
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Malin Zimmerman | -- | 1999 | 2022-04-01 10:52:56 | | | |
| 2 | Bruce Ren | Meta information modification | 1999 | 2022-04-06 10:59:05 | | |
Video Upload Options
Carpal tunnel syndrome (CTS) is the most common compression neuropathy in the general population and is frequently encountered among individuals with type 1 and 2 diabetes. The reason(s) why a peripheral nerve trunk in individuals with diabetes is more susceptible to nerve compression is still not completely clarified, but both biochemical and structural changes in the peripheral nerve are probably implicated. In particular, individuals with neuropathy, irrespective of aetiology, have a higher risk of peripheral nerve compression disorders, as reflected among individuals with diabetic neuropathy. Diagnosis of CTS in individuals with diabetes should be carefully evaluated; detailed case history, thorough clinical examination, and electrophysiological examination is recommended. Individuals with diabetes and CTS benefit from surgery to the same extent as otherwise healthy individuals with CTS.
1. Introduction
2. Neuropathy in Diabetes
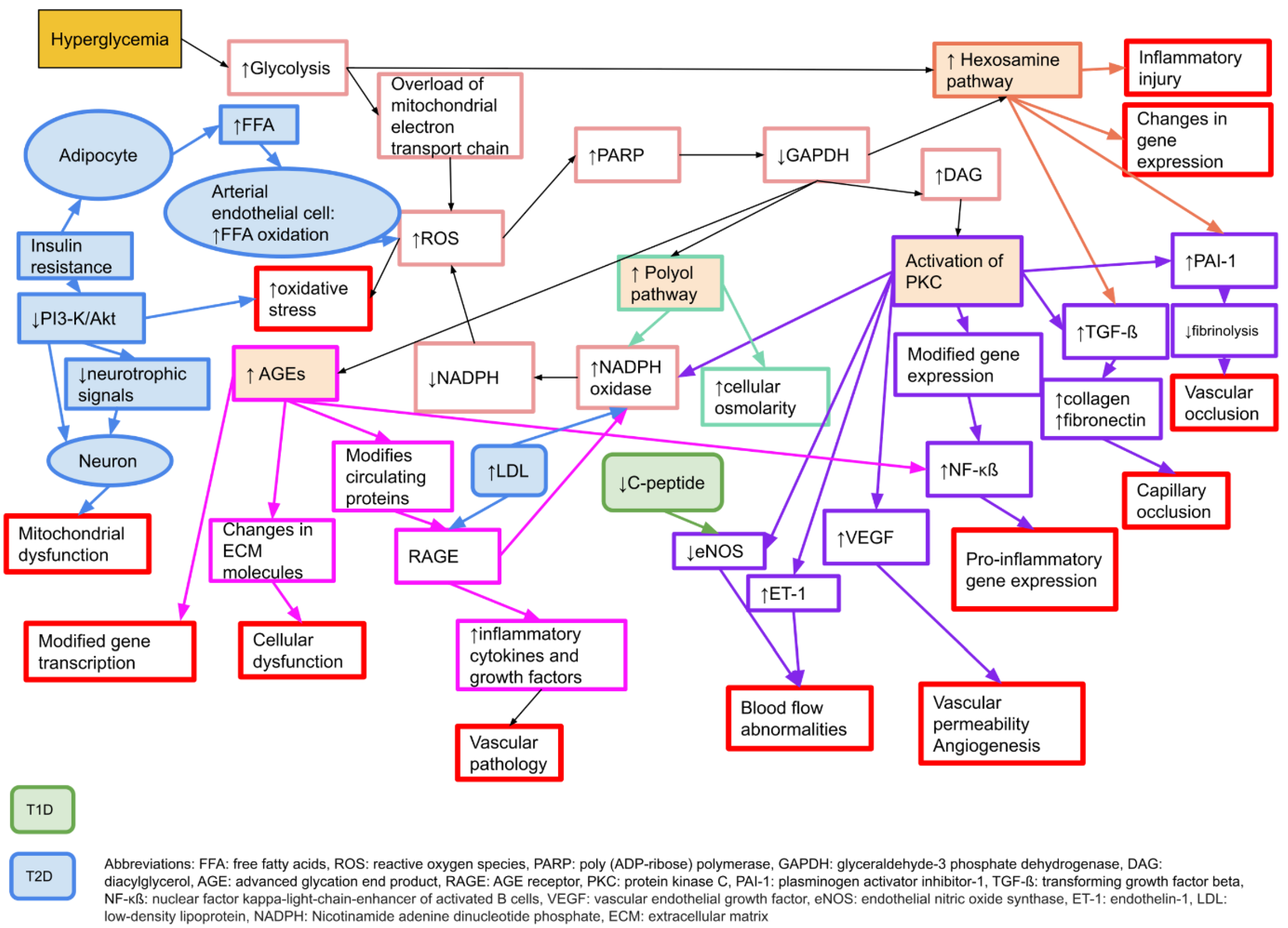
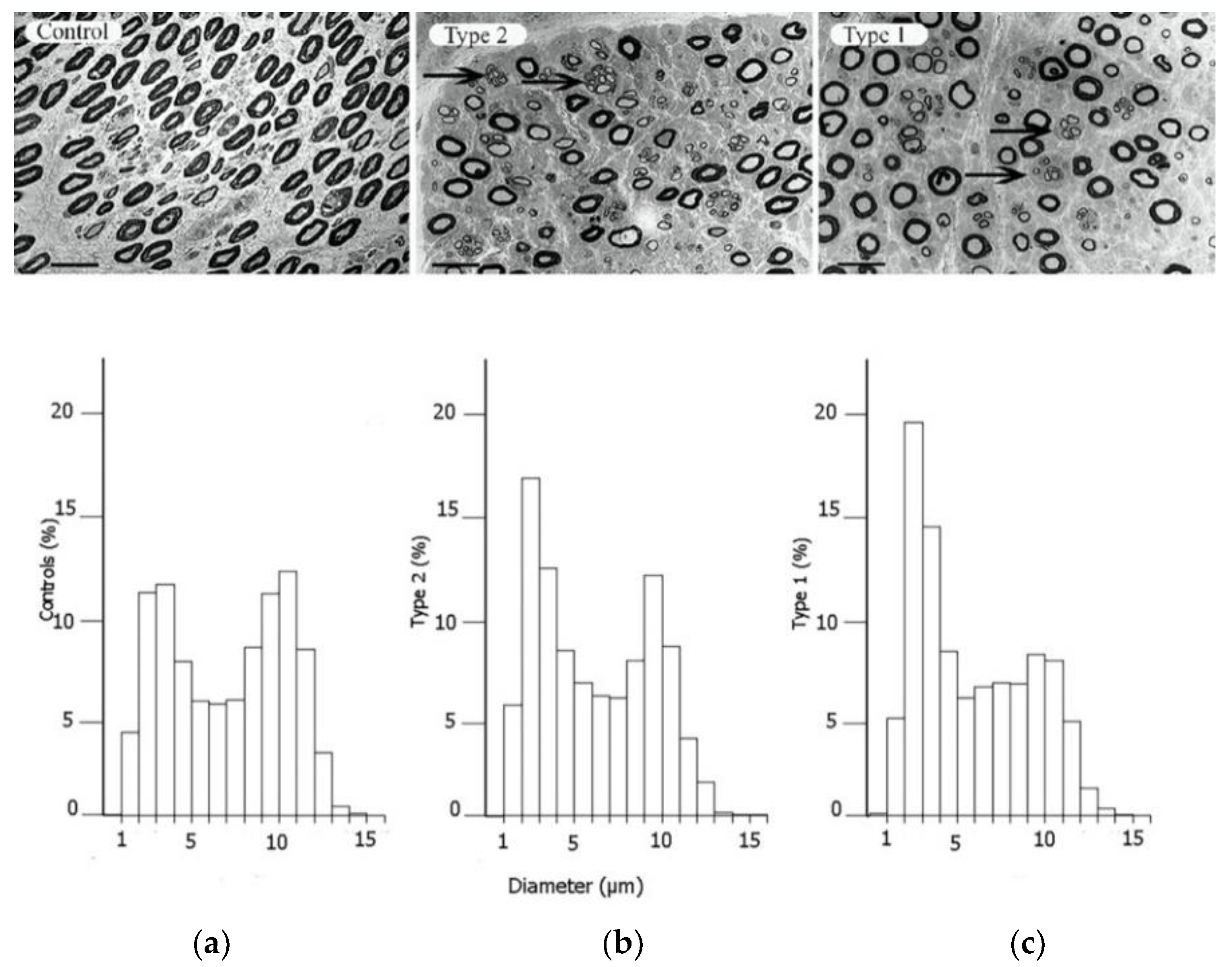
3. The Increased Susceptibility to Nerve Compression in Diabetes
4. Symptoms and Clinical Signs of CTS
5. CTS and Type 1 and Type 2 Diabetes
| OR (95% CI) | Men with Diabetes | Women with Diabetes | ||
|---|---|---|---|---|
| 1.99 (1.81–2.19) | 2.63 (2.42–2.86) | |||
| T1D | T2D | T1D | T2D | |
| Prevalence | 6.8% | 5.0% | 13.5% | 10.1% |
| Incidence rate/ 10,000 person-years |
58.1 | 31.6 | 95.5 | 52.1 |
References
- Latinovic, R.; Gulliford, M.C.; Hughes, R.A. Incidence of common compressive neuropathies in primary care. J. Neurol. Neurosurg. Psychiatry 2006, 77, 263–265.
- Zimmerman, M.; Hall, E.; Carlsson, K.S.; Nyman, E.; Dahlin, L.B. Socioeconomic factors predicting outcome in surgically treated carpal tunnel syndrome: A national registry-based study. Sci. Rep. 2021, 11, 2581.
- Zimmerman, M.; Nyman, E.; Steen Carlsson, K.; Dahlin, L.B. Socioeconomic Factors in Patients with Ulnar Nerve Compression at the Elbow: A National Registry-Based Study. BioMed Res. Int. 2020, 2020, 5928649.
- Atroshi, I.; Gummesson, C.; Johnsson, R.; Ornstein, E.; Ranstam, J.; Rosen, I. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999, 282, 153–158.
- Atroshi, I. Incidence of physician-diagnosed carpal tunnel syndrome in the general population. Arch. Intern. Med. 2011, 171, 943–944.
- Tadjerbashi, K.; Åkesson, A.; Atroshi, I. Incidence of referred carpal tunnel syndrome and carpal tunnel release surgery in the general population: Increase over time and regional variations. J. Orthop. Surg. 2019, 27, 2309499019825572.
- Nordstrom, D.L.; DeStefano, F.; Vierkant, R.A.; Layde, P.M. Incidence of diagnosed carpal tunnel syndrome in a general population. Epidemiology 1998, 9, 342–345.
- Bland, J.D.; Rudolfer, S.M. Clinical surveillance of carpal tunnel syndrome in two areas of the United Kingdom, 1991–2001. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1674–1679.
- Wiberg, A.; Ng, M.; Schmid, A.B.; Smillie, R.W.; Baskozos, G.; Holmes, M.V.; Künnapuu, K.; Mägi, R.; Bennett, D.L.; Furniss, D. A genome-wide association analysis identifies 16 novel susceptibility loci for carpal tunnel syndrome. Nat. Commun. 2019, 10, 1030.
- Renard, E.; Jacques, D.; Chammas, M.; Poirier, J.L.; Bonifacj, C.; Jaffiol, C.; Simon, L.; Allieu, Y. Increased prevalence of soft tissue hand lesions in type 1 and type 2 diabetes mellitus: Various entities and associated significance. Diabete Metab. 1994, 20, 513–521.
- Papanas, N.; Maltezos, E. The diabetic hand: A forgotten complication? J. Diabetes Its Complicat. 2010, 24, 154–162.
- Rydberg, M.; Zimmerman, M.; Gottsäter, A.; Svensson, A.; Eeg-Olofsson, K.; Dahlin, L.B. The Diabetic Hand-prevalence and incidence of diabetic hand problems using data from 1.1 million inhabitants in southern Sweden. BMJ Open Diabetes Res. Care 2022, 10, e002614.
- Rota, E.; Morelli, N. Entrapment neuropathies in diabetes mellitus. World J. Diabetes 2016, 7, 342–353.
- Rydberg, M.; Zimmerman, M.; Gottsäter, A.; Nilsson, P.M.; Melander, O.; Dahlin, L.B. Diabetes mellitus as a risk factor for compression neuropathy: A longitudinal cohort study from southern Sweden. BMJ Open Diabetes Res. Care 2020, 8, e001298.
- Upton, A.R.; McComas, A.J. The double crush in nerve entrapment syndromes. Lancet 1973, 2, 359–362.
- Thomsen, N.O.; Mojaddidi, M.; Malik, R.A.; Dahlin, L.B. Reduced myelinated nerve fibre and endoneurial capillary densities in the forearm of diabetic and non-diabetic patients with carpal tunnel syndrome. Acta Neuropathol. 2009, 118, 785–791.
- Dahlin, L.; Sanden, H.; Dahlin, E.; Zimmerman, M.; Thomsen, N.; Bjorkman, A. Low myelinated nerve-fibre density may lead to symptoms associated with nerve entrapment in vibration-induced neuropathy. J. Occup. Med. Toxicol. 2014, 9, 7.
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154.
- Factors in development of diabetic neuropathy. Baseline analysis of neuropathy in feasibility phase of Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. Diabetes 1988, 37, 476–481.
- Salvotelli, L.; Stoico, V.; Perrone, F.; Cacciatori, V.; Negri, C.; Brangani, C.; Pichiri, I.; Targher, G.; Bonora, E.; Zoppini, G. Prevalence of neuropathy in type 2 diabetic patients and its association with other diabetes complications: The Verona Diabetic Foot Screening Program. J. Diabetes Its Complicat. 2015, 29, 1066–1070.
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41.
- Partanen, J.; Niskanen, L.; Lehtinen, J.; Mervaala, E.; Siitonen, O.; Uusitupa, M. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1995, 333, 89–94.
- Aaberg, M.L.; Burch, D.M.; Hud, Z.R.; Zacharias, M.P. Gender differences in the onset of diabetic neuropathy. J. Diabetes Its Complicat. 2008, 22, 83–87.
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625.
- Albers, J.W.; Pop-Busui, R. Diabetic Neuropathy: Mechanisms, Emerging Treatments, and Subtypes. Curr. Neurol. Neurosci. Rep. 2014, 14, 473.
- Zimmerman, M. The Diabetic Nerve. Studies on Outcome after Open Carpal Tunnel Release and the Development of Autonomic Neuropathy. Ph.D. Dissertation, Lund University, Lund, Sweden, 2018.
- Baptista, F.I.; Pinheiro, H.; Gomes, C.A.; Ambrósio, A.F. Impairment of Axonal Transport in Diabetes: Focus on the Putative Mechanisms Underlying Peripheral and Central Neuropathies. Mol. Neurobiol. 2019, 56, 2202–2210.
- Medori, R.; Autilio-Gambetti, L.; Jenich, H.; Gambetti, P. Changes in axon size and slow axonal transport are related in experimental diabetic neuropathy. Neurology 1988, 38, 597–601.
- Mohseni, S.; Badii, M.; Kylhammar, A.; Thomsen, N.O.B.; Eriksson, K.F.; Malik, R.A.; Rosen, I.; Dahlin, L.B. Longitudinal study of neuropathy, microangiopathy, and autophagy in sural nerve: Implications for diabetic neuropathy. Brain Behav. 2017, 7, e00763.
- Callaghan, B.C.; Cheng, H.; Stables, C.L.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 2012, 11, 521–534.
- Sima, A.A.F.; Zhang, W.; Grunberger, G. Type 1 Diabetic Neuropathy and C-peptide. Exp. Diabesity Res. 2004, 5, 65–77.
- Nathan, D.M. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 2014, 37, 9–16.
- Callaghan, B.C.; Little, A.A.; Feldman, E.L.; Hughes, R.A. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst. Rev. 2012, 6, Cd007543.
- Callaghan, B.; Feldman, E. The metabolic syndrome and neuropathy: Therapeutic challenges and opportunities. Ann. Neurol. 2013, 74, 397–403.
- Callaghan, B.C.; Gallagher, G.; Fridman, V.; Feldman, E.L. Diabetic neuropathy: What does the future hold? Diabetologia 2020, 63, 891–897.
- Eid, S.; Sas, K.M.; Abcouwer, S.F.; Feldman, E.L.; Gardner, T.W.; Pennathur, S.; Fort, P.E. New insights into the mechanisms of diabetic complications: Role of lipids and lipid metabolism. Diabetologia 2019, 62, 1539–1549.
- Ohkubo, Y.; Kishikawa, H.; Araki, E.; Miyata, T.; Isami, S.; Motoyoshi, S.; Kojima, Y.; Furuyoshi, N.; Shichiri, M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: A randomized prospective 6-year study. Diabetes Res. Clin. Pract. 1995, 28, 103–117.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352, 837–853.
- Osman, A.A.; Dahlin, L.B.; Thomsen, N.O.; Mohseni, S. Autophagy in the posterior interosseous nerve of patients with type 1 and type 2 diabetes mellitus: An ultrastructural study. Diabetologia 2015, 58, 625–632.
- Sunderland, S. Nerves and Nerve Injuries, 2nd ed.; Edinburgh; Churchill Livingstone: Philadelphia, PA, USA, 1978.
- Boron, W.F.B.; Emile, L. Medical Physiology, 2nd ed.; Saunders; Elsevier: Hoboken, NJ, USA, 2009.
- King, R. Peripheral Nerve Disorders; Vallat, J.-M., Weis, J., Eds.; International Society of Neuropathology Series; Wiley: Hoboken, NJ, USA, 2014.
- Dahlin, L.B.; Shyu, B.C.; Danielsen, N.; Andersson, S.A. Effects of nerve compression or ischaemia on conduction properties of myelinated and non-myelinated nerve fibres. An experimental study in the rabbit common peroneal nerve. Acta Physiol. Scand. 1989, 136, 97–105.
- Sleigh, J.N.; Rossor, A.M.; Fellows, A.D.; Tosolini, A.P.; Schiavo, G. Axonal transport and neurological disease. Nat. Rev. Neurol. 2019, 15, 691–703.
- Dahlin, L.B.; Meiri, K.F.; McLean, W.G.; Rydevik, B.; Sjostrand, J. Effects of nerve compression on fast axonal transport in streptozotocin-induced diabetes mellitus. An experimental study in the sciatic nerve of rats. Diabetologia 1986, 29, 181–185.
- Snedeker, J.G.; Gautieri, A. The role of collagen crosslinks in ageing and diabetes-the good, the bad, and the ugly. Muscles Ligaments Tendons J. 2014, 4, 303–308.
- Samii, A.; Unger, J.; Lange, W. Vascular endothelial growth factor expression in peripheral nerves and dorsal root ganglia in diabetic neuropathy in rats. Neurosci. Lett. 1999, 262, 159–162.
- Lundborg, G.; Myers, R.; Powell, H. Nerve compression injury and increased endoneurial fluid pressure: A “miniature compartment syndrome”. J. Neurol. Neurosurg. Psychiatry 1983, 46, 1119–1124.
- Lakshminarayanan, K.; Shah, R. Median nerve and carpal arch morphology changes in women with type 2 diabetes: A case–control study. J. Ultrasound 2021.
- Mojaddidi, M.A.; Ahmed, M.S.; Ali, R.; Jeziorska, M.; Al-Sunni, A.; Thomsen, N.O.; Dahlin, L.B.; Malik, R.A. Molecular and pathological studies in the posterior interosseous nerve of diabetic and non-diabetic patients with carpal tunnel syndrome. Diabetologia 2014, 57, 1711–1719.
- Strömberg, T.; Dahlin, L.B.; Brun, A.; Lundborg, G. Structural nerve changes at wrist level in workers exposed to vibration. Occup. Environ. Med. 1997, 54, 307–311.
- Vinik, A.; Mehrabyan, A.; Colen, L.; Boulton, A. Focal entrapment neuropathies in diabetes. Diabetes Care 2004, 27, 1783–1788.
- Mackinnon, S.E. Pathophysiology of nerve compression. Hand Clin. 2002, 18, 231–241.
- Dahlin, L.B. Aspects on pathophysiology of nerve entrapments and nerve compression injuries. Neurosurg. Clin. N. Am. 1991, 2, 21–29.
- Gupta, R.; Rowshan, K.; Chao, T.; Mozaffar, T.; Steward, O. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp. Neurol. 2004, 187, 500–508.
- Aboonq, M.S. Pathophysiology of carpal tunnel syndrome. Neurosciences 2015, 20, 4–9.
- Lundborg, G.; Dahlin, L.B. Anatomy, function, and pathophysiology of peripheral nerves and nerve compression. Hand Clin. 1996, 12, 185–193.
- Tapadia, M.; Mozaffar, T.; Gupta, R. Compressive Neuropathies of the Upper Extremity: Pathophysiology, Classification, Electrodiagnostic Findings. J. Hand Surg. 2010, 35, 668–677.
- Thomsen, N.O.B.; Rosén, I.; Dahlin, L.B. Neurophysiologic recovery after carpal tunnel release in diabetic patients. Clin. Neurophysiol. 2010, 121, 1569–1573.
- Thomsen, N.O.B.; Andersson, G.S.; Bjork, J.; Dahlin, L.B. Neurophysiological recovery 5 years after carpal tunnel release in patients with diabetes. Muscle Nerve 2017, 56, E59–E64.
- Zhang, D.; Collins, J.; Blazar, P.; Earp, B.E. Factors Associated With Advanced Presentation for Carpal Tunnel Release. J. Hand Surg. Am. 2020, 45, 111–116.
- Mackinnon, S.E.; Dellon, A.L.; Hudson, A.R.; Hunter, D.A. Chronic human nerve compression—A histological assessment. Neuropathol. Appl. Neurobiol. 1986, 12, 547–565.
- Wiberg, A.; Smillie, R.W.; Dupré, S.; Schmid, A.B.; Bennett, D.L.; Furniss, D. Replication of epidemiological associations of carpal tunnel syndrome in a UK population-based cohort of over 400,000 people. J. Plast. Reconstr. Aesthet. Surg. 2021; in press.
- Pourmemari, M.H.; Shiri, R. Diabetes as a risk factor for carpal tunnel syndrome: A systematic review and meta-analysis. Diabet. Med. 2016, 33, 10–16.




