
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maximilian Lübke | + 7687 word(s) | 7687 | 2022-02-09 08:38:07 | | | |
| 2 | Angelika S. Thalmayer | Meta information modification | 7687 | 2022-03-27 22:14:13 | | | | |
| 3 | Nora Tang | -73 word(s) | 7614 | 2022-03-28 05:09:17 | | |
Video Upload Options
Diabetes is a chronic and, according to the state of the art, an incurable disease. Therefore, to treat diabetes, regular blood glucose monitoring is crucial since it is mandatory to mitigate the risk and incidence of hyperglycemia and hypoglycemia. Nowadays, it is common to use blood glucose meters or continuous glucose monitoring via stinging the skin, which is classified as invasive monitoring. In recent decades, non-invasive monitoring has been regarded as a dominant research field. Overall, the scientific approaches show a comparable accuracy in the Clarke error grid to that of the commercial ones. However, they are in different stages of development and, therefore, need improvement regarding parameter optimization, temperature dependency, or testing with blood under real conditions. Moreover, the size of scientific sensing solutions must be further reduced for a wearable monitoring system.
1. Introduction
Diabetes mellitus (DM) is a chronic metabolic disease, which is caused by the lack or ineffective use of the insulin produced by the body [1]. According to the report released in December 2020 by the World Health Organisation (WHO), diabetes is among the top 10 causes of death [2]. Overall, patients suffering from diabetes can be categorized into three groups: Type 1 Diabetes Mellitus (T1D), where the body produces no or too little insulin; Type 2 Diabetes Mellitus (T2D), caused by insulin resistance and Gestational Diabetes Mellitus (TGD) during pregnancy [3].
The normal range of fasting blood glucose is between 70 mg/dL and 100 mg/dL [4][5]. Blood glucose levels (BGL) below 70 mg/dL are called hypoglycemia, whereas levels above 120 mg/dL or 140 mg/dL when fasting or two hours after eating, respectively, or in general a value of >180 mg/dL, corresponding to hyperglycemia [6][7]. In case the glucose level differs from the normal range, it can cause an adverse influence on the heart, the blood vessels, the eyes, the kidneys, and the nerves [1] as well as circulatory system problems. Those long-term complications of hyperglycemia can be categorized into macrovascular diabetic complications, such as heart diseases, and microvascular diabetic complications, which cause diseases in organs, such as nephropathy, retinopathy, and neuropathy [8].
In addition, short-term complications of hypoglycemia may lead to coma or death, in the worst cases [9][10][11]. Nevertheless, the complication incidence has declined since the 1990s, benefitting from the better recognition and management of blood glucose levels [12].
Consequently, affected patients must check their blood glucose levels regularly. In 2000, systems for continuous blood glucose monitoring (CGM) became commercially available [13]. These systems automatically measure the blood glucose level and its trend in short time intervals and are, thus, excellent candidates to make the life of a patient suffering from diabetes more comfortable and safe.
From this background, scientists are driven to conduct further research in the field of continuous glucose sensing. For example, in 2014, the biotech-company Verily of Google tried to use a 2-layer smart contact lens combined with a radio chip to monitor blood glucose (BG) variation [14]. However, the results revealed that it is difficult to get a reliable mapping between glucose levels in the blood and the tear fluid [15]. In addition, the Robert Bosch company has owned some patents for the BG sensor, which is a sensor that is implantable in the earlobe [15] .
Various approaches regarding blood glucose sensing have been proposed so far, which can be divided into invasive, minimally invasive, and non-invasive as follows:
- Invasive monitoring: The traditional monitoring method of BG is via pricking the fingertip and then putting the obtained drop of blood on the test stripe multiple times per day [15]. This way is called invasive monitoring measurement. Although such monitoring helps patients greatly with BG management and is highly sensitive and correct, it still brings pain, infection risk, and even damages to the skin tissue over a long time [16]. Moreover, the finger pricking method falls short when it comes to CGM since it is conducted every couple of hours by the affected patient rather than in short time intervals over the length of the day [15].
- Minimal invasive monitoring: Minimal invasive glucose sensing is via microneedles inserted into the skin where the interstitial fluid is located [17]. A probe of this liquid is then chemically evaluated to determine the glucose level. The well-established commercial available glucose sensing systems of Dexcom [18] and FreeStyle Libre [19] are based on this method. Compared to the traditional finger pricking, the determination of the BGL with the FreeStyle Libre or Dexcom sensors is less painful for the patient and yields the significant advantage of enabling CGM. However, the costs for this system are relatively high, since the sensor has to be replaced at last every 10 or 14 days.
- Non-invasive (NI) monitoring: NI blood glucose monitoring aims to produce neither pain nor discomfort during the glucose measurement [20]. These approaches can be classified according to the applied glucose-sensing method. The primary sensing methods for NI methods are the electrochemical [20][21] and the electromagnetic-based methods [22][23][24]. In electrochemical NI glucose sensors, a probe of saliva [25], teardrop [26], or exhaled breath [27] is analyzed. The electromagnetic methods are based on the interaction of {electromagnetic} waves with the human body. The applied wavelengths vary from the m-range (impedance spectroscopy) to the mm-range (microwaves) up to the nm-range (optical frequencies)---compare Figure 1.
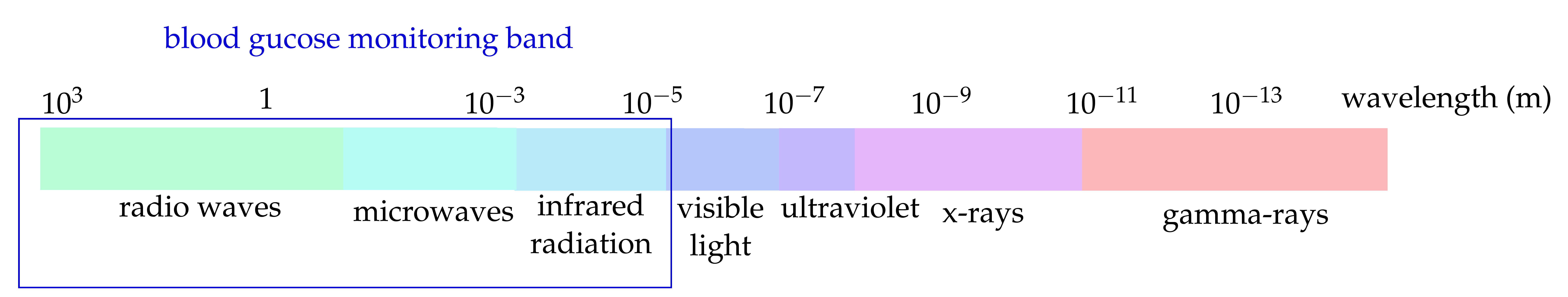
Figure 1. Electromagnetic spectrum.
Subsequently, post-processing is required to find the relation between the measured signal (usually current, voltage, or phase/frequency) and the BG concentration. Indeed, the relation between the measured signal and the blood glucose level (BGL) is often determined by a simple proportionality. However, a calibration step is required to extract the BGL precisely. Nevertheless, since data loss is often a problem, interpolation and extrapolation are also conducted on the data [28]. Furthermore, the rapid development of artificial intelligence (AI) involving machine learning, deep learning, and cognitive computing is promising for more accurate and reliable data processing since AI is able to interpret and process high amounts of data [29], and more or less instantaneously, it can suggest a proper recommended course of action to the patient. In sum, this enables further improvements in screening, diagnosis, and management of the patients’ diabetes [30]. Methods like a hybrid least-squares random sample consensus (LS-RANSAC) [28] or a Principal Component Analysis (PCA) algorithm [31] further enhance the detection sensitivity of the measured sensor data significantly [31].
However, the main focus on using AI in the post-processing of BGL data lies in predicting glucose trends [32]. Thereby, the prediction horizon of the BGL is up to 120 min [33], is based on data-based and hybrid models (e.g., Gaussian process and random forest [34]). Various features such as BGL, insulin, meal, exercise, sleep, and others can be observed individually and combined with each other to improve prediction accuracy [33]. Additionally, AI-based approaches are also investigated for predicting the risk of secondary diseases [35][36]. However, this is out of the scope of the proposed paper, and detailed information can be found in the review papers [32][33][37][38].
2. Non-Invasive Sensor Principles
This focuses on the non-invasive (NI) sensing of glucose. Consequently, in the following, the main principles of state-of-the-art NI glucose sensing are explored.
2.1. Electrochemical NI Sensors
As mentioned in Section 1, NI monitoring can be approached via a medium such as saliva, teardrops, or exhaled breath [39]. This is because these body liquids are easily accessible and collected [20]. Principally, detection of the glucose concentration is also possible via urine, but this is not suitable for a CGM-system [40][41]. Therefore, utilizing saliva, tears and exhaled breath is preferred [46]. In general, such kinds of sensors are called biosensors [21][32].
2.1.1. Saliva Analysis
Saliva contains lots of biological information that reflect the physiology and health status [42]. Thus far it has been widely used in human immunodeficiency virus (HIV) infection diagnosis and drug abuse [43][44][45]. That means saliva indicates physiological functions of the body and can be regarded as an alternative to blood [46].
2.1.2. Ocular Fluid: Tear
Tears also carry information and show similar glucose concentration to blood [26][47]. Tears contain organic molecules, which reflect the health status [42]. Compared to saliva, tear glucose concentration is relatively stable in the range of 0.9–90 mg/dL (0.05–5 mM), while blood glucose concentration is in the range of 90–140 mg/dL [48][49]. Therefore, tears have attracted much attraction for decades [50]. In addition, the worldwide use of contact lenses is a strong motivation for tear glucose measurement [51]. In 2014, Verily, which is regarded as the Google Life Sciences, launched the smart contact lens project. Their challenge was to get reliable tear glucose readings [14]. Even though their development was discontinued because the correlation between blood glucose and tear was too weak [14], it is still encouraging to have further innovation based on tear glucose concentration [52].
2.1.3. Exhaled Breath Analysis
Despite the medium, such as saliva and tear, exhaled breath is another attractive biomarker of diabetes. The relation between diseases and the smell of exhaled breath is well established [27][53]. For instance: the ‘fruity smell’ of acetone in the breath can be regarded as an indicator of diabetes, whereas ‘musty and fishy smell’ can be considered as a hint of advanced liver disease [54]. Therefore, exhaled breath analysis provides potential deep insights on physiological and pathophysiological conditions in terms of related diseases [55]. Similar to the other two media, the exhaled breath is, in general, easy to access and collect. In addition, it is safer, friendlier, and more acceptable for patients compared to the saliva and tears analysis [27][56]. Examples of biomarkers in human breath are acetone, isopropanol (IPA), carbon monoxide, isoprene, and ethanol [27]. The content of acetone is quite large, which can be found both in T1DM and T2DM diabetics, and comes from the increase of acetyl-CoA level in the liver because of the lipolysis [57].
Nevertheless, the correlation between blood glucose level and the detected acetone is controversial as discussed in the literature [58]: positive [59] negative [60][61], some arguing no correlation [62][63][64]. The core problem of BGL-detection based on exhaled breath analysis is that the acetone level is influenced by many factors such as insulin injection, type of diabetes, alcohol intake, exercise, food and beverage intake, etc. [57]. As acetone is produced during fat metabolism, it is also used as a diet marker [65]. Companies, like BIOSENSE, LEVL, Ketonix, Keyto, House of Keto Monitor, and KHC M3, have released their breath acetone meters. However, only BIOSENSE, LEVL, and Ketonix have received the FDA-approval for diet management and diabetes diagnosis [66]. In the context of diabetes care, however, exhaled breath analysis is currently mainly investigated regarding diabetes diagnosis and not as a CGM sensing system [67][68][69].
2.1.4. Summary
Nowadays research on glucose monitoring based on saliva, tears, and exhaled breath is still a hot topic. One of the dominant reasons is that these fluids are easily accessible and can be markers for blood glucose concentration. However, these three MUTs face the same problem: containing various proteins (saliva and tears) or breath biomarkers (exhaled breath), which raises the challenge of having accurate monitoring. This also indicates that in future studies more interference rejection parts are needed for better monitoring. Moreover, the lag time between blood and tear glucose, and exhaled breath is different. The lag time of blood and tear glucose is about 15 min, whereas the lag time of exhaled breath depends on the type of the sensor [70]. Therefore, reducing the lag time during the secondary fluid glucose monitoring is another important research point as well [71][72][73]. Furthermore, since saliva and tears are human parts, the materials also need to be bio-materials, which brings the issue of allergies, such as skin irritation and rejection reaction [74].
2.2. Electromagnetic Non-Invasive Monitoring
Optical techniques utilize the reflection, absorption, and scattering properties of waves. Well-known methods are for example Raman spectroscopy, optical polarimetry (OP), or optical coherence tomography (OCT) [71]. The millimeter and microwave sensing and bioimpedance spectroscopy utilize the dielectric properties of glucose [24][71]. Both techniques are applied mostly over the skin. However, the tissue surface is rough, which is one main factor leading to scattering and energy loss. Such characteristics of tissues lead to another vital point, the so-called penetration depth. If the penetration depth is not high enough, it is hardly possible to reach the vessels, i.e., arteries in the body for sensing the glucose change. In consequence, the monitoring accuracy will be reduced [71].
2.2.1. Raman Spectroscopy
It is a vibrational spectroscopic technique based on Raman scattering. Energy exchange between light and matter leads to the equivalence relation between the frequency change of the scattered light and the vibrational frequency of the scattering molecules. In the other words, the molecules absorb the energy of a photon, transit from the ground state to the excited state, and emit a Raman scattered photon. The rotational and vibrational states among molecules are the dominant factors in Raman spectroscopy, resulting in the so-called Raman peak in the spectrum. Another important feature is the Raman shift (with the unit cm-1), which is the difference between the initial and vibrational wavelengths and is caused by the vibrational frequency of the scattering molecules. It characterizes the intensity of scattered light and reflects the chemical structure of scattering molecules. A basic Raman spectroscopy system consists of 4 parts, namely a monochromatic light source, a lens, a filter, and a detector connected to the computer. The reason why Raman spectroscopy is preferred is that it has high sensitivity to detect tiny changes with a molecular size of 1 μm [75][76]. The general advantages of Raman spectroscopy are higher depth penetration compared to mid-infrared spectroscopy, being less sensitive to temperature changes compared to OCT, wide application, and high specificity [71].
2.2.2. Impedance Spectroscopy-Based Monitoring
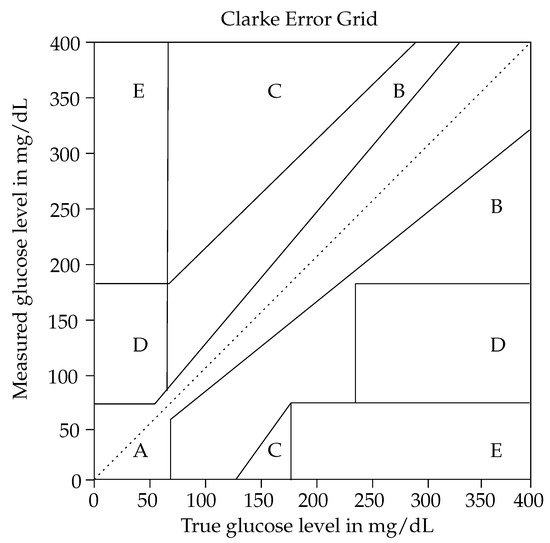
For more than 15 years, impedance spectroscopy or also dielectric spectroscopy has been under research for non-invasive glucose sensing [77]. The research of numerous scientists resulted in a CE approval for such an impedance spectroscopy-based sensor called Pendra in 2003. However, post-marketing studies on six type 1 diabetes patients revealed that 4.3% of the Pendra readings were in the dangerous Zone E of the Clarke error grid. Consequently, Pendra was removed from the market shortly after its CE approval [78]. Moreover, GlucoBand is another impedance spectroscopy-based glucose monitoring system with a similar fate as Pendra and was never released in the commercial market.
In impedance spectroscopy, the impedance Z of human tissue is measured by passing alternating current signals across the skin in the frequency spectrum of 100 Hz to 200 MHz [77][79][80]. The specific reaction of blood and tissue cells to a change in glucose level results in a change in the electrolyte balance across the membranes of blood and underlying tissue. Therefore, the electric conductivity σ, and thus, Z of tissue is sensitive to the glucose level [77]. However, non-invasive glucose sensing with impedance spectroscopy is challenging due to distortions imposed by the movement of the electrodes on the skin surface [81], sweat, and temperature fluctuations as well as skin thickness or moisture variations [80]. Therefore, researchers proposed to combine impedance spectroscopy sensors with multiple sensors to increase the overall accuracy and stability of non-invasive glucose sensing. In [79], a wearable system comprising impedance spectroscopy, temperature, humidity as well as optical sensors. Moreover, in [81], a similar multisensor wearable system was proposed. The system consisted of dielectric, optical, temperature, humidity sensors, and an accelerometer. Both approaches fused several physiological parameters to increase the overall sensor accuracy.
2.2.3. Microwave-Based Monitoring
Reviewing microwave-based noninvasive glucose sensors is one of the main goals of the proposed work since it is expected to have great potential. This is because of its promising characteristics: sufficient penetration depth in human tissue, high sensitivity to subtle variation of glucose concentration, and easy and low-cost fabrication as well as for safety reasons [71][82][83]. In general, the technology can be classified into three parts according to their properties, namely, reflection, transmission, and resonant perturbation [71]. The reflection-based technique is a one-port one, which evaluates the reflection parameter S11, as there is a dependency relation between the intensity and phase variation of the signal and permittivity variation in the blood glucose level [71]. The transmission-based technique on the other hand is a two-port technique, which utilizes the transmission coefficients S21 and reflection coefficients S11, whereas the resonant perturbation-based technique uses the Q-factor [48][71].
3. Post-Processing
There are several methods to perform the data processing of glucose data, such as temporal abstractions, time series analysis, and a combination of symbolic and numerical methods [84]. As the loss of data occurs quite often, interpolation and extrapolation are applied for more reliability. The processed data are then fitted into the regression equations. Such problems will be amplified, especially for individual glucose management. The problem, however, can be dealt with by applying machine learning, which has drawn a great deal of attention for its advantageous data processing performance in optimization [85]. With CGM, the data recording is nowadays straightforward, and on the other hand, a huge amount of data are available. This large dataset is particularly advantageous for the machine learning method to improve the data analysis for better accuracy [28][31][86]. Notably, for the machine learning method, a large training set is needed, which means large biomedical data have to be available. However, the biomedical data are usually complex and disordered [87]. Thus, the pre-processing of the data is mandatory. The performance of machine learning depends not only on the algorithm itself but also on the similarity of the training set and the test set, which means the right choice of the dataset and the test set is essential.
Moreover, machine learning can even enhance the diagnosis as well as the therapy of diabetes by improving the prediction of BGL trends, thus reducing hyperglycemia and hypoglycemia [32][33][37][38]. Furthermore, the risk of getting secondary diseases can be estimated [35][36]. All in all, as machine learning has such a high potential for the postprocessing of BG monitoring, the chosen processing systems using machine learning methods for improving accuracy as well as predicting BGL trends are introduced.
As can be seen, post-processing using AI offers great opportunities in both, increasing the accuracy of measurement data and predicting the trends of blood glucose levels. The latter supports diabetic persons significantly since it helps to avoid hyperglycemia and hypoglycemia. A comparison of different approaches with a prediction horizon of 30 min is given in the discussion summarized in Table 4. A more detailed table can be found in [33][38]. Further discussion regarding the potential, challenges, and concerns (used dataset, external validation) of AI in signal post-processing can be found in Section 5.2.
4. Commercial Devices and Systems
4.1. Commercial Devices
A commercial sensor must satisfy several criteria to get CE and Food and Drug Administration (FDA) approval, which implies satisfying the reliability, consistency, and safety criterion [71]. The corresponding accuracy for the EU, defined by the European Medicine Agency (EMA), and for the USA, defined by the FDA, are listed in Table 1. The FDA requires that 95% of all measurement values should be for BGL≥75mg/dL within the range of ±12% compared to the reference values and, additionally, for BGL<75mg/dL within ±12mg/dL. Furthermore, 98% of the measurement results should not exceed ±15% for BGL≥75mg/dL, and ±15mg/dL for BGL<75mg/dL, respectively [88].
| Reference | Agency | Country | Blood Glucose Level | Min. Accuracy |
| [89] | EMA | EU | ≥ 100 mg/dL | 95% ± 15% |
| [88] | FDA | USA | ≥ 75 mg/dL | 95% ± 12% 98% ± 15% |
| FreeStyle Libre 2 | FreeStyle Libre 3 | Dexcom G6 | |
| Release time | 2020 | 2021 | 2020 |
| Sensor type | CGM using flash glucose monitoring system |
CGM using CGM system |
CGM |
| Sensor principle | electrochemical | electrochemical | electrochemical |
| Regulatory status | CE Mark cleared by FDA |
CE Mark not cleared by FDA |
CE Mark cleared by FDA |
| Sensor size | 5 mm in height 35 mm in diameter |
2.9 mm in height 21 mm in diameter |
45.7 mm × 30.5 mm × 15.2 mm |
| Sensor weight | 5 g | 1 g | 12 g |
| BG measuring range | 40-500 mg/dL | 40-500 mg/dL | 40-400 mg/dL |
| Working period | 14 days | 14 days | 10 days |
| Calibration time | 60 min | 60 min | 120 min |
| Wearing position | back of the upper arm | back of the upper arm | belly (from the age of 2) back of the upper arm (from the age of 2) the upper buttocks (ages from 2 to 17) |
| User age | from the age of 4 | from the age of 4 | from the age of 2 |
| Data reading | mobile phone (FreeStyle LibreLink APP) separate reader |
mobile phone | mobile phone (Dexcom Follow App) |
4.2. Commercial System
5. Discussion
Diabetes is a chronic disease. More precisely speaking, in the case of T1D it is until now an incurable disease. The glucose level must be within a specific range to prevent further damage to a patient by avoiding hyperglycemia and hypoglycemia. Therefore, a glucose monitoring system should continuously track the glucose level with high accuracy (<20%). Thus, research in glucose monitoring has attracted attention for years, from the early conventional diagnosis to intensified diagnosis, which nowadays, it is desired that it be non-invasive. Meanwhile, many commercial devices were in the market to provide a reliable glucose measurement. However, most are unsuccessful in receiving the FDA or CE approval or are discontinued afterward. At present, two commercial systems are dominant in such a field, namely, FreeStyle Libre and Dexcom. In recent years several generations have been released. The latest versions are FreeStlye Libre 3 and Dexcom G6. The advantages of these systems are that both provide continuous glucose measurement and work for at least ten days, which eases the burden of glucose management on both patients and medical specialists. Nevertheless, both commercial systems belong to minimal-invasive technology. Therefore, the risk of infection, pain for the patient and contact allergy, and the cost of sensor replacement are still existing disadvantages.
To satisfy the demand for less costly, more convenient, and more accurate glucose measurement and monitoring devices, much research is being conducted on non-invasive monitoring technology. An overview of exemplary state-of-the-art approaches is listed in Table 3. Furthermore, the opportunities and challenges of advanced post-processing are discussed, and finally, all proposed approaches are characterized and compared using the Clarke error grid.
| Reference | Evaluation Object |
Measuring Method |
Post- Processing |
Detection Range (mg/dL) |
Calibration/ Validation |
Accuracy/ Sensitivity |
Observation Time |
Sensor Size | Influence Factor/ Sensor Limitation/ Further Development |
Dataset |
| Sensor Systems: | ||||||||||
| [25] | real saliva in vivo |
electrochemical | — | 0–180 | Proof of Concept | — | testing: 20 min; monitoring more than 5 h |
25 mm × 5 mm × 0.5 mm | many proteins in the saliva |
1 person |
| [104] | aqueous solution with, glucose, urea, lactate in vitro |
Raman spectroscopy |
filtering, smoothing, least-square fit |
glucose: 18–1081 urea: 18–3604 lactate: 18–3604 |
area under Raman shift peaks |
R2 = 0.97 ≈ 4072 counts/mM |
360 s each meas. 3 × 36 × 360 s |
— | interference due to other blood comp., scattering light |
3 × 36 meas. |
| [105] | real blood with NaCl, water and glucose in vitro |
microwave | — | 0–40.000 (14–16 GHz) |
temperature control |
reflected signal: 0.08° and 3.2 mV (Δ10, 000 mg/dL) transmitted signal: 0.2° and 2 mV (Δ7500 mg/dL) |
— | decimeter range plus VNA |
temperature of the oscillator, sedimentation in the blood samples, water absorption |
50 × 4 meas. |
| [106] | glucose water solution simulation |
microwave | Debye model | 0–500 (19 GHz) |
— | phase of S11 of 2° per 10 mg/dL |
— | 20 × 11.8 × 0.4 mm | tapering, fabrication errors |
simulation |
| [107] | glucose solution in vitro |
microwave | lin. regression | 30–500 (1.5 GHz) |
lin. regression | 0.0049 dB/mg/dL | — | 0.3 × 0.25 mm | optimization for more realistic situation |
— |
| [108] | saline solutions in vitro |
microwave | regression averaging |
0–180 (1.45–1.55 GHz) |
regression | 21.7–23.4 dB/(mg/dL) |
— | diameter: 25 mm thickness: 0.76 mm |
optimization for mobility, data collecting time, data processing time |
10 × 7 meas. |
| [109] | glucose water solution in vitro |
microwave | lin. fitting | 25–300 (0.8, 3.2 GHz) |
lin. fitting, 2-port cal. |
1.38 MHz per mg/dL | — | centimetre range plus VNA |
temperature, geometrical parameters |
12 × 3 meas. |
| [110] | glucose water solution in vitro |
microwave | lin. fitting, averaging |
0–400 (2.26 GHz) |
VNA Cal. | 1.947 mdB per mgdL-1 μL |
1080 s (CGM) | ≈several centimetre plus VNA |
temperature, rel. humidity |
20 × 9 CGM meas. |
| [111] | real blood in vivo |
microwave | lin. interpolation | 89–262 (5.5, 8.5 GHz) |
Comparison with Accu. check and aqueous solution for cal. curve |
8.5 GHz: 0.04 per mg/dL 5.5 GHz: 0.06 per mg/dL |
— | 30 × 18 mm plus VNA |
temperature (skin, environ.), blood pressure, EMV, thickness of skin, pressure, sweat, pollution |
11 persons |
| Sensor Systems and Accuracy Improvement via Post-Processing: | ||||||||||
| [28] | glucose water solution in vitro |
microwave | INNHO, LS-RANSAC, BPNN |
20–500 (0.2–4 GHz) |
Cal.: SOLT Val.: k-fold cross-val. |
0.0045 dB/(mg/dL) RMSE of 5.52 mg/dL |
— | 80 × 30 × 6 mm | measurement uncertainty |
training: 255 × 25 testing: 255 × 5 |
| [31] | aqueous glucose water (in vitro) fingertip (in vivo) |
microwave | PCA classification | 40–140 (2.45 GHz) |
VNA calibrated, internal validation |
0.45–0.9 (dispersed) 0.63–1.25 (compact) each per MHz |
1 h each 10 min | 5.55 × 3 cm | temperature, geometrical parameters |
600 samples (in vitro) 1 healthy P. (in vivo) |
| Sensor Systems and Prediction of Blood Glucose Trends: | ||||||||||
| [112] | pig ears in vivo |
Raman spectroscopy |
Prediction MLR, PLSR |
52–914 | Lin. Regression for calibration, cross-4-fold validation |
MARD: 6.6% R = 0.96 (250–500 mg/dL) R = 0.98 (>500 mg/dL) |
3 × 7 h each 5 min | portable Raman spectrometer fibre bundle: 2 mm diameter |
temperature, heart rate, skin movement, sweat, effective sampling volume |
3 female Yorkshire pig |
| [113] | nail fold in vivo |
Raman spectroscopy |
Prediction PCA, BPNN |
105–216 | Cal. with 2 reference points |
R2 = 0.98 RMSE = 5 mg/dL |
12 × 10 × 2.5 h each 5 min 6 meas. |
Renishaw inVia confocal Raman spectrometer |
temperature dirt, sweat |
12 healthy persons |
| [79] | in vivo | impedance spectroscopy and multiple sensors |
time series analysis sensor fusion |
0–200 (1–150 kHz) (10–60 MHz) |
Comparison with Accu-check and calibration |
average correlation factor = 0.8314 NRMSE = 14.6064 |
3 × 72 h (diabetic, CGM) healthy: during lunch |
flexible wrist band ≈ several cm |
movement artifacts sweat |
6 healthy, 3 diabetic persons |
| [114] | real blood in vivo |
microwave | Prediction linear regression |
60–400 (1.3 GHz) |
Pre-processing Accu check as reference |
MARD: 22.98% (without sub-band) 4.204% (with sub-band) |
— | 6.8 × 4.8 cm | object movement, temperature, pressure, humidity |
75 non-diabetic 50 pre-diabetic 125 diabetic persons |
Table 3: Review and comparison of different sensor systems with and without AI-based post-processing.
5.1. Non-Invasive Sensor Principles
For electrochemical-based measuring of the glucose level, different biological mediums such as saliva, tears, exhaled breath, and blood are regarded as the MUT. However, many distortion factors (e.g., foot or beverage intake) exist in saliva, tear, and exhaled breath, as the molecules inside are various and complex. Therefore, an additional layer was proposed as a standard solution to such a problem, although the function is different. The authors of [25], added a membrane to filter the molecules inside the saliva to increase the glucose measurement accuracy, based on the design in [115]. Similarly, an additional layer with different supporting polymers was proposed in [116] or for condensation of VOCs in [67]. The difference is that such a layer is not for filtering but for sensing. Especially, sensing using exhaled breath is regarded as controversial in the discussion regarding the correlation between BGL and measured acetone because of the significant influence of various factors. In consequence, exhaled breath is currently commercially investigated for diet management or diabetes diagnosis and not as a CGM sensing system [67][68][69]. However, for example, GLUCAIR™ is currently working on a commercial solution for non-invasive blood glucose monitoring using exhaled breath [39]. In general, secondary liquids, such as saliva, urine, teardrops, or interstitial fluid, face the problem of latency compared to glucose variations in blood. For example, the duration of the glucose observation in [25] and [116] is about 20 min. The requirement for CGM is, however, a short latency between the measured and real glucose value. Additionally, the biosensor material must be waterproof and manufactured to be biocompatible, which is crucial for avoiding skin irritation or rejections but increases the costs of a biosensor.
Blood is more commonly used than saliva and tears as MUT for electromagnetic-based glucose sensors. In [112][117][118] Raman Spectroscopy was applied. A linear relation was observed between the signal intensities and the glucose concentration difference in [112]. The minimal detectable change was reported to be 29–78 mg/dL, which is not suitable for real application. Moreover, their relatively bulky structure makes it difficult to integrate into a wearable glucose sensing system.
The impedance spectroscopy-based glucose sensors with operating frequencies in the kilohertz to megahertz range have been extensively explored by researchers over the past years [77][79][80][81]. This effort resulted in a shortly commercially available impedance spectroscopy-based glucose monitoring system called Pendra. However, post-market studies revealed that the accuracy partially fell in the dangerous Zone C of the Clarke error grid, and thus, it was removed from the market. The impedance of human tissue is affected by several factors such as temperature, sweat, skin thickness, and moisture, which vary over the day and also from patient to patient. Moreover, since electrodes are placed on the skin, relative movement between the skin and the electrodes may change the measured impedance as well as have the potential to lead to allergic reactions. To increase the overall accuracy and stability of impedance spectroscopy-based glucose sensing, recent studies combined those sensors with multiple sensors, such as temperature, humidity, and optical ones [79][81] and also with accelerometers [81]. Thereby, promising results were achieved in in-vivo experiments. Moreover, the time lag between the physiological parameters and the estimated glucose values was reduced by using time series analysis and sensor fusion [79]. However, the study population of 9 [79] and 20 [81] was still relatively small and in [81] 12.1% of sensor readings fell in the potential dangerous Zone D of the Clarke error grid. Therefore, impedance spectroscopy-based sensors are promising and yield the potential to be integrated into a wearable sensors system to enhance the overall performance and stability of non-invasive glucose sensing in the daily life situations of a patient.
The microwave-based approaches to miniaturization show a quite promising performance with low fabrication costs. The sizes of the sensors are in the range of centimeters to millimeters and even go down to the micrometer range. Consequently, these sensors are highly applicable to wearable systems for a patient’s daily life. However, one of the most crucial design criteria is getting the measurement signal to the area of interest (blood vessels) through the different tissues (e.g., skin, fat) and back to the sensor, respectively. If the signal levels are too low, changes in BGL will not be detectable. This significantly depends on the chosen operating frequency, which defines the penetration depth. The working frequencies are mostly in the range of 0–6 GHz [28][107][108][119][109][110][111][114][120][121][122], and the others are in the range of 10–20 GHz [105][106]. In general, the higher the frequency, the lower the penetration depth. For the other working frequency range, [106] works better than [105], as the phase change to the glucose change is larger to 2° per 10 mg/dL. In addition, different from other approaches, [114] introduced sub-band processing. V.V. Deshmukh et al. proposed different frequency bands for diabetes situations (with diabetes, without diabetes, and pre-diabetes) to increase the accuracy. In addition, the choice of the antenna and its corresponding tapering is essential as this will also define the available signal strength.
In general, the proposed scientific microwave approaches are in different development stages: conducting only simulations or developing a corresponding (mathematical) simulation-based model and validating this with experiments under ideal and under realistic conditions. Besides this classification, the scientific approaches can also be divided into those that only investigate the sensors system and those that utilize advanced signal processing for improving sensor accuracy. Depending on the desired mounting position, several tissue models were analyzed. Other researchers prefer to use the fingertip for measuring the BGL since a low penetration depth is needed to reach the intravascular blood in the microvessels of this region [31][111]. Fingertip approaches provide great prospects of success since they outperform systems using other detection areas. These are mostly resonator-based such as in the cases of Omer et al. [31] or Kiani et al. [111]. However, they are based on placing the fingertip on the sensor area, and therefore, they can be a good alternative to finger pricking but are not suitable for a CGM system. Furthermore, the proposed methods differ in the evaluated liquid. Most approaches use water with glucose or dextrose solutions [106][107][108], whereas a minority conduct measurements with real blood in the lab or even with humans.
In the literature, there are three methods discussed for detecting the change of the permittivity and the corresponding BGL: shift of resonance frequency [107][123][124][125][126], reflection (S11) [28][106][108][121][122] or transmission (S21) [28][127][128] of the amplitude or phase of the S-Parameters. S. Zeising et al. [106] stated that the phase variation of S11 is more sensitive than of S21. X. Xiao et al. [28] utilized both, S11 and S21, plus advanced signal processing improving the performance of RSME to 5.53 mg/dL. Modern signal processing techniques like machine learning can improve the performance of the BGL-detection significantly.
5.2. Post-Processing
Overall, post-processing using AI offers excellent opportunities to increase the accuracy of measurement data and predict the trends of blood glucose levels. The latter supports diabetic persons significantly since it helps to avoid hyperglycemia and hypoglycemia. However, in the prediction, there is a trade-off between accuracy and the prediction horizon [33]. This gets particularly interesting for predicting, warning, and thus avoiding hypoglycemia during sleep, which can be highly dangerous for the patients [129][130]. A comparison of different approaches with a prediction horizon of 30 min is given in Table 4.
| Reference | Model | RMSE in mg/dL | Data Set |
| [118] | RNN | 18.87 | Ohio T1DM |
| [131] | RNN | 19.04 | Ohio T1DM |
| [132] | RNN | 18.22 | Ohio T1DM |
| [133] | Autoregression with exogenous inputs (ARX) | 19.48 | Ohio T1DM |
| [134] | Grammatical evolution (GE) | 21.19 | Ohio T1DM |
| [135] | Physiological models | 19.33 | Ohio T1DM |
| [136] | XGBoost | 19.32 | Ohio T1DM |
| [137] | Convolutional Neural Network (CNN) | 21.72 | Ohio T1DM |
| [38] | Ensemble MMS (3 aggregated NNs) | 19.57 | Ohio T1DM |
| [138] | Long short-term memory (LSTM) | 18.23 | Ohio T1DM |
| [139] | RNN and Restricted Boltzmann Machines (RNN-RBM) | 15.59 | DirecNet [140] |
| [141] | Support Vector Regression (SVR) | 18.0 | own data set |
| [142] | LSTM | 21.4 | described in [141] |
Table 4. Performance comparison for 30 min prediction horizon.
Moreover, the results of AI in general strongly depend on the dataset and the model design (training, validating, and testing). Next to unrecognized biases in the dataset, the accuracy of the results can be distorted by a wrong validation. Thus, appropriate testing combined with external validation is crucial. However, the available datasets are often limited by a small-sized samples or missing data regarding reference BGL or features such as eating, physical activities, or insulin injection. In consequence, generalization is a problem. Additionally, reproducibility is a fundamental problem, as only a few researchers share their codes and/or datasets [29].
Overall, AI-based post-processing offers opportunities such as being able to predict BGL trends, and furthermore, it outperforms traditional signal processing approaches by enhancing the sensitivity of sensor systems. For the approaches using AI to increase their sensor performance validation is critical. For the required reproducibility, the study design must be defined for all the influential factors such as conditions of the sensor itself (hardware, fabrication errors), measurement environment (temperature, humidity), generalizability of tested persons (gender, age, type of diabetes) and algorithm.
5.3. Evaluation with Clarke Error Grid
Based on the different levels of development of the proposed sensor systems in the literature, the archived accuracy varies significantly. Several researchers demonstrated only a proof of concept of their approach, by simulating [98] and measuring [105][108][119][120][122] physical parameters such as S11 or S21. All of them showed that the amplitude and phase, as well as the resonance frequency, depend on the glucose concentration; however, a specific determination on their measured BGL was missing. In consequence, their methods cannot be evaluated by means of the Clarke error grid. An overview of the other approaches regarding the Clarke error grid is listed in Table 5: The sensor readings of commercial glucose monitoring systems fell, particularly in zones A and B. However, the results of [143][144][145] revealed that up to 2.1% of the sensor readings were in the dangerous zone D.
| Reference | Measuring Method | Detection Range in mg/dL |
Dataset | Clarke Error Grid: | ||||
| A | B | C | D | E | ||||
| Commercial Sensor Systems: | ||||||||
| [143] | Dexcom G6 | 40–400 | 25P T1D (resistance), 30 min each (aerobic), 30 min each | 85.4% 74.0% | 12.5% 26.0% |
0% 0% |
2.1% 0% |
0% 0% |
| [144] | FreeStyle Libre | 30–400 | 24P T1D, 11P T2D, 39P TGD, all pregnant, 4207 data points | 83.6% | 15.5% | 0% | 0.8% | 0% |
| [145] | FreeStyle Libre | 40–500 | 30P T2D, 1353 data points | 88.54% | 11.01% | 0% | 0.45% | 0% |
| Optical Sensor Systems: | ||||||||
| [117] | Raman Spectroscopy | 50–400 | 10.000 synthetic generated spectra | 93.0% | NA | NA | NA | |
| [113] | Raman Spectroscopy | 105–216 | 30 meas. × 12P | 100% | 0% | 0% | 0% | |
| Microwave-Based Sensor Systems: | ||||||||
| [114] | Microwave | 60–400 | 205P without categorization 205P with categorization |
80.91% 95.12% |
19.09% 4.88% |
0% 0% |
0% 0% |
0% 0% |
| [107] | Microwave | 30–500 | 6 × 6 meas. | 100% | 0% | 0% | 0% | 0% |
| [108] | Microwave | 0–180 | 7 × 10 meas. | 85.7% | 14.3% | 0% | 0% | 0% |
| [110] | Microwave | 0–400 | 10 min in total, 2 min each concentraation level (CGM) | 100% | 0% | 0% | 0% | 0% |
| [146] | Microwave | 50–500 | 5 × 6 meas. for: 1. Silver-painted device 2. Adhesive copper tape device |
44.45% 68.97% |
40.74% 24.14% |
3.70% 0% |
11.11% 6.89% |
0% 0% |
| Sensor Systems with Advanced Post-Processing: | ||||||||
| [28] | Microwave Post Processing INNHO |
20–500 | 255 × 5 × 7 data points | 100% | 0% | 0% | 0% | 0% |
| [79] | Impedance Spectr./ Sensor Fusion Post-Proc.: Time Series Analysis |
0-200 | 3 T1D P and 6 healthy P | 100% | 0% | 0% | 0% | 0% |
| [147] | Flash CGM Post Processing xgboost model |
60-180 | 198 TGD, 37 healthy P 3240 data points |
100% | 0% | 0% | 0% | 0% |
| [118] | Medtronic Enlite CGM sensors Post processing RNN |
30-400 | Ohio T1DM dataset [118] 2514. 2791 data points |
patients dependent, >90% in A and B | ||||
| [148] | Photoplethysmography (PPG) Monte Carlo Simulation |
50–150 80-200 |
synthetic real data (35P) |
80% 91.8% |
20% 5.05% |
0% 0% |
0% 3.15% |
0% 0% |
| [132] | RNN | 30-400 | Ohio T1DM dataset | 90% | 9% | 0% | 1% | 0% |
| [134] | Grammatical Evolution (GE) | 30-400 | Ohio T1DM dataset | 87.1% | 11.5% | 0% | 1.4% | 0% |
Table 5. Overview of the accuracy according to the Clarke error grid. Abbreviations: P = Persons, T1D = diabetes mellitus type 1, T2D = diabetes mellitus type 2, TGD = gestational diabetes mellitus, NA = Not a Number.
In addition, Table 12 shows the results of an impedance spectroscopy-based sensor combined with multiple different sensors [79]. Herein, 100% of the sensor readings fell in zones A and B, which is considered as clinical accuracy. The results were significantly improved by adding humidity, temperature, and optical sensors to the wearable system and applying time series analysis as post-processing. However, the study population was relatively small, with nine subjects. Furthermore, the observed detection range was insufficiently limited to 0–200 mg/dL. According to the requirements of the FDA, an appropriate sensor system used outside the hospital must be able to detect the BGL in a range of 20–500 mg/dL [88]. Most of the systems proposed in Table 12 cover this required range approximately. However, approaches such as that of [108][113][147][148] are also limited in their analyzed detection range. The Clarke error grid results of those have to be interpreted with caution since it is easier to design a precise sensor system for a narrow detection range than for a broad one.
5.3 Summary
The microwave approaches show the most promising results regarding miniaturization and low fabrication cost. Moreover, the accuracy of those approaches is comparable to that of commercial sensors. Kumar et al. [107] achieved a microwave-based sensor that is independent of temperatures between 10 and 50 °C. However, they conducted their measurements with a DI water drop, and therefore, it is questionable if the temperature independence is also fulfilled for a blood drop. Furthermore, using a drop of blood is invasive and, thus, cannot be seen as an improvement compared to traditional finger pricking. In addition, in the microwave-based approaches, the sensor must have direct contact with the skin, with no air gap in between, since the impedances of the sensors are designed to match those of the skin. Impedance matching is the most critical point in measurement scenarios, especially in daily life, since it also depends on immutable factors such as the temperature and the humidity of the skin or the sensor mounting compression. If the sensor loses contact with the skin, there is a jump in the impedance, which leads to high losses, and thus, the change of the BGL is no longer detectable. The impedance spectroscopy-based sensors suffer from the same problem. Since electrodes are fixed on the skin, relative movement between the skin and the electrodes results in an impedance change. Moreover, the impedance of the tissue is affected by the temperature, sweat, or moisture level of the tissue. Therefore, they are usually combined with other sensors. The optical-based sensors are in the early stage of development and are not suitable for wearable systems due to their bulky size. Moreover, optical-based sensors significantly depend on the temperature. Overall, the accuracy of scientific solutions for non-invasive glucose monitoring is comparable with commercial sensors. Nevertheless, it is worth mentioning that gold standard finger pricking—which is often used as a reference value for sensors characterization [79][111][114][149]—also does not have 100% of the measurement results in Zone A of the Clarke error grid as shown in [150].
In future scientific approaches to non-invasive glucose sensing systems, additional criteria (apart from the sensor sensitivity, safety, and accuracy), such as sensor size, battery lifetime and convenience of the sensor should be considered. Since nowadays it is common to track the fitness level via a smartwatch, a glucose sensor is proposed to be assembled in the electric watch instead of as a separate device, such as Apple is planning to do with the AppleWatch [151]. On the other hand, some new approaches are underway through cell therapy with b-cells [152]. Some companies are actively involved, e.g., Sernova, which is a regenerative medicine company, is developing new therapeutic technologies. Recently, they proposed Sernova’s Cell Pouch System™, which is an implantable and scalable medical device [153]. Additionally, a clinical trial, the so-called functional cure, is now in process, which captured a great deal of attention at the beginning of the year [154].
6. Conclusions
The lives of those who face diabetes differ significantly from those of non-diabetics. Patients must test their blood glucose levels at least several times a day. Although people nowadays can use insulin pumps with integrated blood glucose sensing systems as an automatic way to monitor and control their blood glucose, there is still an increased risk of infection. That is why non-invasive methods, such as those using saliva, tear, or electromagnetic-based sensors embedded in wearable devices, are attracting increased attention. Since electromagnetic sensors offer several advantages, such as low fabrication cost and independence in terms of temperature, they are the most promising approaches to non-invasive blood glucose monitoring. However, they are highly sensitive to penetration depth, operating frequency and tapering. On the other hand, artificial intelligence is gaining importance in signal processing to improve accuracy and predict the development of the blood glucose level precisely. Moreover, combining microwave-based sensors with multiple sensors such as temperature, humidity, or impedance spectroscopy-based sensors could improve the overall accuracy and stability of a non-invasive glucose sensing system. The state of the art is reviewed, focusing on comparing scientific electrochemical and electromagnetic non-invasive approaches to already existing commercial solutions. Companies such as Sernova are introducing novel diabetes therapies, whereas sensors like the ones of FreeStyle Libre or Dexcom are already commercially available and are being steadily enhanced. However, considering the current situation, under the COVID-19 pandemic, a reliable, low-cost blood glucose monitoring sensor enabling telemedical care is in high demand. Thus, there is still substantial room for improvements in terms of better accuracy, stability, safety, efficiency, simplicity, lab-on-chip compatibility, and miniaturization.
References
- WHO. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 5 March 2021).
- WHO. WHO Reveals Leading Causes of Death and Disability Worldwide: 2000–2019. Available online: https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019 (accessed on 8 March 2021).
- IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021.
- Yunos, M.F.A.M.; Nordin, A.N. Non-invasive glucose monitoring devices: A review. Bull. Electr. Eng. Inform. 2020, 9, 2609–2618.
- WHO. Mean Fasting Blood Glucose. Available online: https://www.who.int/data/gho/indicator-metadata-registry/imrdetails/2380 (accessed on 8 March 2021).
- David, S.O. The Science of Hypoglycemia in Patients with Diabetes. Curr. Diabetes Rev. 2013, 9, 195–208.
- Mouri, M.; Badireddy, M. Hyperglycemia; StatPearls [Internet]: Treasure Island, FL, USA, 2021.
- Fowler, M.J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 2008, 26, 77–82.
- Vriesendorp, T.M.; DeVries, J.H.; van Santen, S.; Moeniralam, H.S.; de Jonge, E.; Roos, Y.B.; Schultz, M.J.; Rosendaal, F.R.; Hoekstra,J.B. Evaluation of short-term consequences of hypoglycemia in an intensive care unit. Crit. Care Med. 2006, 34, 2714–2718.
- Cappon, G.; Acciaroli, G.; Vettoretti, M.; Facchinetti, A.; Sparacino, G. Wearable continuous glucose monitoring sensors: Arevolution in diabetes treatment. Electronics 2017, 6, 65.
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHOConsultation. Part 1, Diagnosis and Classification of Diabetes Mellitus; Technical Report; World Health Organization: Geneva,Switzerland, 1999.
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of currentevidence. Diabetologia 2019, 62, 3–16.
- Gross, T.M.; Mastrototaro, J.J. Efficacy and reliability of the continuous glucose monitoring system. Diabetes Technol. Ther. 2000, 2,19–26.
- Ma, X.; Ahadian, S.; Liu, S.; Zhang, J.; Liu, S.; Cao, T.; Lin, W.; Wu, R.; Barros, N.; Zare, M.R.; et al. Smart Contact Lenses forBiosensing Applications. Adv. Intell. Syst. 2021, 3, 2000263, doi:10.1002/aisy.202000263.
- Sensor, Method and Test Kit for Measuring Glucose. Available online: https://patents.google.com/patent/DE102013216886A1/en (accessed on 12 December 2021).
- So, C.F.; Choi, K.S.;Wong, T.K.; Chung, J.W. Recent advances in non-invasive glucose monitoring. Med. Devices 2012, 5, 45–52.
- Sharma, S.; Huang, Z.; Rogers, M.; Boutelle, M.; Cass, A.E. Evaluation of a minimally invasive glucose biosensor for continuoustissue monitoring. Anal. Bioanal. Chem. 2016, 408, 8427–8435.
- Dexcom G6 CGM Users Guide. Available online: https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf(accessed on 11 December 2021).
- FreeStyle Libre 3 User Handbook. Available online: https://freestyleserver.com/Payloads/IFU/2021/q1/ART42968-001_rev-B.pdf (accessed on 17 June 2021).
- Smith, J.L. The Pursuit of non-invasive Glucose: Hunting the Deceitful Turkey; Revised and Expanded; Self-published;Availableonline: https://www.researchgate.net/publication/215519631_The_Pursuit_of_Noninvasive_Glucose_Hunting_the_Deceitful_Turkey (accessed on 31 December 2021); 2015.
- Hassan, M.H.; Vyas, C.; Grieve, B.; Bartolo, P. Recent Advances in Enzymatic and Non-Enzymatic Electrochemical GlucoseSensing. Sensors 2021, 21, 4672, doi:10.3390/s21144672.
- Alsunaidi, B.; Althobaiti, M.; Tamal, M.; Albaker, W.; Al-Naib, I. A Review of Non-Invasive Optical Systems for ContinuousBlood Glucose Monitoring. Sensors 2021, 21, 6820, doi:10.3390/s21206820.
- Zhang, R.; Liu, S.; Jin, H.; Luo, Y.; Zheng, Z.; Gao, F.; Zheng, Y. non-invasive ElectromagneticWave Sensing of Glucose. Sensors2019, 19, 1151, doi:10.3390/s19051151.
- Juan, C.G.; Potelon, B.; Quendo, C.; Bronchalo, E. Microwave Planar Resonant Solutions for Glucose Concentration Sensing: ASystematic Review. Appl. Sci. 2021, 11, 7018, doi:10.3390/app11157018.
- Arakawa, T.; Tomoto, K.; Nitta, H.; Toma, K.; Takeuchi, S.; Sekita, T.; Minakuchi, S.; Mitsubayashi, K. A wearable celluloseacetate-coated mouthguard biosensor for in vivo salivary glucose measurement. Anal. Chem. 2020, 92, 12201–12207.
- Geelhoed-Duijvestijn, P.; Vegelyte, D.; Kownacka, A.; Anton, N.; Joosse, M.; Wilson, C. Performance of the prototype NovioSensenon-invasive biosensor for tear glucose in type 1 diabetes. J. Diabetes Sci. Technol. 2020, 15, 1932296820964844.
- Dixit, K.; Fardindoost, S.; Ravishankara, A.; Tasnim, N.; Hoorfar, M. Exhaled Breath Analysis for Diabetes Diagnosis andMonitoring: Relevance, Challenges and Possibilities. Biosensors 2021, 11, 476, doi:10.3390/bios11120476.
- Xiao, X.; Yu, Q.; Li, Q.; Song, H.; Kikkawa, T. Precise non-invasive estimation of glucose using UWB microwave with improvedneural networks and hybrid optimization. IEEE Trans. Instrum. Meas. 2020, 70, 1–10.
- Dankwa-Mullan, I.; Rivo, M.; Sepulveda, M.; Park, Y.; Snowdon, J.; Rhee, K. Transforming Diabetes Care Through ArtificialIntelligence: The Future Is Here. Popul. Health Manag. 2018, 22, 229–242, doi:10.1089/pop.2018.0129.
- López, B.; Martin, C.; Herrero, P. Special section on artificial intelligence for diabetes. Artif. Intell. Med. 2018, 85, 26–27,doi:10.1016/j.artmed.2017.09.008.
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Kokabi, H.; Alquié, G.; Deshours, F.; Shubair, R. Low-cost Portable Microwave Sensorfor Non-Invasive Monitoring of Blood Glucose Level: Novel Design Utilizing a Four-Cell CSRR Hexagonal Configuration. Nature2020, 10, 15200, doi:10.1038/s41598-020-72114-3.
- Zhang, Y.; Sun, J.; Liu, L.; Qiao, H. A review of biosensor technology and algorithms for glucose monitoring. J. Diabetes Complicat.2021, 35, 107929, doi:10.1016/j.jdiacomp.2021.107929.
- Felizardo, V.; Garcia, N.; Pombo, N.; Megdiche, I. Data-based algorithms and models using diabetics real data forblood glucose and hypoglycaemia prediction—A systematic literature review. Artif. Intell. Med. 2021, 118, 102120,doi:10.1016/j.artmed.2021.102120.
- Georga, E.; Protopappas, V.; Polyzos, D.; Fotiadis, D. Evaluation of short-term predictors of glucose concentration in type 1diabetes combining feature ranking with regression models. Med. Biol. Eng. Comput. 2015, 53, 1305–1318, doi:10.1007/s11517-015-1263-1.
- Ooka, T.; Johno, H.; Nakamoto, K.; Yoda, Y.; Yokomichi, H.; Yamagata, Z. Random forest approach for determining risk predictionand predictive factors of type 2 diabetes: Large-scale health check-up data in Japan. BMJ Nutr. Prev. Health 2021, 4, pp. 140–148doi:10.1136/bmjnph-2020-000200.
- Dworzynski, P.; Aasbrenn, M.; Rostgaard, K.; Melbye, M.; Gerds, T.; Hjalgrim, H.; Pers, T. Nationwide prediction of type 2diabetes comorbidities. Sci. Rep. 2020, 10, 1776, doi:10.1038/s41598-020-58601-7.
- Das, S.K.; Roy, P.; Mishra, A.K. Deep Learning Techniques Dealing with Diabetes Mellitus: A Comprehensive Study. In HealthInformatics: A Computational Perspective in Healthcare; Patgiri, R., Biswas, A., Roy, P., Eds.; Springer: Singapore, 2021; pp. 295–323,doi:10.1007/978-981-15-9735-0_15.
- Tena, F.; Garnica, O.; Lanchares, J.; Hidalgo, J.I. Ensemble Models of Cutting-Edge Deep Neural Networks for Blood GlucosePrediction in Patients with Diabetes. Sensors 2021, 21, 7090, doi:10.3390/s21217090.
- Bolla, A.S.; Priefer, R. Blood glucose monitoring- an overview of current and future non-invasive devices. Diabetes Metab. Syndr.Clin. Res. Rev. 2020, 14, 739–751, doi:10.1016/j.dsx.2020.05.016.
- Mohammadifar, M.; Tahernia, M.; Choi, S. An Equipment-Free, Paper-Based Electrochemical Sensor for Visual Monitoring ofGlucose Levels in Urine. SLAS Technol. Transl. Life Sci. Innov. 2019, 24, 499–505, doi:10.1177/2472630319846876.
- Zhang, J.; Liu, J.; Su, H.; Sun, F.; Lu, Z.; Su, A. A wearable self-powered biosensor system integrated with diaper for detecting theurine glucose of diabetic patients. Sens. Actuators B Chem. 2021, 341, 130046, doi:10.1016/j.snb.2021.130046.
- Zhao, M.; Leung, P.S. Revisiting the use of biological fluids for non-invasive glucose detection. Future Med. Chem. 2020, 12,645–647.
- Soares Nunes, L.A.; Mussavira, S.; Sukumaran Bindhu, O. Clinical and diagnostic utility of saliva as a non-invasive diagnosticfluid: A systematic review. Biochem. Medica 2015, 25, 177–192.
- Marley, G.; Kang, D.; Wilson, E.C.; Huang, T.; Qian, Y.; Li, X.; Tao, X.; Wang, G.; Xun, H.; Ma, W. Introducing rapid oral–fluid HIVtesting among high risk populations in Shandong, China: Feasibility and challenges. BMC Public Health 2014, 14, 422.
- Kaufman, E.; Lamster, I.B. The diagnostic applications of saliva—A review. Crit. Rev. Oral Biol. Med. 2002, 13, 197–212.
- Caixeta, D.C.; Aguiar, E.M.; Cardoso-Sousa, L.; Coelho, L.M.; Oliveira, S.W.; Espindola, F.S.; Raniero, L.; Crosara, K.T.; Baker,M.J.; Siqueira, W.L.; et al. Salivary molecular spectroscopy: A sustainable, rapid and non-invasive monitoring tool for diabetesmellitus during insulin treatment. PLoS ONE 2020, 15, e0223461.
- Jones, L.; Hui, A.; Phan, C.M.; Read, M.; Azar, D.; Buch, J.; Ciolino, J.; Naroo, S.; Pall, B.; Romond, K.; et al. CLEAR—Contact lenstechnologies of the future. Contact Lens Anterior Eye 2021, 44, 398–430, doi:10.1016/j.clae.2021.02.007.
- Kim, S.; Jeon, H.J.; Park, S.; Lee, D.Y.; Chung, E. Tear glucose measurement by reflectance spectrum of a nanoparticle embeddedcontact lens. Sci. Rep. 2020, 10, 8254.
- Makaram, P.; Owens, D.; Aceros, J. Trends in nanomaterial-based non-invasive diabetes sensing technologies. Diagnostics 2014,4, 27–46.
- Xiong, C.; Zhang, T.; Kong,W.; Zhang, Z.; Qu, H.; Chen,W.;Wang, Y.; Luo, L.; Zheng, L. ZIF-67 derived porous Co3O4 hollownanopolyhedron functionalized solution-gated graphene transistors for simultaneous detection of glucose and uric acid in tears.Biosens. Bioelectron. 2018, 101, 21–28.
- Baca, J.T.; Finegold, D.N.; Asher, S.A. Tear glucose analysis for the non-invasive detection and monitoring of diabetes mellitus.Ocul. Surf. 2007, 5, 280–293.
- Bamgboje, D.; Christoulakis, I.; Smanis, I.; Chavan, G.; Shah, R.; Malekzadeh, M.; Violaris, I.; Giannakeas, N.; Tsipouras, M.;Kalafatakis, K.; et al. Continuous Non-Invasive Glucose Monitoring via Contact Lenses: Current Approaches and FuturePerspectives. Biosensors 2021, 11, 189, doi:10.3390/bios11060189.
- Mule, N.M.; Patil, D.D.; Kaur, M. A comprehensive survey on investigation techniques of exhaled breath (EB) for diagnosis ofdiseases in human body. Inform. Med. Unlocked 2021, 26, 100715, doi:10.1016/j.imu.2021.100715.
- Di Francesco, F.; Fuoco, R.; Trivella, M.G.; Ceccarini, A. Breath analysis: Trends in techniques and clinical applications. Microchem.J. 2005, 79, 405–410.
- Das, S.; Pal, S.; Mitra, M. Significance of exhaled breath test in clinical diagnosis: A special focus on the detection of diabetesmellitus. J. Med. Biol. Eng. 2016, 36, 605–624.
- Chen, T.; Liu, T.; Li, T.; Zhao, H.; Chen, Q. Exhaled breath analysis in disease detection. Clin. Chim. Acta 2021, 515, 61–72.
- Tang, L.; Chang, S.J.; Chen, C.J.; Liu, J.T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925,doi:10.3390/s20236925.
- Wang, C.; Mbi, A.; Shepherd, M. A Study on Breath Acetone in Diabetic Patients Using a Cavity Ringdown Breath Analyzer:Exploring Correlations of Breath Acetone With Blood Glucose and Glycohemoglobin A1C. IEEE Sens. J. 2010, 10, 54–63,doi:10.1109/JSEN.2009.2035730.
- Wang, Z.; Sun, M.; Zhao, X.; Jiang, C.; Li, Y.; Wang, C. Study of Breath Acetone in a Rat Mode of 126 Rats with Type 1 Diabetes. J.Anal. Bioanal. Tech. 2017, 8, 1–7, doi:10.4172/2155-9872.1000344.
- Rydosz, A. A Negative Correlation Between Blood Glucose and Acetone Measured in Healthy and Type 1 Diabetes MellitusPatient Breath. J. Diabetes Sci. Technol. 2015, 9, 881–884, doi:10.1177/1932296815572366.
- Wang, Z.; Sun, M.; Zhao, X.; Jiang, C.; Li, Y.; Wang, C. Study of Breath Acetone in a Rat Mode of 126 Rats with Type 1 Diabetes. J.Anal. Bioanal. Tech. 2017, 8, 1–7, doi:10.4172/2155-9872.1000344.
- Wilson, A.D. Advances in Electronic-Nose Technologies for the Detection of Volatile Biomarker Metabolites in the Human Breath.Metabolites 2015, 5, 140–163, doi:10.3390/metabo5010140.
- Sun, M.; Chen, Z.; Gong, Z.; Zhao, X.; Jiang, C.; Yuan, Y.; Wang, Z.; Li, Y.X.; Wang, C. Determination of breath acetone in 149 Type2 diabetic patients using a ringdown breath-acetone analyzer. Anal. Bioanal. Chem. 2015, 407, 1641–1650, doi:10.1007/s00216-014-8401-8.
- Andrews, B.T.E.; Denzer, W.; Hancock, G.; Lunn, A.D.; Peverall, R.; Ritchie, G.A.D.; Williams, K. Measurement of breath acetonein patients referred for an oral glucose tolerance test. J. Breath Res. 2018, 12, 036015, doi:10.1088/1752-7163/aabd88.
- Alkedeh, O.; Priefer, R. The Ketogenic Diet: Breath Acetone Sensing Technology. Biosensors 2021, 11, 26, doi:10.3390/bios11010026.
- Kricka, L.J.; Wiencek, J.; Fortina, P. Breath Acetone. IFCC Emerging Technologies Division. 2021. Available online: https://www.ifcc.org/media/479112/wg-vol_point-of-care-volatolomics_.pdf (accessed on 18 December 2021).
- Pathak, A.K.; Viphavakit, C. VOC Biomarker Monitoring for Diabetes Through Exhaled Breath Using Ag/P-TiO2 CompositePlasmonic Sensor. IEEE Sens. J. 2021, 21, 22631–22637.
- Usman, F.; Dennis, J.O.; Ahmed, A.Y.; Meriaudeau, F.; Ayodele, O.B.; Rabih, A.A.S. A Review of Biosensors for Non-InvasiveDiabetes Monitoring and Screening in Human Exhaled Breath. IEEE Access 2019, 7, 5963–5974, doi:10.1109/ACCESS.2018.2887066.
- Zhang, J.; Lei, C.; Liang, T.; Liu, R.; Zhao, Z.; Qi, L.; Ghaffar, A.; Xiong, J. Acetone Sensor Based on FAIMS-MEMS. Micromachines2021, 12, 1531.
- Das, S.; Pal, M. Non-invasive monitoring of human health by exhaled breath analysis: A comprehensive review. J. Electrochem.Soc. 2020, 167, 037562.
- Villena Gonzales, W.; Mobashsher, A.T.; Abbosh, A. The progress of glucose monitoring—A review of invasive to minimally andnon-invasive techniques, devices and sensors. Sensors 2019, 19, 800.
- La Belle, J.T.; Engelschall, E.; Lan, K.; Shah, P.; Saez, N.; Maxwell, S.; Adamson, T.; Abou-Eid, M.; McAferty, K.; Patel, D.R.; et al.A Disposable Tear Glucose Biosensor—Part 4: Preliminary Animal Model Study Assessing Efficacy, Safety, and Feasibility. J.Diabetes Sci. Technol. 2014, 8, 109–116.
- Yan, Q.; Peng, B.; Su, G.; Cohan, B.E.; Major, T.C.; Meyerhoff, M.E. Measurement of tear glucose levels with amperometric glucosebiosensor/capillary tube configuration. Anal. Chem. 2011, 83, 8341–8346.
- Lee, I.; Probst, D.; Klonoff, D.; Sode, K. Continuous glucose monitoring systems-Current status and future perspectives of theflagship technologies in biosensor research. Biosens. Bioelectron. 2021, 181, 113054.
- Parachalil, D.R.; McIntyre, J.; Byrne, H.J. Potential of Raman spectroscopy for the analysis of plasma/serum in the liquid state:Recent advances. Anal. Bioanal. Chem. 2020, 412, 1993–2007.
- Petry, R.; Schmitt, M.; Popp, J. Raman spectroscopy—A prospective tool in the life sciences. ChemPhysChem 2003, 4, 14–30.
- Caduff, A.; Hirt, E.; Feldman, Y.; Ali, Z.; Heinemann, L. First human experiments with a novel non-invasive, non-opticalcontinuous glucose monitoring system. Biosens. Bioelectron. 2003, 19, 209–217, doi:10.1016/s0956-5663(03)00196-9.
- Wentholt, I.M.E.; Hoekstra, J.B.L.; Zwart, A.; DeVries, J.H. Pendra goes Dutch: Lessons for the CE mark in Europe. Diabetologia2005, 48, 1055–1058, doi:10.1007/s00125-005-1754-y.
- Geng, Z.; Tang, F.; Ding, Y.; Li, S.; Wang, X. Noninvasive Continuous Glucose Monitoring Using a Multisensor-Based Glucometerand Time Series Analysis. Sci. Rep. 2017, 7, 12650, doi:10.1038/s41598-017-13018-7.
- Caduff, A.; Talary, M.S.; Mueller, M.; Dewarrat, F.; Klisic, J.; Donath, M.; Heinemann, L.; Stahel, W.A. Non-invasive glucosemonitoring in patients with Type 1 diabetes: A Multisensor system combining sensors for dielectric and optical characterisationof skin. Biosens. Bioelectron. 2009, 24, 2778–2784, doi:10.1016/j.bios.2009.02.001.
- Zanon, M.; Mueller, M.; Zakharov, P.; Talary, M.S.; Donath, M.; Stahel, W.A.; Caduff, A. First Experiences With a WearableMultisensor Device in a non-invasive Continuous Glucose Monitoring Study at Home, Part II: The Investigators’ View. J. DiabetesSci. Technol. 2017, 12, 554–561, doi:10.1177/1932296817740591.
- Narang, R.; Mohammadi, S.; Ashani, M.M.; Sadabadi, H.; Hejazi, H.; Zarifi, M.H.; Sanati-Nezhad, A. Sensitive, real-timeand non-intrusive detection of concentration and growth of pathogenic bacteria using microfluidic-microwave ring resonatorbiosensor. Sci. Rep. 2018, 8, 15807.
- Choi, H.; Naylon, J.; Luzio, S.; Beutler, J.; Birchall, J.; Martin, C.; Porch, A. Design and in vitro interference test of microwavenon-invasive blood glucose monitoring sensor. IEEE Trans. Microw. Theory Tech. 2015, 63, 3016–3025.
- Deutsch, T.; Gergely, T.; Trunov, V. A computer system for interpreting blood glucose data. Comput. Methods Programs Biomed.2004, 76, 41–51.
- Khanam, J.J.; Foo, S.Y. A comparison of machine learning algorithms for diabetes prediction. ICT Express 2021, 7, 432–439.doi:10.1016/j.icte.2021.02.004.
- Gusev, M.; Poposka, L.; Spasevski, G.; Kostoska, M.; Koteska, B.; Simjanoska, M.; Ackovska, N.; Stojmenski, A.; Tasic, J.; Trontelj,J. non-invasive glucose measurement using machine learning and neural network methods and correlation with heart ratevariability. J. Sens. 2020, 2020, 9628281.
- Mujahid, O.; Contreras, I.; Vehi, J. Machine learning techniques for hypoglycemia prediction: Trends and challenges. Sensors2021, 21, 546.
- FDA. Blood Glucose Monitoring Test Systems for Prescription Point-of-Care Use. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/blood-glucose-monitoring-test-systems-prescription-point-care-use(accessed on 27 December 2021).
- Anand, P.K.; Shin, D.R.; Memon, M.L. Adaptive Boosting Based Personalized Glucose Monitoring System (PGMS) for Non-Invasive Blood Glucose Prediction with Improved Accuracy. Diagnostics 2020, 10, 285.
- Freckmann, G.; Pleus, S.; Grady, M.; Setford, S.; Levy, B. Measures of Accuracy for Continuous Glucose Monitoring and BloodGlucose Monitoring Devices. J. Diabetes Sci. Technol. 2018, 13, 193229681881206, doi:10.1177/1932296818812062.
- Clarke, W.L.; Cox, D.; Gonder-Frederick, L.A.; Carter, W.; Pohl, S.L. Evaluating Clinical Accuracy of Systems for Self-Monitoringof Blood Glucose. Diabetes Care 1987, 10, 622–628, doi:10.2337/diacare.10.5.622.
- Shang, T.; Zhang, J.Y.; Thomas, A.; Arnold, M.A.; Vetter, B.N.; Heinemann, L.; Klonoff, D.C. Products for Monitoring GlucoseLevels in the Human Body with non-invasive Optical, non-invasive Fluid Sampling, or Minimally Invasive Technologies. J.Diabetes Sci. Technol. 2021, doi:10.1177/19322968211007212.
- Abbott. Available online: https://www.freestylelibre.de/produkte/freestyle-libre-3-sensor.html (accessed on 21 November2021).
- Dexcom. Available online: https://uk.store.dexcom.com/en-GB/dexcom-g6/g6-sensor-single/STS-GS-002.html (accessed on21 November 2021).
- FreeStyle Libre 2 User Handbook. Available online: https://freestyleserver.com/Payloads/IFU/2021/q1/ART41007-201_rev-A_Web.pdf (accessed on 17 June 2021).
- Blum, A. Freestyle libre glucose monitoring system. Clin. Diabetes 2018, 36, 203–204.
- FDA. Summary of Safety and Effectiveness Data—Freestyle Libre Pro Flash Glucose Monitoring System. Available online:https://www.accessdata.fda.gov/cdrh_docs/pdf15/p150021b.pdf (accessed on 21 June 2021).
- Fokkert, M.; Van Dijk, P.; Edens, M.; Abbes, S.; De Jong, D.; Slingerland, R.; Bilo, H. Performance of the FreeStyle Libre Flashglucose monitoring system in patients with type 1 and 2 diabetes mellitus. BMJ Open Diabetes Res. Care 2017, 5, e000320.
- Boscari, F.; Galasso, S.; Facchinetti, A.; Marescotti, M.; Vallone, V.; Amato, A.; Avogaro, A.; Bruttomesso, D. FreeStyle Libre andDexcom G4 Platinum sensors: Accuracy comparisons during two weeks of home use and use during experimentally inducedglucose excursions. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 180–186.
- Tsoukas, M.; Rutkowski, J.; El-Fathi, A.; Yale, J.F.; Bernier-Twardy, S.; Bossy, A.; Pytka, E.; Legault, L.; Haidar, A. Accuracy ofFreeStyle Libre in adults with type 1 diabetes: The effect of sensor age. Diabetes Technol. Ther. 2020, 22, 203–207.
- Oyagüez, I.; Merino-Torres, J.F.; Brito, M.; Bellido, V.; Cardona-Hernandez, R.; Gomez-Peralta, F.; Morales-Perez, F. Cost analysisof the flash monitoring system (FreeStyle Libre 2) in adults with type 1 diabetes mellitus. BMJ Open Diabetes Res. Care 2020,8, e001330.
- Press Release Details. Available online: https://dexcom.gcs-web.com/news-releases/news-release-details/dexcom-looksfuture-continuous-glucose-monitoring (accessed on 12 December 2021).
- Martens, T.; Beck, R.W.; Bailey, R.; Ruedy, K.J.; Calhoun, P.; Peters, A.L.; Pop-Busui, R.; Philis-Tsimikas, A.; Bao, S.; Umpierrez, G.;et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin:A Randomized Clinical Trial. JAMA 2021, 325, 2262–2272, doi:10.1001/jama.2021.7444.
- Golparvar, A.; Boukhayma, A.; Loayza, T.; Caizzone, A.; Enz, C.; Carrara, S. Very Selective Detection of Low PhysiopathologicalGlucose Levels by Spontaneous Raman Spectroscopy with Univariate Data Analysis. BioNanoScience 2021, 11, 871–877,doi:10.1007/s12668-021-00867-w.
- Hofmann, M.; Fischer, G.; Weigel, R.; Kissinger, D. Microwave-based non-invasive concentration measurements for biomedicalapplications. IEEE Trans. Microw. Theory Tech. 2013, 61, 2195–2204.
- Zeising, S.; Kirchner, J.; Khalili, H.F.; Ahmed, D.; Lübke, M.; Thalmayer, A.; Fischer, G. Towards Realisation of a Non-InvasiveBlood Glucose Sensor Using Microstripline. In Proceedings of the 2020 IEEE International Instrumentation and MeasurementTechnology Conference (I2MTC), Dubrovnik, Croatia, May 2020; pp. 1–6.
- Kumar, A.;Wang, C.; Meng, F.Y.; Zhou, Z.L.; Zhao, M.; Yan, G.F.; Kim, E.S.; Kim, N.Y. High-sensitivity, quantified, linear andmediator-free resonator-based microwave biosensor for glucose detection. Sensors 2020, 20, 4024.
- Gorst, A.; Zavyalova, K.; Mironchev, A. Non-Invasive Determination of Glucose Concentration Using a Near-Field Sensor.Biosensors 2021, 11, 62.
- Yue, W.; Kim, E.S.; Zhu, B.H.; Chen, J.; Liang, J.G.; Kim, N.Y. Permittivity-Inspired Microwave Resonator-Based Biosensor Basedon Integrated Passive Device Technology for Glucose Identification. Biosensors 2021, 11, 508, doi:10.3390/bios11120508.
- Jang, C.; Park, J.K.; Lee, H.J.; Yun, G.H.; Yook, J.G. Sensitivity-Enhanced Fluidic Glucose Sensor Based on a Microwave ResonatorCoupled with an Interferometric System for non-invasive and Continuous Detection. IEEE Trans. Biomed. Circuits Syst. 2021, 15,1017–1026.
- Kiani, S.; Rezaei, P.; Fakhr, M. Dual-Frequency Microwave Resonant Sensor to Detect non-invasive Glucose-Level ChangesThrough the Fingertip. IEEE Trans. Instrum. Meas. 2021, 70, 1–8, doi:10.1109/TIM.2021.3052011.
- Kang, J.W.; Park, Y.S.; Chang, H.; Lee, W.; Singh, S.P.; Choi, W.; Galindo, L.H.; Dasari, R.R.; Nam, S.H.; Park, J.; et al. Directobservation of glucose fingerprint using in vivo Raman spectroscopy. Sci. Adv. 2020, 6, eaay5206.
- Li, N.; Zang, H.; Sun, H.; Jiao, X.; Wang, K.; Liu, T.C.Y.; Meng, Y. A non-invasive Accurate Measurement of Blood Glucose Levelswith Raman Spectroscopy of Blood in Microvessels. Molecules 2019, 24, 1500, doi:10.3390/molecules24081500.
- Deshmukh, V.V.; Chorage, S.S. Non-invasive determination of blood glucose level using narrowband microwave sensor. J.Ambient. Intell. Humaniz. Comput. 2021, 1–16.
- Arakawa, T.; Kuroki, Y.; Nitta, H.; Chouhan, P.; Toma, K.; Sawada, S.I.; Takeuchi, S.; Sekita, T.; Akiyoshi, K.; Minakuchi, S.; et al.Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor. Biosens. Bioelectron. 2016,84, 106–111.
- Duong, H.D.; Sohn, O.J.; Rhee, J.I. Development of a Ratiometric Fluorescent Glucose Sensor Using an Oxygen-Sensing MembraneImmobilized with Glucose Oxidase for the Detection of Glucose in Tears. Biosensors 2020, 10, 86.
- Park, Y.S.; Ahn, S.; Chang, H.; Lee, W.; Nam, S.H. Influence of Raman Spectrometer Collection Efficiency on Performance of noninvasiveBlood Glucose Detection for Device Miniaturization. In Proceedings of the 2020 42nd Annual International Conferenceof the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 6139–6142.
- Martinsson, J.; Schliep, A.; Eliasson, B.; Mogren, O. Blood glucose prediction with variance estimation using recurrent neuralnetworks. J. Healthc. Inform. Res. 2020, 4, 1–18.
- Hofmann, M.; Fersch, T.; Weigel, R.; Fischer, G.; Kissinger, D. A novel approach to non-invasive blood glucose measurementbased on RF transmission. In Proceedings of the 2011 IEEE International Symposium on Medical Measurements and Applications,Bari, Italy, 30–31 May 2011; pp. 39–42.
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S. Portable radar-driven microwave sensor for intermittent glucose levels monitoring.IEEE Sens. Lett. 2020, 4, 3500604.
- Deshmukh, V.V.; Chorage, S.S. Microstrip antennas used for non-invasive determination of blood glucose level. In Proceedingsof the 2020 4th International Conference on Intelligent Computing and Control Systems (ICICCS), Madurai, India, May 2020;pp. 720–725.
- Zapasnoy, A.S.; Belichenko, V.P.; Yakubov, V.P.; Gorst, A.V.; Mironchev, A.S.; Klokov, A.V.; Zavyalova, K.V. Application ofBroadband Microwave Near-Field Sensors for Glucose Monitoring in Biological Media. Appl. Sci. 2021, 11, 1470.
- Kim, N.; Dhakal, R.; Adhikari, K.; Kim, E.; Wang, C. A reusable robust radio frequency biosensor using microwave resonator byintegrated passive device technology for quantitative detection of glucose level. Biosens. Bioelectron. 2015, 67, 687–693.
- Jang, C.; Park, J.K.; Lee, H.J.; Yun, G.H.; Yook, J.G. Temperature-corrected fluidic glucose sensor based on microwave resonator.Sensors 2018, 18, 3850.
- Ebrahimi, A.; Scott, J.; Ghorbani, K. Microwave reflective biosensor for glucose level detection in aqueous solutions. Sens.Actuators A Phys. 2020, 301, 111662.
- Odabashyan, L.; Babajanyan, A.; Baghdasaryan, Z.; Kim, S.; Kim, J.; Friedman, B.; Lee, J.H.; Lee, K. Real-time non-invasivemeasurement of glucose concentration using a modified Hilbert shaped microwave sensor. Sensors 2019, 19, 5525.
- Saha, S.; Cano-Garcia, H.; Sotiriou, I.; Lipscombe, O.; Gouzouasis, I.; Koutsoupidou, M.; Palikaras, G.; Mackenzie, R.; Reeve, T.;Kosmas, P.; et al. A glucose sensing system based on transmission measurements at millimetre waves using micro strip patchantennas. Sci. Rep. 2017, 7, 6855.
- Hu, S.; Nagae, S.; Hirose, A. Millimeter-wave adaptive glucose concentration estimation with complex-valued neural networks.IEEE Trans. Biomed. Eng. 2018, 66, 2065–2071.
- Bertachi, A.; Viñals, C.; Biagi, L.; Contreras, I.; Vehí, J.; Conget, I.; Gimenez, M. Prediction of Nocturnal Hypoglycemia in Adultswith Type 1 Diabetes under Multiple Daily Injections Using Continuous Glucose Monitoring and Physical Activity Monitor.Sensors 2020, 20, 1705, doi:10.3390/s20061705.
- Vu, L.H.; Kefayati, S.; Idé, T.; Pavuluri, V.N.; Jackson, G.P.; Latts, L.; Zhong, Y.; Agrawal, P.; Chang, Y.C. Predicting NocturnalHypoglycemia from Continuous Glucose Monitoring Data with Extended Prediction Horizon. AMIA Annu. Symp. Proc. 2019,2019, 874–882.
- Chen, J.; Li, K.; Herrero, P.; Zhu, T.; Georgiou, P. Dilated Recurrent Neural Network for Short-time Prediction of GlucoseConcentration. In Proceedings of the 3rd International Workshop on Knowledge Discovery in Healthcare Data co-located withthe 27th International Joint Conference on Artificial Intelligence and the 23rd European Conference on Artificial Intelligence(IJCAI-ECAI 2018), Stockholm, Schweden, 13 July 2018; pp. 69–73.
- Rubin-Falcone, H.; Fox, I.; Wiens, J. Deep Residual Time-Series Forecasting: Application to Blood Glucose Prediction. InProceedings of the 5th International Workshop on Knowledge Discovery in Healthcare Data Co-located with 24th EuropeanConference on Artificial Intelligence, KDH@ECAI 2020, Santiago de Compostela, Spain, 9–30 August 2020.
- Xie, J.;Wang, Q. Benchmarking Machine Learning Algorithms on Blood Glucose Prediction for Type I Diabetes in ComparisonWith Classical Time-Series Models. IEEE Trans. Biomed. Eng. 2020, 67, 3101–3124, doi:10.1109/TBME.2020.2975959.
- Contreras, I.; Bertachi, A.; Biagi, L.; Vehí, J.; Oviedo, S. Using Grammatical Evolution to Generate Short-term Blood GlucosePrediction Models. In Proceedings of the 3rd InternationalWorkshop on Knowledge Discovery in Healthcare Data co-locatedwith the 27th International Joint Conference on Artificial Intelligence and the 23rd European Conference on Artificial Intelligence(IJCAI-ECAI 2018), Stockholm, Schweden, 13 July 2018; pp. 91–96.
- Bertachi, A.; Biagi, L.; Contreras, I.; Luo, N.; Vehí, J. Prediction of Blood Glucose Levels And Nocturnal Hypoglycemia UsingPhysiological Models and Artificial Neural Networks. In Proceedings of the 3rd InternationalWorkshop on Knowledge Discoveryin Healthcare Data co-located with the 27th International Joint Conference on Artificial Intelligence and the 23rd EuropeanConference on Artificial Intelligence (IJCAI-ECAI 2018), Stockholm, Schweden, 13 July 2018; pp. 85–90.
- Midroni, C.; Leimbigler, P.; Baruah, G.; Kolla, M.; Whitehead, A.; Fossat, Y. Predicting glycemia in type 1 diabetes patients:Experiments with xg-boost. In Proceedings of the 3rd International Workshop on Knowledge Discovery in Healthcare Dataco-located with the 27th International Joint Conference on Artificial Intelligence and the 23rd European Conference on ArtificialIntelligence (IJCAI-ECAI 2018), Stockholm, Schweden, 13 July 2018.
- Zhu, T.; Li, K.; Herrero, P.; Chen, J.; Georgiou, P. A Deep Learning Algorithm for Personalized Blood Glucose Prediction. InProceedings of the 3rd InternationalWorkshop on Knowledge Discovery in Healthcare Data co-located with the 27th InternationalJoint Conference on Artificial Intelligence and the 23rd European Conference on Artificial Intelligence (IJCAI-ECAI 2018),Stockholm, Schweden, 13 July 2018; pp. 64–78.
- Bevan, R.; Coenen, F. Experiments in Non-Personalized Future Blood Glucose Level Prediction. In Proceedings of the 5thInternationalWorkshop on Knowledge Discovery in Healthcare Data Co-located with 24th European Conference on ArtificialIntelligence, KDH@ECAI 2020, Santiago de Compostela, Spain, 9–30 August 2020.
- Nasser, A.R.; Hasan, A.M.; Humaidi, A.J.; Alkhayyat, A.; Alzubaidi, L.; Fadhel, M.A.; Santamaría, J.; Duan, Y. IoT and CloudComputing in Health-Care: A NewWearable Device and Cloud-Based Deep Learning Algorithm for Monitoring of Diabetes.Electronics 2021, 10, 2719, doi:10.3390/electronics10212719.
- JAEB Center for Health Research. Diabetes Research in Childern Network (DirecNet). Available online: https://public.jaeb.org/direcnet/stdy (accessed on 14 December 2021).
- Bunescu, R.; Struble, N.; Marling, C.; Shubrook, J.; Schwartz, F. Blood Glucose Level Prediction Using Physiological Models andSupport Vector Regression. In Proceedings of the 2013 12th International Conference on Machine Learning and Applications,Miami, FL, USA, 4–7 December 2013; Volume 1, pp. 135–140, doi:10.1109/ICMLA.2013.30.
- Sun, Q.; Jankovic, M.; Bally, L.; Mougiakakou, S. Predicting Blood Glucose with an LSTM and Bi-LSTM Based Deep NeuralNetwork. In Proceedings of the 2018 14th Symposium on Neural Networks and Applications (NEUREL), Belgrade, Serbia, 20–21November 2018; pp. 1–5, doi:10.1109/NEUREL.2018.8586990.
- Guillot, F.H.; Jacobs, P.G.; Wilson, L.M.; Youssef, J.E.; Gabo, V.B.; Branigan, D.L.; Tyler, N.S.; Ramsey, K.; Riddell, M.C.; Castle, J.R.Accuracy of the Dexcom G6 Glucose Sensor during Aerobic, Resistance, and Interval Exercise in Adults with Type 1 Diabetes.Biosensors 2020, 10, 138.
- Scott, E.M.; Bilous, R.W.; Kautzky-Willer, A. Accuracy, User Acceptability, and Safety Evaluation for the FreeStyle Libre FlashGlucose Monitoring System When Used by Pregnant Women with Diabetes. Diabetes Technol. Ther. 2018, 20, 180–188.
- Costa, D.; Lourenço, J.; Monteiro, A.M.; Castro, B.; Oliveira, P.; Tinoco, M.C.; Fernandes, V.; Marques, O.; Gonçalves, R.; Rolanda,C. Clinical Performance of Flash Glucose Monitoring System in Patients with Liver Cirrhosis and Diabetes Mellitus. Sci. Rep.2020, 10, 7460.
- Juan, C.G.; Potelon, B.; Quendo, C.; García-Martínez, H.; Ávila Navarro, E.; Bronchalo, E.; Sabater-Navarro, J.M. Study ofQu-Based Resonant Microwave Sensors and Design of 3-D-Printed Devices Dedicated to Glucose Monitoring. IEEE Trans. Instrum.Meas. 2021, 70, 8005716.
- Pustozerov, E.A.; Tkachuk, A.S.; Vasukova, E.A.; Anopova, A.D.; Kokina, M.A.; Gorelova, I.V.; Pervunina, T.M.; Grineva, E.N.;Popova, P.V. Machine learning approach for postprandial blood glucose prediction in gestational diabetes mellitus. IEEE Access2020, 8, 219308–219321.
- Haque, C.A.; Hossain, S.; Kwon, T.H.; Kim, K.D. non-invasive In Vivo Estimation of Blood-Glucose Concentration by MonteCarlo Simulation. Sensors 2021, 21, 4918, doi:10.3390/s21144918.
- Kandwal, A.; Nie, Z.; Igbe, T.; Li, J.; Liu, Y.; Liu, L.W.; Hao, Y. Surface Plasmonic Feature Microwave Sensor With HighlyConfined Fields for Aqueous-Glucose and Blood-Glucose Measurements. IEEE Trans. Instrum. Meas. 2021, 70, 8000309,doi:10.1109/TIM.2020.3017038.
- Setford, S.; Grady, M.; Phillips, S.; Miller, L.; Mackintosh, S.; Cameron, H.; Corrigall, K. Seven-Year Surveillance of the Clinical Performanceof a Blood Glucose Test Strip Product. J. Diabetes Sci. Technol. 2017, 11, 1932296817703133, doi:10.1177/1932296817703133.
- Apple Plans FasterWatch, Future Temperature and Glucose Sensors. Available online: https://www.bloomberg.com/news/articles/2021-06-14/apple-plans-faster-watch-future-temperature-and-glucose-sensors (accessed on 9 October 2021).
- Maxwell, K.G.; Augsornworawat, P.; Velazco-Cruz, L.; Kim, M.H.; Asada, R.; Hogrebe, N.J.; Morikawa, S.; Urano, F.; Millman, J.R.Gene-edited human stem cell–derived b cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci.Transl. Med. 2020, 12, doi:10.1126/scitranslmed.aax9106.
- Sernova Technology. Available online: https://www.sernova.com/technology//#Indications (accessed on 26 March 2021).
- London, Ont. Company Makes Big Leap Forward in the Fight to Cure Type 1 Diabetes 980 CFPL Omny.fm. Available online:https://omny.fm/shows/am980/london-ont-company-makes-big-leap-forward-in-the-f (accessed on 27 March 2021).




