepared from crab shell had a significantly higher nitrogen content, degree of deacetylation, solubility, and viscosity and improved antibacterial activity than chitosan prepared from crab legs
[29].
On the other hand, chitosan oligomers and polymers from different sources present different antibacterial activities. They have been tested against food borne pathogens, and the results demonstrate that the source, degree of deacetylation, and molecular size of chitosan must be selectively chosen to control food borne target pathogens
[33].
3. Influence of Chitosan Concentration on the Antibacterial Effect
A lot of experiments suggested that chitosan can inhibit bacterial growth at different concentrations
[43]. Usually, the required concentration of chitosan to inhibit bacterial growth is correlated with the acetylation degree of chitosan; a solution exhibiting 7.5% acetylation degree was more effective than that exhibiting 15% acetylation degree
[43,44][43][44].
Only at
lower concentrations does chitosan bind to the negatively charged cell surface, especially of Gram-negative bacteria. In this case, it interferes with the cell membrane permeability; thus, intracellular components will be externalized, leading to cellular death. On the other hand,
at higher concentrations, chitosan that is positively charged due to amino groups may coat the cellular surface, and the intracellular components are blocked in the cell. In addition, in the case of Gram-positive bacteria, the positively charged bacterial cells and the positively charged chitosan will have a repelling effect on each other, preventing agglutination
[33,45][33][45].
Liu et al. evaluated the effects of the molecular weight and concentration of chitosan against
E. coli. Different molecular weight chitosans (5.5 × 10
4 to 15.5 × 10
4 Da) in various concentrations (20 to 1000 ppm) were used. All chitosan samples with molecular weight from 5.5 × 10
4 to 15.5 × 10
4 Da had good antimicrobial activities at high concentrations (>200 ppm), and all samples at low concentration (20 ppm) could promote the growth of
E. coli [32]. This might be explained by the fact that bactericidal effects of high concentrations of chitosan can occur through the flocculation of bacteria. Low concentrations of chitosan did not exert this effect, promoting instead the survival of bacteria by favoring their reproduction
[32,46][32][46].
4. Environment pH Influence on the Antibacterial Effect of Chitosan
Environmental pH is one of the most important factors affecting the antimicrobial activity of chitosan and its derivatives.
pH primarily affects the solubility of chitosan, but it also affects the electrical charges of the chitosan molecule. This property enables chitosan molecules to bind via electrical interactions
[47].
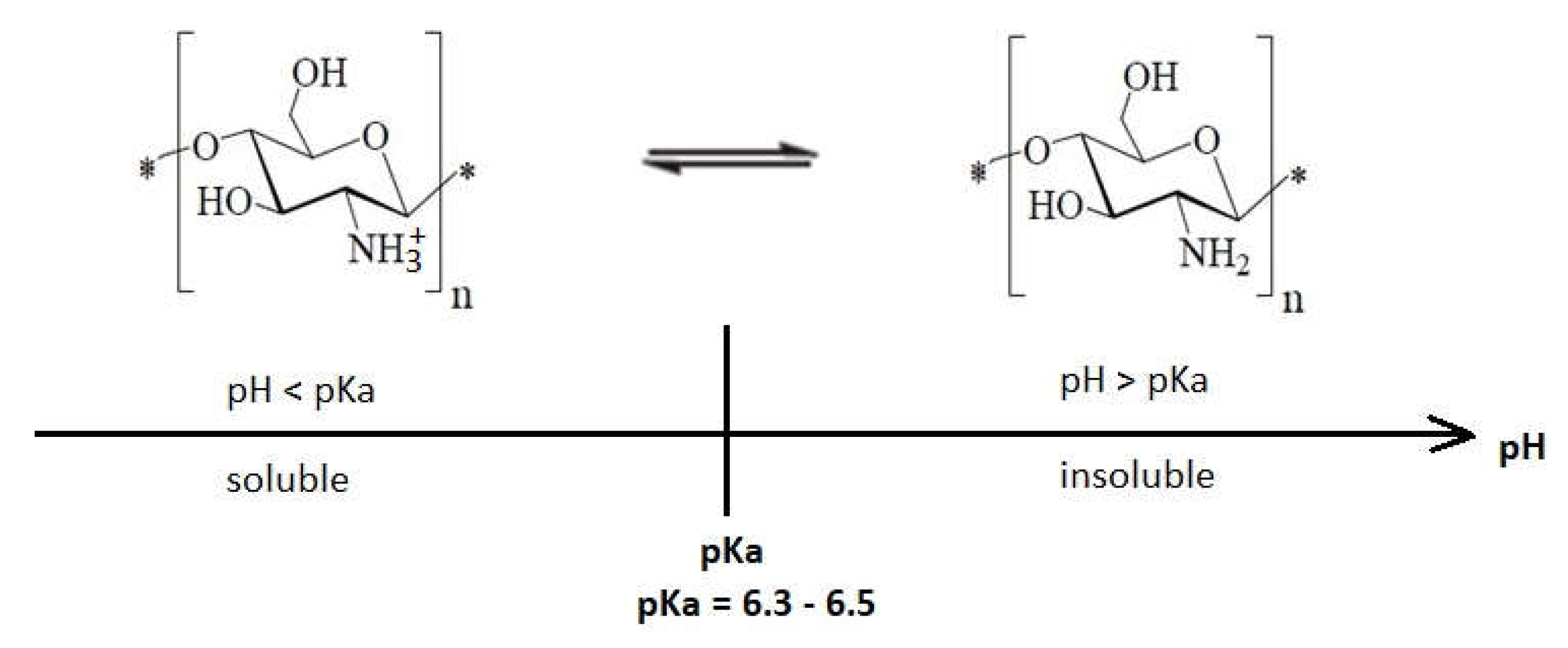
It is well known that native chitosan is soluble in organic acids at pH lower than 6, but it is insoluble in water, organic solvents, and alkaline medium (
Figure 6). Preparation of different water-soluble chitosan salts is possible by its neutralization with hydrochloric acid, acetic acid, lactic acid, or formic acid
[43]. Its solubility in diluted aqueous solutions can be correlated with the conversion of glucosamine units into the soluble form of R-NH
3+. Experimental data proved that water insoluble chitosan shows antimicrobial activity in acidic medium, being appropriate for use as a preservative in acidic foods
[48].
Figure 6. The pH influence upon chitosan solubility according to
[57][49].
At
pH lower than pKa, chitosan molecules are protonated due to the high density of amino groups (–NH
3+) that get converted to the quaternary form, giving a positive charge to the polymer, which increases the intermolecular electric repulsion, resulting in a polycationic macromolecule
[38]. Chitosan has polycationic behavior at pH < 6, which makes it soluble in water. It was observed that while pH decreases, the adsorption of chitosan on bacterial surfaces will increase. These are the requirements to interact with negatively charged substances like proteins, fatty acids, and phospholipids, which are components of the bacterial cell
[33,38,49][33][38][50]. Thus, the interaction between positively charged chitosan molecules with negatively charged residues on the bacterial surface is possible, and in this way, the cell permeability is perturbed, which ultimately leads to bacterial death
[16,33,40,50][16][33][40][51]. In all mechanisms that explain the antibacterial effects of chitosan molecules, the cationic charge is considered to be responsible for efficient binding of chitosan to the anionic components that are present at the level of bacterial membranes
[45,48,51,52,53,54,55,56][45][48][52][53][54][55][56][57]. The interaction between protonated chitosan and negatively charged cell membranes is the most common mechanism that explains cellular death, referred as the “antibacterial effect” of chitosan.
There is the problem of the pH difference between the physiological pH of most bacterial cells (which is a neutral pH) and the pH at which chitosan is soluble. The physiological pH of the cell being neutral, chitosan molecules precipitate, and chitosan molecules remain on the surface of the bacterial cell, acting like a layer that blocks the ion exchange channels from the cellular wall, which are essential for the good survival of the microbial cell. This mechanism destabilizes the cell wall morphology and functions, causing severe damage to cell constituents and, in the end, the cell will die
[48].
This mechanism of action is not unique; the antibacterial effect of chitosan is the result of a string of molecular processes that cause multiple damages, leading to cellular death.
Most research has shown that when the pH of the medium is below pKa, the polymer has an increased antimicrobial effect
[35,43][35][43]. It seems that the presence of positive charge on the structure of the polymer, rather than its solubility depending on the pH range, is the critical factor for expressing antimicrobial activity
[58].
A series of studies has shown that chitosan derivatives are active towards Gram-positive and Gram-negative bacteria only when the degree of substitution is low; chitosan derivates should have a higher number of protonated amino groups
[14,39,59,60,61,62,63][14][39][59][60][61][62][63].
Not only the presence of positive charge is enough, but a decisive role in exerting the bactericidal effect of chitosan derivatives is played by the location of the cationic charge, compared to the polymer backbone structure. In the case of a series of compounds with different spacing lengths of substituted carbon, the inhibitory effect of the compounds decreased if the distance between the backbone of the polymer and the cationic position increased. Most researchers conclude that the antimicrobial effect seems to be highest if cationic fragments are closer to the polymer backbone, and it tends to decrease when the functional groups are present at an increasing distance from the polymer chain. This effect is clearly observed when the same functional group is bounded to the polymer by chains of different lengths
[8,62,64][8][62][64].
At
pH higher than pKa, chitosan tends to lose its positive charge, and it precipitates due to deprotonation of amino groups, becoming insoluble. This is explained by the fact that the majority of amino groups become uncharged at pH close to 7
[38,58][38][58]. Although at pH > pKa chitosan is deprotonated, it still remains reactive, having the possibility to form gels or protective films
[56,65,66][57][65][66].
Another study concluded that at pH = 6.2, a stronger biocidal effect was observed, which is related to the particular pKa of chitosan (pKa = 6.3–6.5)
[67], obviously very close to a pH of 6.2. At this pH, the amount of positively charged amino groups is about 75% in chitosan, while at pH 7.4, this quantity represents approximately 10%
[66].
5. The Molecular Weight Contribution to the Antibacterial Effect of Chitosan
Based on molecular weight, there are three types of chitosan:
- ✓
-
low molecular weight (LMw) chitosan, named also “oligo-chitosan” or “short chain chitosan” (molecular weight < 50 kDa);
-
- ✓
-
medium molecular weight (MMw) chitosan, with molecular weight between 50 kDa and 250 kDa;
-
- ✓
-
high molecular weight (HMw) chitosan, with molecular weight > 250 kDa
[6,35,38][6][35][38].
-
Almost all studies reported a correlation between the bactericidal effect of chitosan and its molecular weight
[68,69][68][69]. Oligosaccharides and D-glucosamine have no antibacterial activity. In some studies it is suggested that a minimum molecular weight of 10 kDa is required for a bactericidal effect
[70].
The relation between the bactericidal effect and the molecular weight is influenced by the type of implied bacteria
[36]. HMw chitosan cannot cross microbial membranes; thus, it remains on the cellular surface, blocking nutrients to be transported inside microbial cells, leading to cell lysis. A dissociated solution of HMw chitosan molecules can bind to the cell membrane, modifying its permeability. Dissociated solutions of LMw chitosan molecules can bind to DNA while penetrating the cell nucleus, inhibiting the synthesis of mRNA
[14,35,68,69][14][35][68][69].
In various studies on several bacteria, such as
Staphylococcus aureus, Bacillus cereus, Klebsiella pneumoniae, and
E. coli, it was found that the lower the molecular weight (LMW) of chitosan, the higher the antibacterial effect
[14]. This is attributed to the size and conformation of chitosan particles that appear to play an essential role in understanding the effectiveness of low molecular weight chitosan. The mobility, attraction, and ionic interaction of small chains are easier than those of large ones. The priority is to facilitate the adoption of large conformations, which can be efficiently bound on the membrane surface
[34,71][34][71].
6. The Contribution of the Type of Bacteria to the Antibacterial Effect of Chitosan
The first step in understanding the antibacterial activity of chitosan on different types of bacteria was made by Allan and Hadwinger in 1979
[77][72]. Over time, the entire antibacterial activity of chitosan itself was attributed to the amino groups that are directly influenced by DP degree and DD degree.
Due to the different composition of the Gram-positive and Gram-negative cell walls, the interaction of chitosan is different with these two types of bacteria. In some studies, researchers found that the bactericidal effect was more important on Gram-negative bacteria than on Gram-positive ones
[40] because of the higher affinity of amino groups for anionic radicals present in the cell wall. In other studies, Gram-positive bacteria were considered to be more sensitive to the antimicrobial activity of chitosan, as a consequence of the Gram-negative outer membrane barrier
[16,78][16][73]. These discrepancies should not surprise
uthe researchers, given that the bactericidal activity of chitosan is influenced by so many factors, both intrinsic and extrinsic.
Antimicrobial activity of chitosan is considered to occur when the compounds are absorbed onto the
surface of the bacterial cells. This is followed by an increase in the permeability of the lipid cell membrane, and essential compounds leave the cell, leading to cellular death
[33].
Although several mechanisms for the antibacterial activity of chitosan and chitosan derivatives have been suggested, the exact mode of action is still not known in detail. However, there is clear evidence that these compounds express molecular-level interactions with the cell membrane
[14,61,79][14][61][74]. Usually, ionic and/or hydrophobic interactions are considered to be responsible for the damage or breakage of cell membranes
[69,80][69][75]. Based on these considerations, the hydrophilicity of Gram-negative bacteria is significantly higher than that of Gram-positive bacteria, making them more sensitive to the action of chitosan. Thus, following the action of an antibacterial agent, the cell wall of Gram-negative bacteria passes several morphological changes compared to Gram-positive ones. Determinant for this is the density of electrical charges on the surface of the bacterial cell, which is actually crucial for the amount of adsorbed chitosan. The more chitosan is adsorbed, the more obvious are the changes in the structure and permeability of the cell membrane
[81][76].
Electronic microscopy proved that chitosan induces extensive cell surface alteration while covering the bacterial outer membrane with vesicular structures, causing alterations of the barrier function of the cell membrane
[69,82,83][69][77][78].
Chitosan possesses a polycationic character and interacts predominantly with the anionic wall components of bacteria (especially lipopolysaccharides and proteins). The consequences of this interaction are represented by externalization of intracellular components and the impossibility for nutrients to enter into the bacterial cell, nutrients being bound to metals
[84][79].
Amino groups, via chelating mechanisms, can react with the metal ions. Because amino groups are protonated in an acidic medium, they can cause electrostatic attraction of anionic compounds, such as anionic residues or proteins, by altering their normal functioning; but, simultaneously, the affinity of the absorbent to bind metal cations is reduced
[85][80].
The membrane of
Gram-negative bacteria is a thin two-dimensional structure containing a peptidoglycan layer that forms a hydrophilic surface (
Figure 7). The cytoplasmic membrane is made of lipopolysaccharides, lipoproteins, and phospholipids. When the protonated amino groups of chitosan meet a certain anionic bacterial surface (carboxylic residues, phosphate residues, etc.), electrostatic binding is possible, especially if chitosan is of a low molecular weight type. Subsequently, cell permeability is affected, and osmotic stability of the bacterial wall is decreased. The complexes resulting from this interaction affect the barrier properties of the cytoplasmic membrane; they enter into the cell and interfere with the physiological bacterial processes or cause a leakage of enzymes and nucleotides from bacteria
[16]. In conclusion, in Gram-negative bacteria, the external membrane layer works as a barrier against hydrophobic residues and macromolecules, explaining the resistance of the Gram-negative bacteria against hydrophobic antibiotics.
Figure 7. Models proposed for chitosan action upon Gram-positive and Gram-negative bacteria (from Kravanja et al., 2019 [55]). Models proposed for chitosan action upon Gram-positive and Gram-negative bacteria (from Kravanja et al., 2019 [56]).
Chitosan exerts a chelating effect
[85][80], binding essential metals and thereby inhibiting microbial growth. It is well known that chitosan has excellent metal-binding abilities, using charged amino groups that interact with metals
[85,86,87][80][81][82]. This type of interaction between amino functions and divalent ions within the microorganism cell wall (Ca
2+ or Mg
2+) prevents the production of toxins and inhibits bacterial growth
[69,88][69][83].
Bacterial membrane permeability is importantly influenced by the molecular size of chitosan and the pH of the medium. These factors were described above.
The cellular wall of
Gram-positive bacteria is a three-dimensional layer, consisting especially of peptidoglycans. Gram-positive bacteria have no outer membrane (
Figure 7).
One proposed mechanism for the bactericidal effect of chitosan on Gram-positive bacteria is its direct blocking capacity, preventing nutrients and oxygen from entering the intracellular space
[33]. This mechanism is suitable for higher molecular weight chitosan, which forms a polymer membrane on the surface of the bacterial cell.
Another bactericidal mechanism of chitosan with low molecular weight derives from the interaction with DNA that inhibits mRNA and protein synthesis after entering the nuclei of microorganisms. This was demonstrated in the case of
E. coli, where intracellular chito-oligomers were observed, and these probably prevent DNA transcription
[89][84].
In conclusion, the different sensitivities of Gram-positive bacteria compared with Gram-negative ones, as a result of chitosan action, is primarily due to the difference between the cell wall structures of the two categories of bacteria. Gram-negative bacteria have three barrier membranes (the hydrophobic outer membrane, the peptidoglycan layer, and the cell membrane), while Gram-positive bacteria have only a thick peptidoglycan layer (that contain teichoic acid and is negatively charged). In addition to these structural differences, intrinsic (molecular mass, degree of deacetylation) and extrinsic factors (concentration, pH, contact time) always occur in the manifestation and magnitude of the bactericidal effect of chitosan or its derivatives on different types of bacteria.
7. The Chitosan Derivatives’ Contributions to the Antibacterial Effect
Chitosan solubility in water is limited. It has been synthetically modified, resulting in improved aqueous solubility and increased antibacterial activity. Based on the type of functional groups that are attached to the polymer backbone, many antibacterial chitosan derivatives exist.
In most cases, functionality of chitosan is provided by chemical reactions that require certain conditions to be accomplished. However, there are some situations where it is sufficient to create reaction conditions that do not actually involve a chemical reaction between components. This is the case for chitosan functionalized by impregnation with various pendant groups; it is necessary to put chitosan in contact with the extractant for a certain time, after which the material is filtered, washed, and dried.
In further sections,
wresearche
rs will describe the most important methods of chemical functionalization of chitosan, referring especially to the hanging groups that can be attached to the chitosan chain.
OurThe objective is a better understanding of the mechanisms that can explain the bactericidal effects of chitosan functionalized by impregnation.
The antibacterial effects of chitosan and its derivatives have been reported by many authors
[10,14,90,91][10][14][85][86]. It was demonstrated that both native chitosan and its derivates have bactericidal effects, but there are evident differences among them.
The presence of –NH
2 and –OH nucleophilic functional groups allow chitosan to be modified either at the amino group or at the hydroxyl group (
Figure 8). In this way, chitosan becomes a support material for the synthesis of another material (chitosan derivative) that will exhibit superior properties.
Figure 8. Schematic chitosan functionalization.
Chitosan derivatives with substituted functional groups for both the –OH and –NH
2 reactive center have an increased bactericidal effect against both Gram-positive and Gram-negative bacteria compared to chitosan functionalized only at a single reactive center
[83][78].
Due to the poor solubility of chitosan in aqueous medium, synthetic modifications of chitosan have mostly been carried out in acidic–aqueous media or under heterogeneous conditions where the polymer is only partially dissolved in the reaction medium. These conventional methods usually allow one to obtain products that can be substituted at all three reactive centers of chitosan (the 2-amino group and the 3- and 6-hydroxyl groups), and this ultimately results in a heterogeneous product or a product having a low degree of substitution. To overcome these issues, different types of groups were introduced to protect either the amino group or the hydroxyl groups
[62]. In this case the synthesis of chemically modified chitosan derivatives can be done selectively by multiple step reactions.
To protect the amino group, the most used protecting group is phtaloyl
[92,93][87][88].
To protect the hydroxyl groups, the most useful protecting groups are acetyl, triphenylmethyl, and trimethylsilyl groups
[93,94,95][88][89][90].
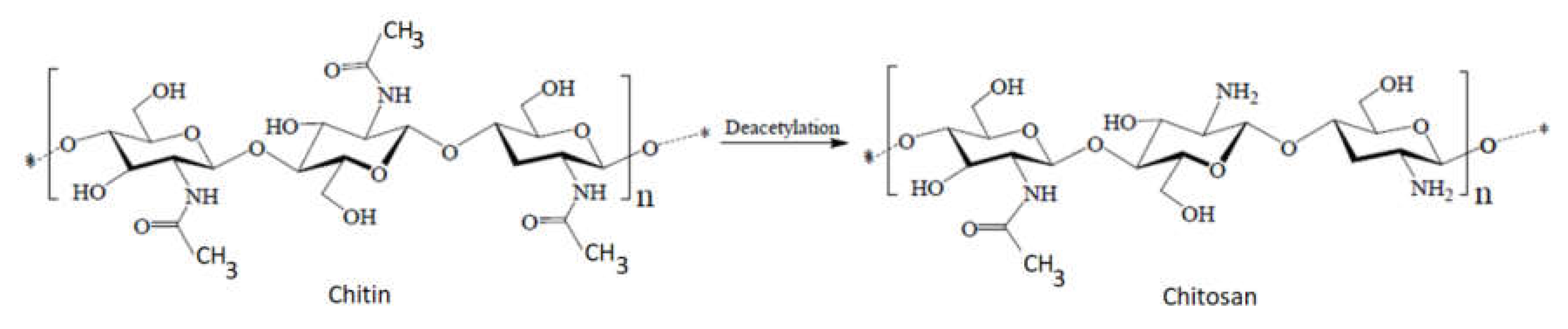
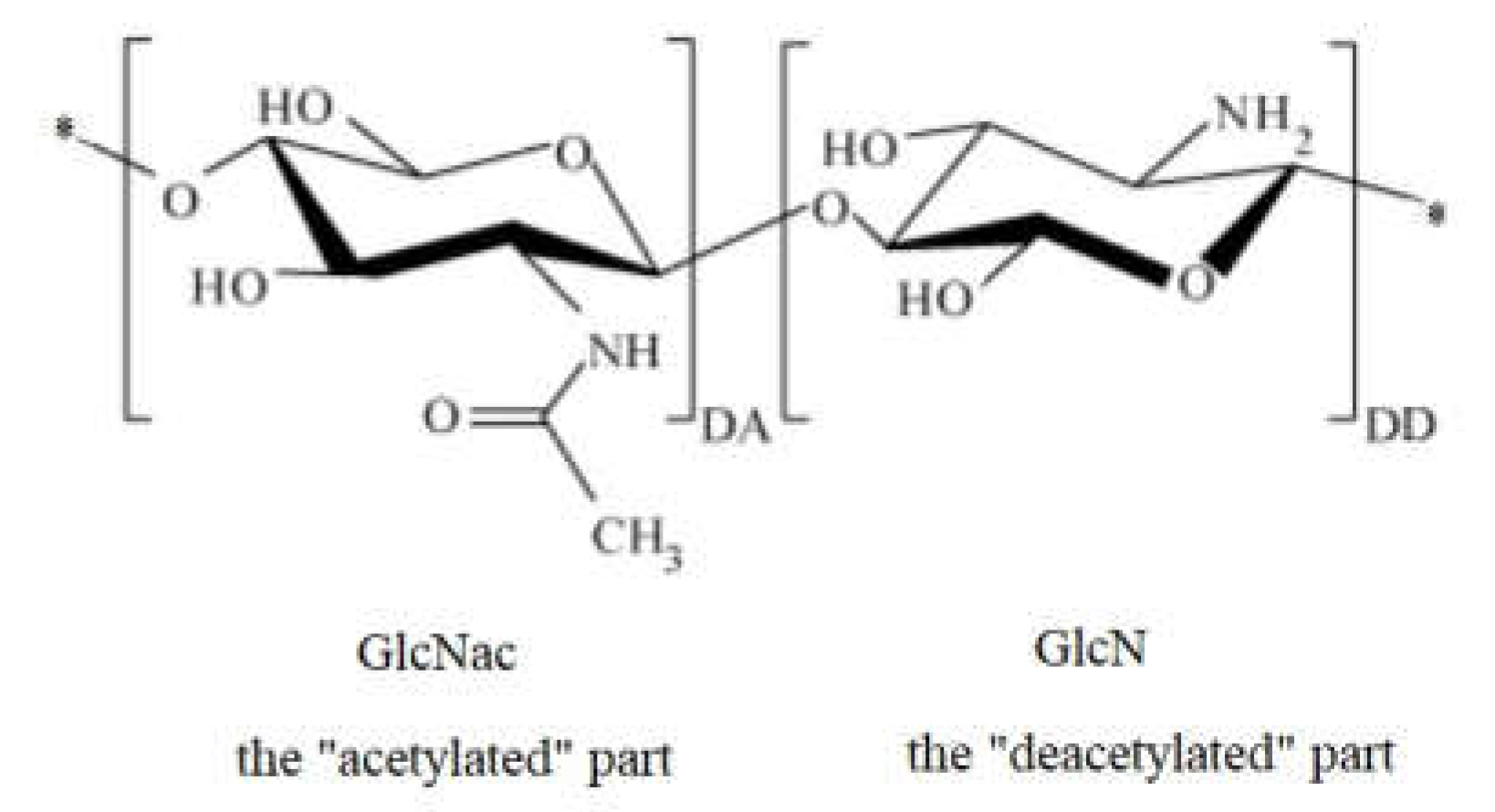
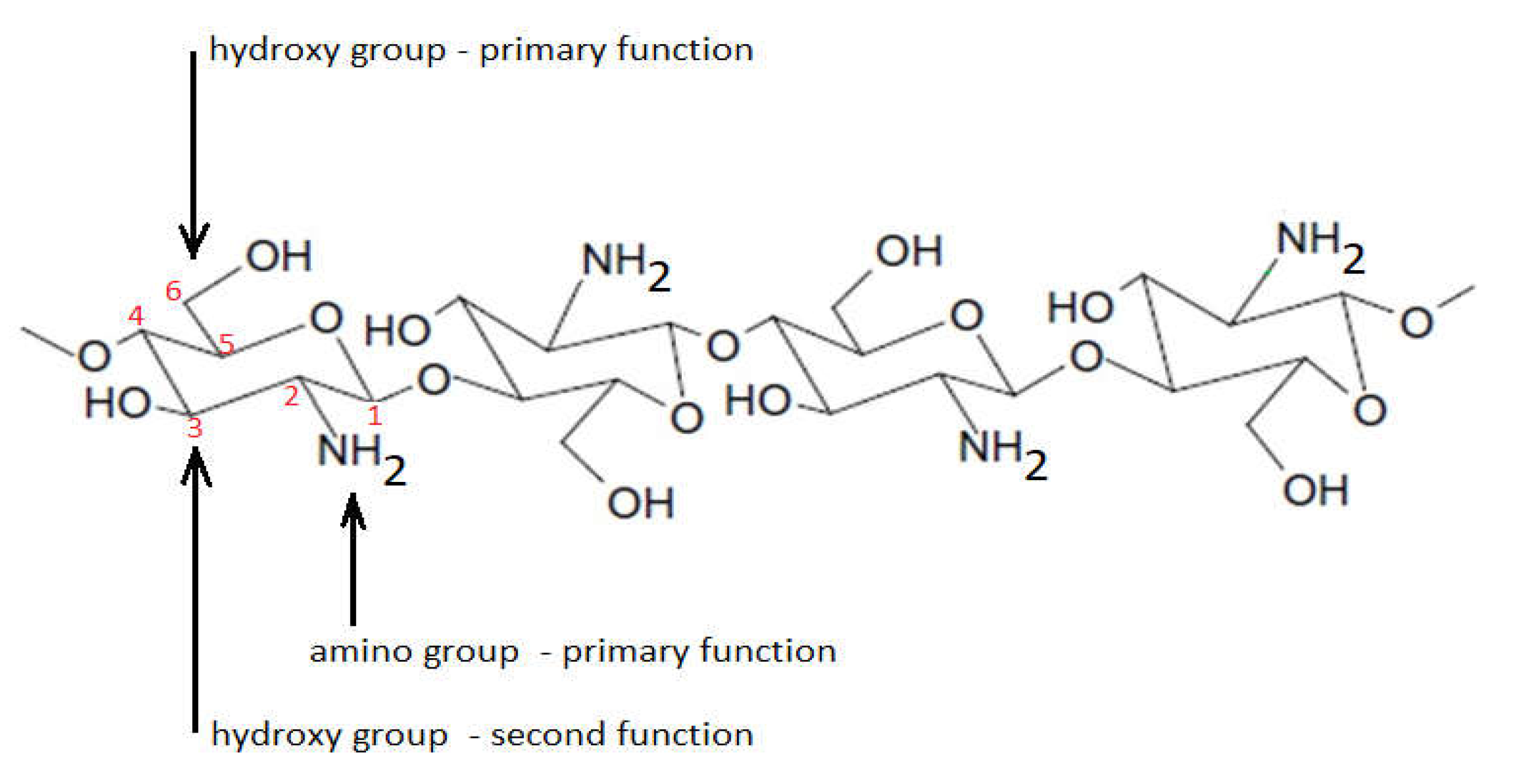
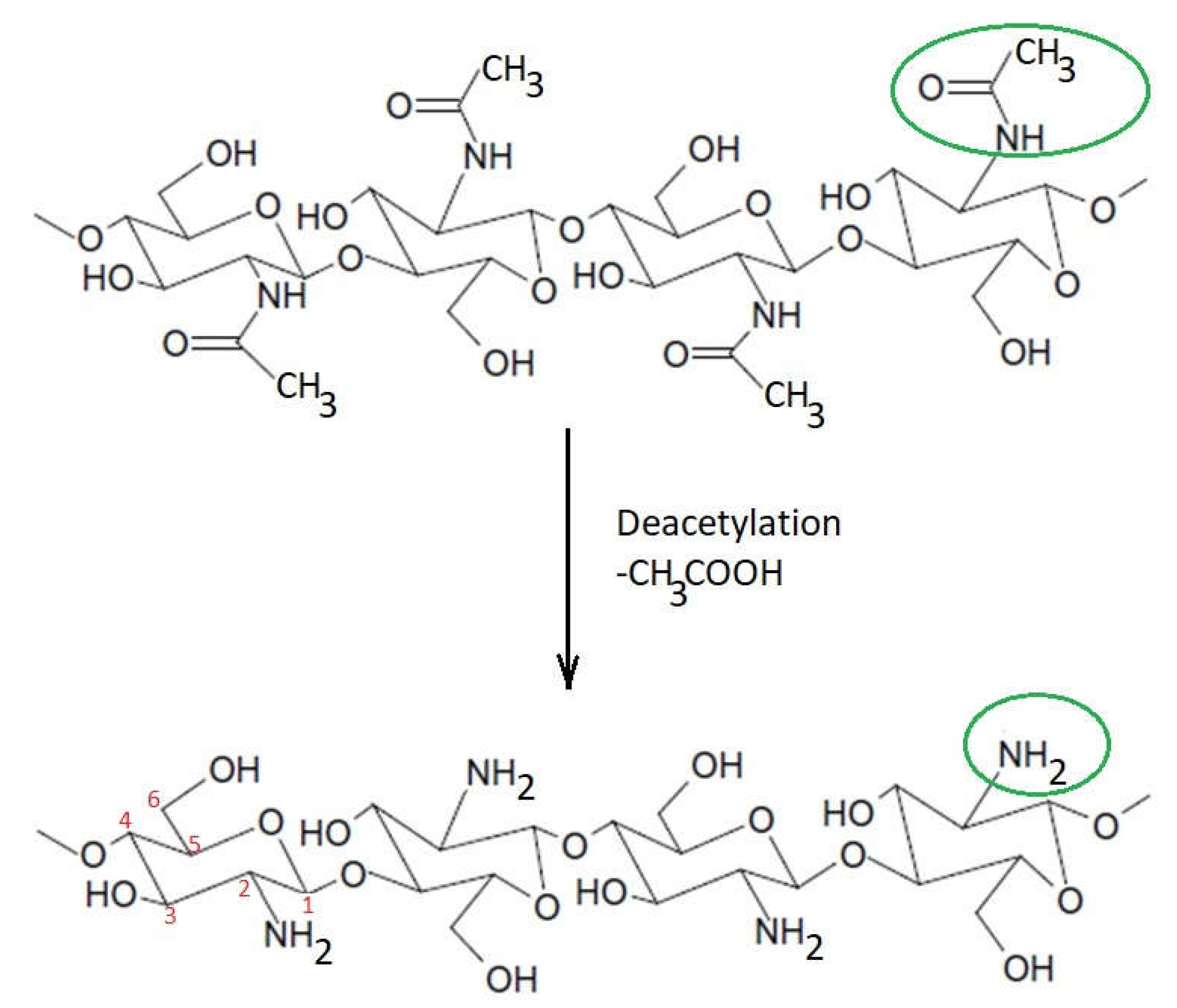
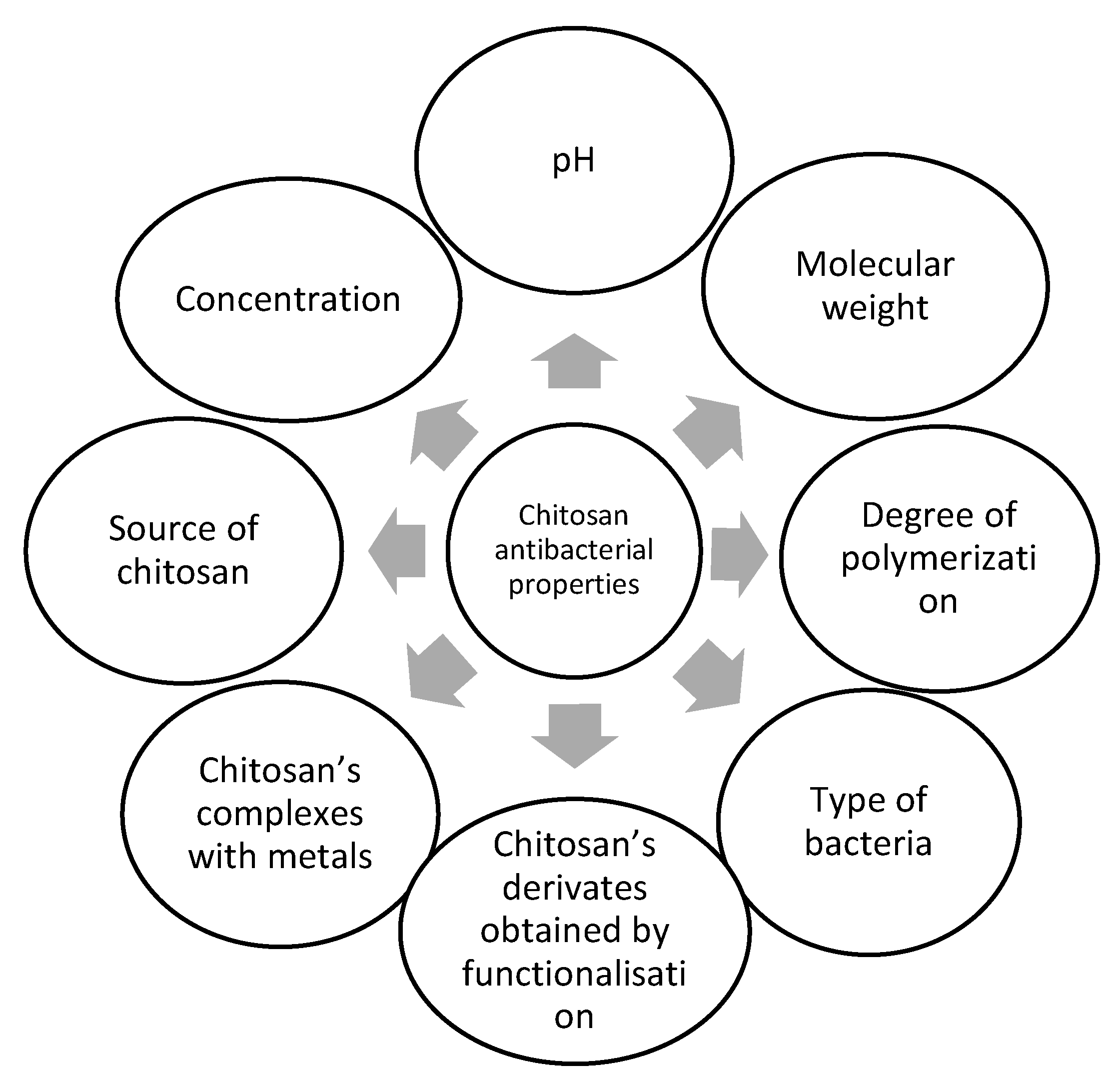
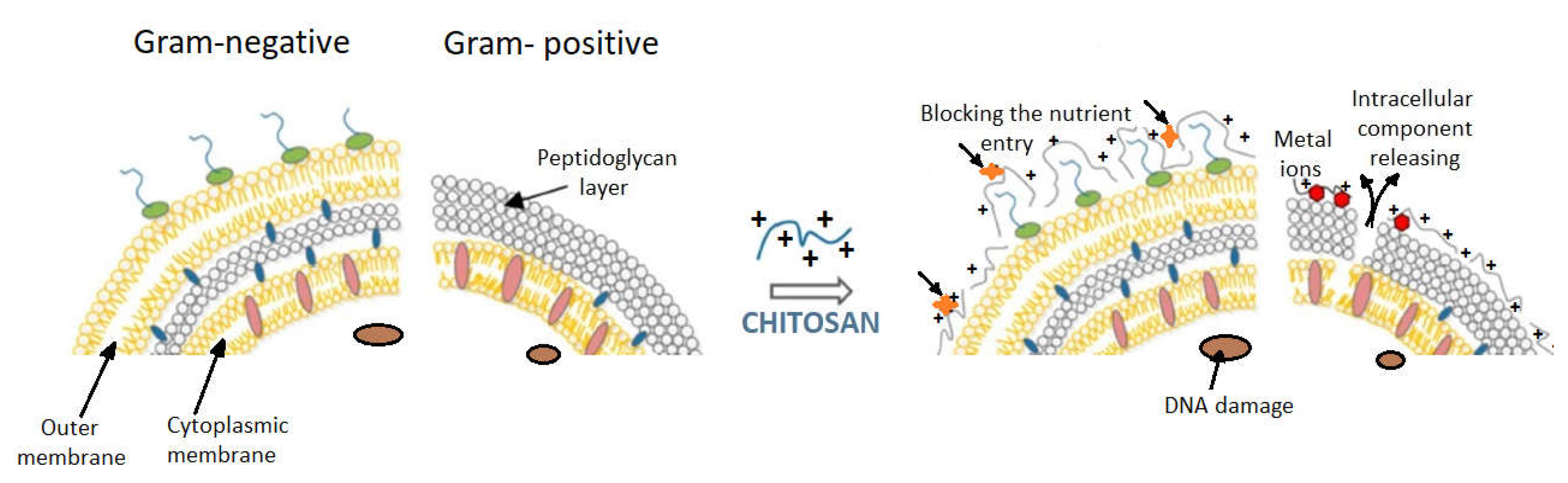 Figure 7. Models proposed for chitosan action upon Gram-positive and Gram-negative bacteria (from Kravanja et al., 2019 [55]).Models proposed for chitosan action upon Gram-positive and Gram-negative bacteria (from Kravanja et al., 2019 [56]).Chitosan exerts a chelating effect [85][80], binding essential metals and thereby inhibiting microbial growth. It is well known that chitosan has excellent metal-binding abilities, using charged amino groups that interact with metals [85,86,87][80][81][82]. This type of interaction between amino functions and divalent ions within the microorganism cell wall (Ca2+ or Mg2+) prevents the production of toxins and inhibits bacterial growth [69,88][69][83].Bacterial membrane permeability is importantly influenced by the molecular size of chitosan and the pH of the medium. These factors were described above.The cellular wall of Gram-positive bacteria is a three-dimensional layer, consisting especially of peptidoglycans. Gram-positive bacteria have no outer membrane (Figure 7).One proposed mechanism for the bactericidal effect of chitosan on Gram-positive bacteria is its direct blocking capacity, preventing nutrients and oxygen from entering the intracellular space [33]. This mechanism is suitable for higher molecular weight chitosan, which forms a polymer membrane on the surface of the bacterial cell.Another bactericidal mechanism of chitosan with low molecular weight derives from the interaction with DNA that inhibits mRNA and protein synthesis after entering the nuclei of microorganisms. This was demonstrated in the case of E. coli, where intracellular chito-oligomers were observed, and these probably prevent DNA transcription [89][84].In conclusion, the different sensitivities of Gram-positive bacteria compared with Gram-negative ones, as a result of chitosan action, is primarily due to the difference between the cell wall structures of the two categories of bacteria. Gram-negative bacteria have three barrier membranes (the hydrophobic outer membrane, the peptidoglycan layer, and the cell membrane), while Gram-positive bacteria have only a thick peptidoglycan layer (that contain teichoic acid and is negatively charged). In addition to these structural differences, intrinsic (molecular mass, degree of deacetylation) and extrinsic factors (concentration, pH, contact time) always occur in the manifestation and magnitude of the bactericidal effect of chitosan or its derivatives on different types of bacteria.
Figure 7. Models proposed for chitosan action upon Gram-positive and Gram-negative bacteria (from Kravanja et al., 2019 [55]).Models proposed for chitosan action upon Gram-positive and Gram-negative bacteria (from Kravanja et al., 2019 [56]).Chitosan exerts a chelating effect [85][80], binding essential metals and thereby inhibiting microbial growth. It is well known that chitosan has excellent metal-binding abilities, using charged amino groups that interact with metals [85,86,87][80][81][82]. This type of interaction between amino functions and divalent ions within the microorganism cell wall (Ca2+ or Mg2+) prevents the production of toxins and inhibits bacterial growth [69,88][69][83].Bacterial membrane permeability is importantly influenced by the molecular size of chitosan and the pH of the medium. These factors were described above.The cellular wall of Gram-positive bacteria is a three-dimensional layer, consisting especially of peptidoglycans. Gram-positive bacteria have no outer membrane (Figure 7).One proposed mechanism for the bactericidal effect of chitosan on Gram-positive bacteria is its direct blocking capacity, preventing nutrients and oxygen from entering the intracellular space [33]. This mechanism is suitable for higher molecular weight chitosan, which forms a polymer membrane on the surface of the bacterial cell.Another bactericidal mechanism of chitosan with low molecular weight derives from the interaction with DNA that inhibits mRNA and protein synthesis after entering the nuclei of microorganisms. This was demonstrated in the case of E. coli, where intracellular chito-oligomers were observed, and these probably prevent DNA transcription [89][84].In conclusion, the different sensitivities of Gram-positive bacteria compared with Gram-negative ones, as a result of chitosan action, is primarily due to the difference between the cell wall structures of the two categories of bacteria. Gram-negative bacteria have three barrier membranes (the hydrophobic outer membrane, the peptidoglycan layer, and the cell membrane), while Gram-positive bacteria have only a thick peptidoglycan layer (that contain teichoic acid and is negatively charged). In addition to these structural differences, intrinsic (molecular mass, degree of deacetylation) and extrinsic factors (concentration, pH, contact time) always occur in the manifestation and magnitude of the bactericidal effect of chitosan or its derivatives on different types of bacteria.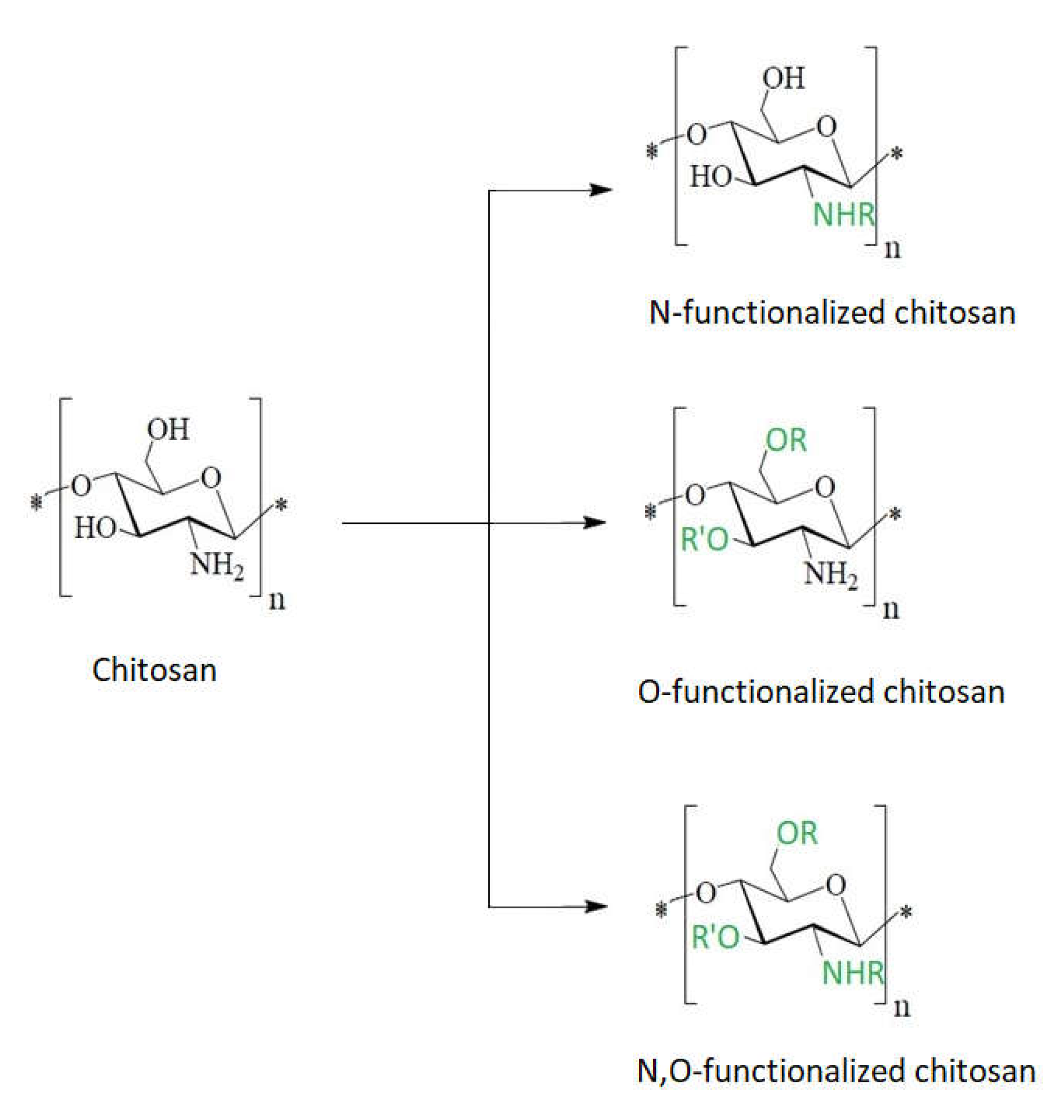 Figure 8. Schematic chitosan functionalization.Chitosan derivatives with substituted functional groups for both the –OH and –NH2 reactive center have an increased bactericidal effect against both Gram-positive and Gram-negative bacteria compared to chitosan functionalized only at a single reactive center [83][78].Due to the poor solubility of chitosan in aqueous medium, synthetic modifications of chitosan have mostly been carried out in acidic–aqueous media or under heterogeneous conditions where the polymer is only partially dissolved in the reaction medium. These conventional methods usually allow one to obtain products that can be substituted at all three reactive centers of chitosan (the 2-amino group and the 3- and 6-hydroxyl groups), and this ultimately results in a heterogeneous product or a product having a low degree of substitution. To overcome these issues, different types of groups were introduced to protect either the amino group or the hydroxyl groups [62]. In this case the synthesis of chemically modified chitosan derivatives can be done selectively by multiple step reactions.
Figure 8. Schematic chitosan functionalization.Chitosan derivatives with substituted functional groups for both the –OH and –NH2 reactive center have an increased bactericidal effect against both Gram-positive and Gram-negative bacteria compared to chitosan functionalized only at a single reactive center [83][78].Due to the poor solubility of chitosan in aqueous medium, synthetic modifications of chitosan have mostly been carried out in acidic–aqueous media or under heterogeneous conditions where the polymer is only partially dissolved in the reaction medium. These conventional methods usually allow one to obtain products that can be substituted at all three reactive centers of chitosan (the 2-amino group and the 3- and 6-hydroxyl groups), and this ultimately results in a heterogeneous product or a product having a low degree of substitution. To overcome these issues, different types of groups were introduced to protect either the amino group or the hydroxyl groups [62]. In this case the synthesis of chemically modified chitosan derivatives can be done selectively by multiple step reactions.