
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ibrahim Sadek | -- | 1574 | 2022-03-31 11:54:25 | | | |
| 2 | Yvaine Wei | -5 word(s) | 1569 | 2022-04-01 04:44:56 | | |
Video Upload Options
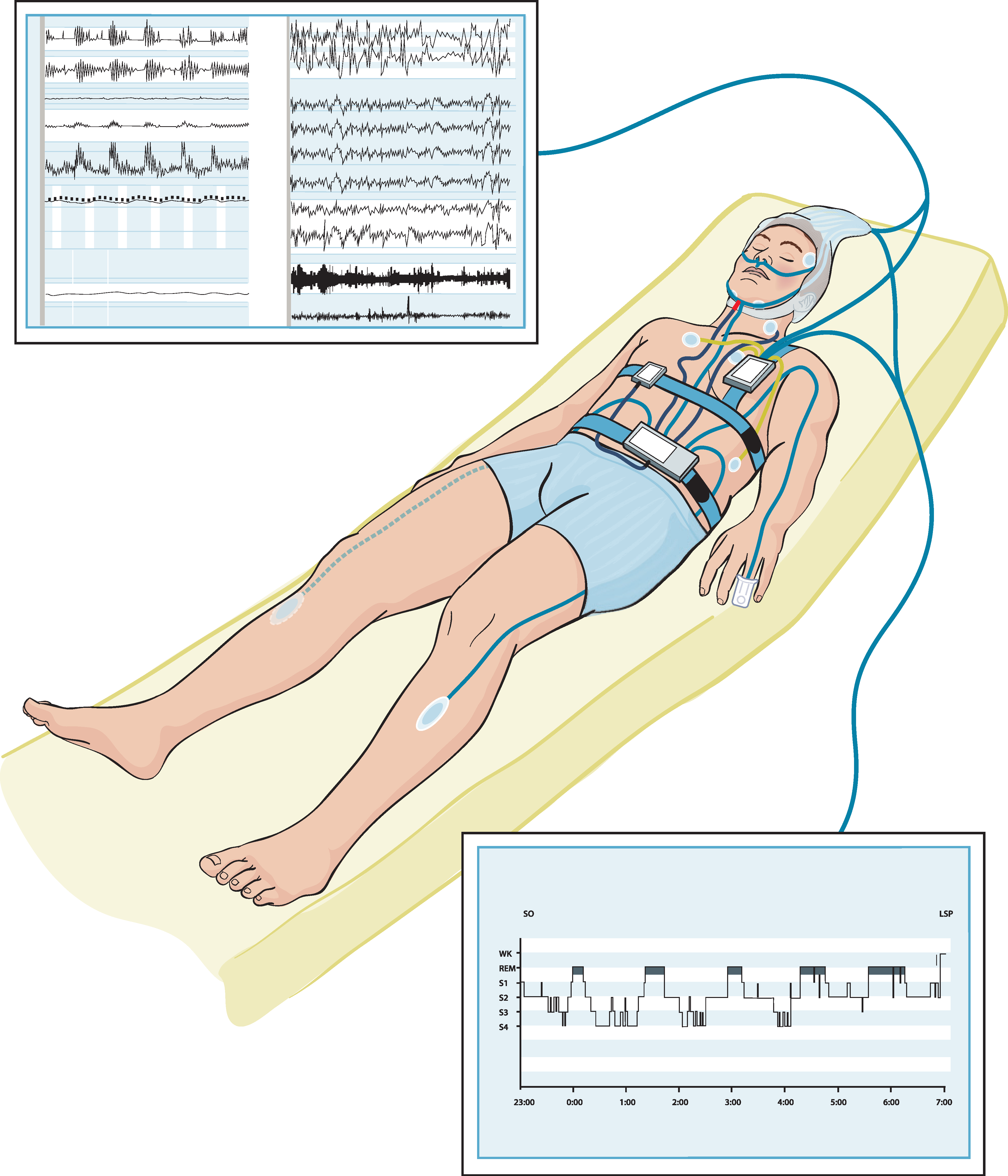
Polysomnography is the gold-standard method for measuring sleep but is inconvenient and limited to a laboratory or a hospital setting. As a result, the vast majority of patients do not receive a proper diagnosis. In an attempt to solve this issue, sleep experts are continually looking for unobtrusive and affordable alternatives that can provide longitudinal sleep tracking. Collecting longitudinal data on sleep can accelerate epidemiological studies exploring the effect of sleep on health and disease. These alternatives can be in the form of wearables (e.g., actigraphs) or nonwearable (e.g., under-mattress sleep trackers).
1. Introduction
2. Current Insights of Sleep Monitoring Methods
It can be noted that there is an increasing interest in sleep monitoring (in particular, sleep cycles) using unobtrusive sensors. Due to the unsuitability of PSG for in-home monitoring, researchers have developed and are developing various unobtrusive systems as alternatives, leveraging on recent technological advancements. Generally, proposed sleep monitoring methods are based on one or a combination of the following: respiratory cycle, cardiac cycle, body movement [20]. It was also observed that the majority of the proposed systems are limited to three-stage sleep classification [20]. Although the results from the abovementioned studies are encouraging in terms of accuracy, commercial devices cannot produce identical results to PSG. It makes sense because EEG-based systems are the most accurate for detecting all the stages of sleep [21].
That said, the ease and relative performance of actigraphy-based devices for sleep and sleep-cycle monitoring has given rise to numerous wearable devices and smartphone-based technologies. Actigraphy devices enable the user to wear dedicated sensors to help track vital signs and movements while sleeping. It is shown that the use of wearable devices for sleep-cycle monitoring is feasible but inaccurate compared to the gold standard PSG [22][23][24]. This is because even in healthy adults accelerometry has high sensitivity but low specificity for sleep detection. These devices often tend to underestimate or overestimate some key parameters such as TST, sleep efficiency, wake, or the transition between the sleep stages [22][23][24]. Patients with sleep disorders, or those who are chronically sleep-deprived, are more likely to suffer from fragmented sleep and reduced ability to understand their functional impairment. Therefore, wearing sleep trackers with incorrect readings could have adverse effects on these patients. This happens because most patients do not realize that the claims of these devices typically outweigh the science to support them as devices to measure and improve sleep. As a result, the importance of precise measurements cannot be overstated [23].
A recent study by Chinoy et al. [25] has shown that off-the-shelf sleep trackers (i.e., Fatigue Science Readiband, Fitbit Alta HR, EarlySense Live, ResMed S+, SleepScore Max) provided mixed results for sleep stage classification and the trackers tended to perform worse on nights with poorer/disrupted sleep. Similarly, Roomkham et al. [26] have come to the same conclusion that further studies are needed to assess the longer-term performance of sleep trackers, namely, the Apple Watch in natural conditions, and against PSG in clinical settings. Furthermore, Kholghi et al. [27] concluded that EMFIT QS failed to distinguish sleep stages against PSG and additional development is needed before using EMIFT QS in clinical settings. Moreover, studies have shown that although smartphone-based sensing systems are simpler and less expensive, they correlate poorly with the PSG [28].
3. Conclusions
References
- Chattu, V.K.; Sakhamuri, S.M.; Kumar, R.; Spence, D.W.; BaHammam, A.S.; Pandi-Perumal, S.R. Insufficient Sleep Syndrome: Is It Time to Classify It as a Major Noncommunicable Disease? Sleep Sci. 2018, 11, 57–64.
- Schutte-Rodin, S.; Deak, M.C.; Khosla, S.; Goldstein, C.A.; Yurcheshen, M.; Chiang, A.; Gault, D.; Kern, J.; O’Hearn, D.; Ryals, S.; et al. Evaluating Consumer and Clinical Sleep Technologies: An American Academy of Sleep Medicine Update. J. Clin. Sleep Med. 2021, 17, 2275–2282.
- Pan, Q.; Brulin, D.; Campo, E. Current Status and Future Challenges of Sleep Monitoring Systems: Systematic Review. JMIR Biomed. Eng. 2020, 5, e20921.
- Worley, S.L. The Extraordinary Importance of Sleep: The Detrimental Effects of Inadequate Sleep on Health and Public Safety Drive an Explosion of Sleep Research. Pharm. Ther. 2018, 43, 758–763.
- Hussain, Z.; Sheng, Q.Z.; Zhang, W.E.; Ortiz, J.; Pouriyeh, S. A Review of the Non-Invasive Techniques for Monitoring Different Aspects of Sleep. arXiv 2021, arXiv:2104.12964.
- Roomkham, S.; Lovell, D.; Cheung, J.; Perrin, D. Promises and Challenges in the Use of Consumer-Grade Devices for Sleep Monitoring. IEEE Rev. Biomed. Eng. 2018, 11, 53–67.
- Tataraidze, A.B.; Anishchenko, L.N.; Korostovtseva, L.S.; Bochkarev, M.V.; Sviryaev, Y.V. Non-Contact Respiratory Monitoring of Subjects with Sleep-Disordered Breathing. In Proceedings of the 2018 International Conference “Quality Management, Transport and Information Security, Information Technologies”, St. Petersburg, Russia, 24–28 September 2018; pp. 736–738.
- Liang, Z.; Chapa-Martell, M.A. A Multi-Level Classification Approach for Sleep Stage Prediction With Processed Data Derived From Consumer Wearable Activity Trackers. Front. Digit. Health 2021, 3, 665946.
- Andrew, T.L.; Rostaminia, S.; Homayounfar, S.Z.; Ganesan, D. Perspective—Longitudinal Sleep Monitoring for All: Payoffs, Challenges and Outlook. ECS Sens. Plus 2022.
- Perez-Pozuelo, I.; Zhai, B.; Palotti, J.; Mall, R.; Aupetit, M.; Garcia-Gomez, J.M.; Taheri, S.; Guan, Y.; Fernandez-Luque, L. The Future of Sleep Health: A Data-Driven Revolution in Sleep Science and Medicine. Npj Digit. Med. 2020, 3, 42.
- Dixon, M.; Schneider, L.D.; Yu, J.; Hsu, J.; Pathak, A.; Shin, D.; Lee, R.S.; Malhotra, M.; Mixter, K.; Mcconnell, M.V.; et al. Sleep-Wake Detection with a Contactless, Bedside Radar Sleep Sensing System; Google LLC: Seattle, WA, USA, 2021.
- Zhang, G.; Vahia, I.V.; Liu, Y.; Yang, Y.; May, R.; Cray, H.V.; McGrory, W.; Katabi, D. Contactless In-Home Monitoring of the Long-Term Respiratory and Behavioral Phenotypes in Older Adults With COVID-19: A Case Series. Front. Psychiatry 2021, 12, 754169.
- Schütz, N.; Saner, H.; Botros, A.; Pais, B.; Santschi, V.; Buluschek, P.; Gatica-Perez, D.; Urwyler, P.; Müri, R.; Nef, T. Contactless Sleep Monitoring for Early Detection of Health Deteriorations in Community-Dwelling Older Adults: Exploratory Study. JMIR mHealth uHealth 2021, 9, e24666.
- Yu, B.; Wang, Y.; Niu, K.; Zeng, Y.; Gu, T.; Wang, L.; Guan, C.; Zhang, D. WiFi-Sleep: Sleep Stage Monitoring Using Commodity Wi-Fi Devices. IEEE Internet Things J. 2021, 8, 13900–13913.
- Korhonen, I.; Iivainen, T.; Lappalainen, R.; Tuomisto, T.; Kööbi, T.; Pentikäinen, V.; Tuomisto, M.; Turjanmaa, V. TERVA: System for Long-Term Monitoring of Wellness at Home. Telemed. J. e-Health 2001, 7, 61–72.
- Sadek, I.; Bellmunt, J.; Kodyš, M.; Abdulrazak, B.; Mokhtari, M. Novel Unobtrusive Approach for Sleep Monitoring Using Fiber Optics in an Ambient Assisted Living Platform. In Enhanced Quality of Life and Smart Living; Mokhtari, M., Abdulrazak, B., Aloulou, H., Eds.; Springer: Cham, Switzerland, 2017; Volume 10461 LNCS, pp. 48–60. Available online: https://link.springer.com/chapter/10.1007/978-3-319-66188-9_5 (accessed on 7 March 2022).
- Lima, F.; Albukhari, A.; Zhu, R.; Mescheder, U. Contactless Sleep Monitoring Measurement Setup. Proceedings 2018, 2, 1083.
- Koyama, Y.; Nishiyama, M.; Watanabe, K. Smart Textile Using Hetero-Core Optical Fiber for Heartbeat and Respiration Monitoring. IEEE Sens. J. 2018, 18, 6175–6180.
- Zhou, Z.; Padgett, S.; Cai, Z.; Conta, G.; Wu, Y.; He, Q.; Zhang, S.; Sun, C.; Liu, J.; Fan, E.; et al. Single-Layered Ultra-Soft Washable Smart Textiles for All-around Ballistocardiograph, Respiration, and Posture Monitoring during Sleep. Biosens. Bioelectron. 2020, 155, 112064.
- Inan, O.T.; Migeotte, P.F.; Park, K.S.; Etemadi, M.; Tavakolian, K.; Casanella, R.; Zanetti, J.; Tank, J.; Funtova, I.; Prisk, G.K.; et al. Ballistocardiography and Seismocardiography: A Review of Recent Advances. IEEE J. Biomed. Health Inform. 2015, 19, 1414–1427.
- Imtiaz, S.A. A Systematic Review of Sensing Technologies for Wearable Sleep Staging. Sensors 2021, 21, 1562.
- Tuominen, J.; Peltola, K.; Saaresranta, T.; Valli, K. Sleep Parameter Assessment Accuracy of a Consumer Home Sleep Monitoring Ballistocardiograph Beddit Sleep Tracker: A Validation Study. J. Clin. Sleep Med. 2019, 15, 483–487.
- Baron, K.G.; Abbott, S.; Jao, N.; Manalo, N.; Mullen, R. Orthosomnia: Are Some Patients Taking the Quantified Self Too Far? J. Clin. Sleep Med. 2017, 13, 351–354.
- Sadek, I.; Biswas, J.; Abdulrazak, B. Ballistocardiogram Signal Processing: A Review. Health Inf. Sci. Syst. 2019, 7, 10.
- Chinoy, E.D.; Cuellar, J.A.; Huwa, K.E.; Jameson, J.T.; Watson, C.H.; Bessman, S.C.; Hirsch, D.A.; Cooper, A.D.; Drummond, S.P.A.; Markwald, R.R. Performance of Seven Consumer Sleep-Tracking Devices Compared with Polysomnography. Sleep 2021, 44, 291.
- Roomkham, S.; Hittle, M.; Cheung, J.; Lovell, D.; Mignot, E.; Perrin, D. Sleep Monitoring with the Apple Watch: Comparison to a Clinically Validated Actigraph . F1000Research 2019, 8, 754.
- Kholghi, M.; Szollosi, I.; Hollamby, M.; Bradford, D.; Zhang, Q. A Validation Study of a Ballistocardiograph Sleep Tracker EMFIT QS against Polysomnography. J. Clin. Sleep Med. 2021; online ahead of print.
- Bhat, S.; Ferraris, A.; Gupta, D.; Mozafarian, M.; De Bari, V.A.; Gushway-Henry, N.; Gowda, S.P.; Polos, P.G.; Rubinstein, M.; Seidu, H.; et al. Is There a Clinical Role for Smartphone Sleep Apps? Comparison of Sleep Cycle Detection by a Smartphone Application to Polysomnography. J. Clin. Sleep Med. 2015, 11, 709–715.
- Wiens, A.D.; Carek, A.M.; Inan, O.T. Sternal Vibrations during Head-out Immersion: A Preliminary Demonstration of Underwater Wearable Ballistocardiography. J. Acoust. Soc. Am. 2015, 138, 342–346.
- Carlson, C.; Turpin, V.R.; Suliman, A.; Ade, C.; Warren, S.; Thompson, D.E. Bed-Based Ballistocardiography: Dataset and Ability to Track Cardiovascular Parameters. Sensors 2021, 21, 156.
- Cappuccio, F.P.; Stranges, S.; Kandala, N.B.; Miller, M.A.; Taggart, F.M.; Kumari, M.; Ferrie, J.E.; Shipley, M.J.; Brunner, E.J.; Marmot, M.G. Gender-Specific Associations of Short Sleep Duration with Prevalent and Incident Hypertension: The Whitehall II Study. Hypertension 2007, 50, 693–700.
- Sadek, I.; Abdulrazak, B.; Mokhtari, M. Evaluating an IoT Under-Mattress Sensor Mat for Detecting Anomalies in Sleep Parameters: A Pilot Study. In Proceedings of the 2021 IEEE Canadian Conference on Electrical and Computer Engineering (CCECE), Ottawa, ON, Canada, 12–17 September 2021; pp. 1–5.
- Sadek, I.; Abdulrazak, B. A Comparison of Three Heart Rate Detection Algorithms over Ballistocardiogram Signals. Biomed. Signal Process. Control 2021, 70, 103017.
- Ye, B.; Khan, S.S.; Chikhaoui, B.; Iaboni, A.; Martin, L.S.; Newman, K.; Wang, A.; Mihailidis, A. Challenges in Collecting Big Data in A Clinical Environment with Vulnerable Population: Lessons Learned from A Study Using A Multi-Modal Sensors Platform. Sci. Eng. Ethics 2019, 25, 1447–1466.




