Grain size is a quantitative trait that is controlled by multiple genes. It is not only a yield trait, but also an important appearance quality of rice. In addition, grain size is easy to be selected in evolution, which is also a significant trait for studying rice evolution. In recent years, many quantitative trait loci (QTL)/genes for rice grain size were isolated by map-based cloning or genome-wide association studies, which revealed the genetic and molecular mechanism of grain size regulation in part.
1. Introduction
Rice, one of the most important staple foods in the world, is a model plant for knowing plant functional genetics. However, it is still urgent to improve rice grain yield with the continuous increase in the world’s population, the deterioration in the environment, and the decrease in the area of arable land. Rice grain yield is composed of three major factors, including effective panicles per plant, grain number per panicle and 1000-grain weight. The 1000-grain weight is affected by grain shape, grain size and the filling of kernels [1]. Grain size, as specified by its three-dimensional structure of seeds (length, width and thickness), is a key determinant of both yield and appearance quality in rice [2]. In recent years, many quantitative trait loci (QTL)/genes for grain size were isolated in rice by map-based cloning and genome-wide association study (GWAS) and revealed the genetic and molecular mechanisms of grain size regulation in part [3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24]. These QTL/genes were involved in multiple signaling pathways, including G protein signaling, the mitogen-activated protein kinase (MAPK) signaling pathway, the ubiquitin–proteasome pathway, phytohormone signaling, transcriptional regulatory factors and so on. [25][26]. At present, grain size has become an important target trait for the high-yield and high-quality molecular design breeding of rice. Therefore, summarizing the QTL/genes and analyzing the molecular regulatory pathways of grain size can provide an important theoretical foundation for rice breeding.
2. Research Progress on the QTL/Genes of Grain Size in Rice
Rice grain size is largely determined by a combination of spikelet hulls, the degree of filling and the development of endosperm. It is a quantitative trait, controlled by multiple genes and genetic systems
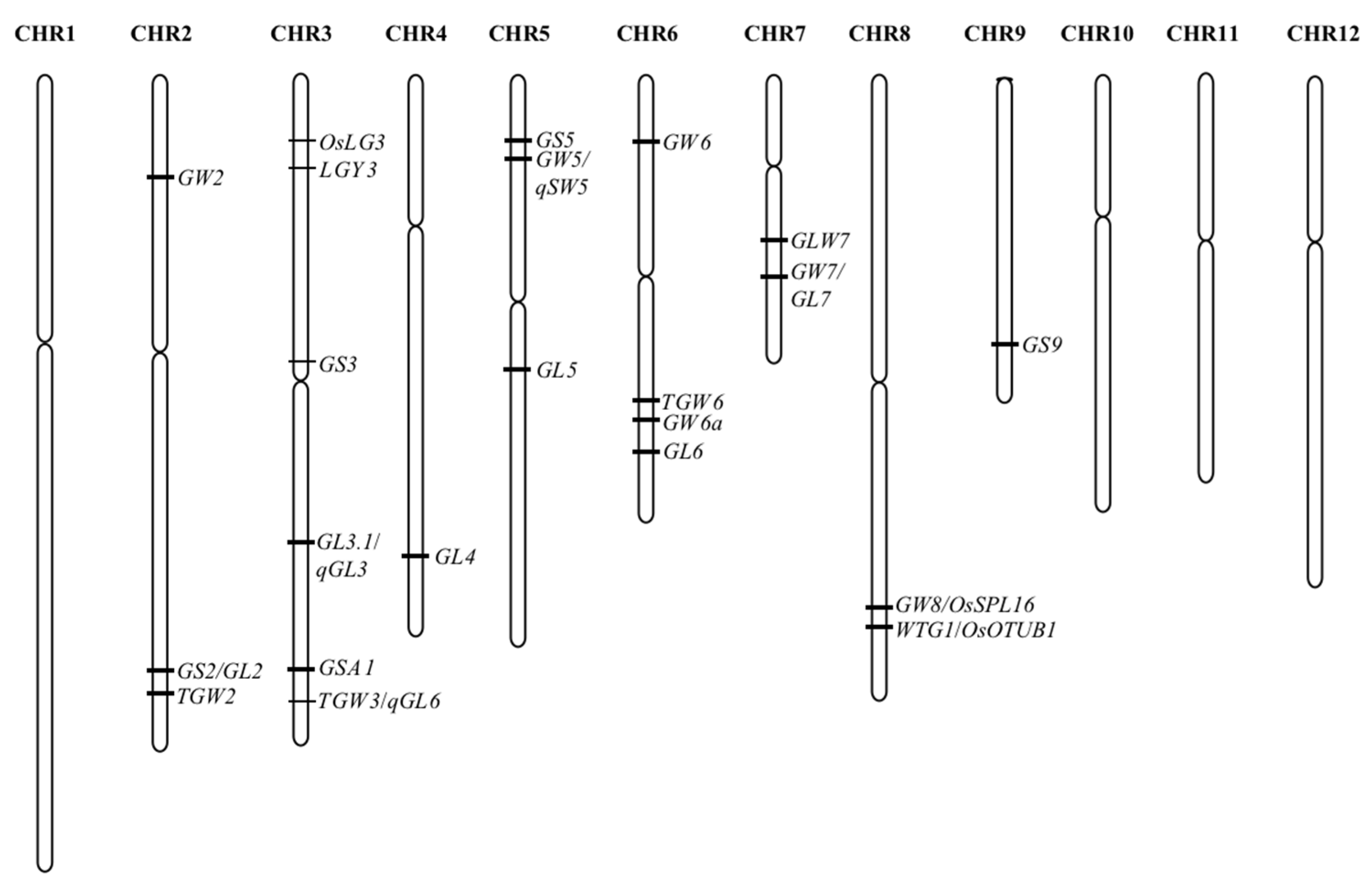
[3]. Therefore, analyzing the QTL and regulatory network is an important method to know grain size in rice. It was addressed only QTL affecting hull size. With the development of rice genome sequencing and functional genomics, more and more functional genes have been gradually resolved. Coupled with cost reduction in the sequencing technologies, a large amount of rice materials have been re-sequenced, and in combination with MutMap and GWAS, the cloning of functional genes related to rice grain size has accelerated, and nearly 200 genes with a direct or indirect role have been cloned. On the other hand, some of the genes were cloned from mutant material with extreme phenotype variation, and it is difficult to meet the actual production demands. Up to now, there are at least 22 grain size-related QTL that have been isolated from natural variation (
Figure 1,
Table 1). Among these QTLs, most concern grain length and width.
Table 1. Identified QTL for grain size in rice.
| QTL |
Gene ID |
Main Regulatory Traits |
Signalling Pathways |
References |
| GW2 |
Os02g0244100 |
Width |
Ubiquitin–proteasome pathway |
[3] |
| GS2 |
Os02g0701300 |
Size |
Phytohormone signaling and transcriptional regulatory factor |
[12] |
| TGW2 |
Os02g0763000 |
Width |
Unclear |
[23] |
| OsLG3 |
Os03g0183000 |
Length |
Phytohormone signaling and transcriptional regulatory factor |
[27] |
| LGY3 |
Os03g0215400 |
Size |
Transcriptional regulatory factor |
[28] |
| GS3 |
Os03g0407400 |
Width |
G protein signaling |
[4] |
| qGL3 |
Os03g0646900 |
Size |
Phytohormone signaling |
[6][8][29] |
| GSA1 |
Os03g0757500 |
Size |
Phytohormone signaling |
[30] |
| qTGW3 |
Os03g0841800 |
Size |
Phytohormone signaling |
[18] |
| GL4 |
ORGLA04G0254300 |
Length |
Transcriptional regulatory factor |
[31] |
| GS5 |
Os05g0158500 |
Size |
Phytohormone signaling |
[5] |
| GW5 |
Os05g0187500 |
Width |
Phytohormone signaling and ubiquitin–proteasome pathway |
[17][26] |
| GL5 |
Os05g0447200 |
Length |
Phytohormone signaling |
[24] |
| GW6 |
Os06g0266800 |
Width and length |
Phytohormone signaling |
[32] |
| TGW6 |
Os06g0623700 |
Length |
Phytohormone signaling |
[9] |
| GW6a |
Os06g0650300 |
Length |
transcriptional regulatory factor |
[13] |
| GL6 |
Os06g0666100 |
Length |
unclear |
[33] |
| GLW7 |
Os07g0505200 |
Length |
transcriptional regulatory factor |
[16] |
| GW7/GL7 |
Os07g0603300 |
Width |
transcriptional regulatory factor |
[14] |
| GW8 |
Os08g0531600 |
Width |
transcriptional regulatory factor |
[14] |
| WTG1 |
Os08g0537800 |
Width and thickness |
Ubiquitin–proteasome pathway |
[34][35] |
| GS9 |
Os09g0448500 |
Length |
transcriptional regulatory factor |
[21] |
Figure 1. The QTLs isolated for grain size in rice.
2.1. QTLs Mainly Associated with Grain Length
Grain length refers to the distance from the base of the lowest part of the grain to the longest part. To date, many QTLs related to grain length have been cloned in rice, including GS3, qGL3, GL4, TGW6, GL7 and so on.
GS3 is the first major QTL identified in rice which regulates grain length and grain weight
[36]. This gene encodes a transmembrane protein consisting of five exons. A nonsense mutation in the second exon in all large-grain cultivars resulted in a deletion of 178 amino acids from the C-terminus of the GS3 protein and was accompanied by the early termination of protein translation, indicating that
GS3 negatively regulates grain length and weight
[4][36].
qGL3/GL3.1 encodes a serine/threonine phosphatase belonging to the PPKL family of protein phosphatases which can control rice grain length and yield by directly dephosphorylating substrates to regulate cell cycle proteins
[6][8][29].
GL4 controls the grain length on chromosome 4 in African rice (
Oryza glaberrima Steud.), which regulates longitudinal cell elongation of the outer and inner glumes. Interestingly,
GL4 also controls the seed shattering phenotype like its orthologue
SH4 gene in Asian rice
[31].
TGW6 encodes a novel protein with indole-3-acetic acid (IAA)-glucose hydrolase activity. In sink organs, the Nipponbare
tgw6 allele affects the timing of the transition from the syncytial to the cellular phase by controlling the IAA supply and limiting cell number and grain length. Most notably, loss of function of the Kasalath allele enhances grain weight through pleiotropic effects on source organs, and this leads to significant yield increases
[9].
GL7 is a major QTL that controls rice grain length. It is located on chromosome 7 and encodes a protein homologous to the Arabidopsis LONGFOLIA protein. It can up-regulate the expression level of
GL7 and simultaneously down-regulate the expression level of its neighboring negative factors to make the cells elongated longitudinally, thereby increasing the grain length of rice seeds and changing their appearance quality
[14].
GS5 encodes a serine carboxypeptidase that positively regulates rice grain size
[5]. Two key single nucleotide polymorphisms (SNPs) in the
GS5 promoter region cause its differential expression in young spikelets, which determines the differences in grain size. Enhanced expression of
GS5 competitively inhibits the interaction between OsBAK1-7 and OsMSBP1 by occupying the extracellular leucine-rich repeat (LRR) domain of OsBAK1-7, thus preventing OsBAK1-7 from endocytosis caused by interacting with OsMSBP1
[37].
GLW7 encodes an
OsSPL13 protein, which makes the grains larger mainly by increasing cell size. Further population genetic analysis revealed that during the genetic improvement of rice, the large grain gene
GLW7 penetrated from indica to tropical japonica and to a lesser extent temperate japonica through genetic drift, thereby improving the 1000- grain weight and yield of japonica rice
[16].
GS9 encodes a protein without a known conserved functional domain. It regulates grain shape by altering cell division. The
gs9 null mutant has slender grains, while overexpression of
GS9 results in round grains
[21].
The rice grain yields QTL
qLGY3, which encodes the transcription factor OsMADS1 and contains the MADS domain, and is a key effector in the downstream of the G protein βγ dimer. The variable splicing protein OsMADS1
lgy3 produces longer grains and improves the quality and yield of rice grains
[28].
OsLG3 is a member of ERF family transcription factor, and it can positively regulate rice grain length with no effect on grain quality
[27].
Loss of function at the QTL
qGL5 (
OsAUX3) could lead to more significant grain length and weight. Research showed that transcription factor
OsARF6 binds directly to the auxin response elements of the
OsAUX3 promoter and controls grain length by altering longitudinal expansion and auxin distribution/content in glume cells. Moreover,
miR167a positively regulates grain length and weight by directing
OsARF6 mRNA silencing. These results indicated that a novel
miR167a-
OsARF6-
OsAUX3 module regulates grain length and weight, providing a potential target for the improvement of rice yield
[24].
2.2. QTLs Mainly Associated with Grain Width
Grain width refers to the distance between the widest parts on both sides of the inner and outer glume, which is one of the main grain shape traits of rice. Cloning the QTLs related to its growth and development is particularly important for knowing rice yield and appearance quality. Currently, there are not many cloned QTLs for grain width in rice, mainly GW2, GW5, GW7, GW8, TGW2 and so on.
GW2 is the first grain width QTL cloned in rice. It is located on the short arm of chromosome 2 and encodes a ubiquitin ligase that negatively regulates cell division by anchoring substrates to the proteasome for degradation
[3]. The mutation of this gene could not recognize the substrate which should be degraded, thus it activated the division of the glume shell cells and increased the width of the glume shell. This indirectly increased the filling rate and consequently expanded the size of the endosperm, and ultimately increased glume shell width, grain weight and yield
[3].
GW5 encodes a calmodulin binding protein
[11], as studies have shown that
GW5 can interact with polyubiquitin, suggesting that it may regulate grain width and weight via the ubiquitin proteasome pathway
[38].
OsSPL16/GW8 is a transcription factor containing an SBP structural domain, which is able to bind directly to the
GW7 promoter and repress its expression, and then regulate rice grain width
[14].
GW6 encodes a GA-regulated GAST family protein and positively regulates grain width and weight. It is highly expressed in the young panicle and increases grain width by promoting cell expansion in the spikelet hull. Knockout of
GW6 exhibits reduced grain size and weight, whereas overexpression of
GW6 results in increased grain size and weight
[32].
TGW2 encodes the cell number regulator 1(OsCNR1). The TGW2 protein interacts with the KRP1 protein, which regulates cell cycle and affects cell proliferation and expansion in glumes, and negatively regulates grain width and grain weight in rice
[23].
2.3. QTLs Mainly Associated with Thickness
Grain thickness refers to the distance between the thickest parts on both sides of the inner and outer glume. Few studies have been reported on rice grain thickness. Most researchers agree that thickness is a quantitative trait which is also regulated by multiple genes.
WTG1 encodes a deubiquitinating enzyme with homology to human OTUB1 and is a functional deubiquitinating enzyme
[34]. The
wtg1-1 mutant exhibits wide, thick, short and heavy grains and also shows an increased number of grains per panicle. Corresponding, overexpression of
WTG1 results in narrow, thin, long grains
[35].