Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Brian Ceresa | + 2777 word(s) | 2777 | 2022-03-24 10:50:33 | | | |
| 2 | Vivi Li | Meta information modification | 2777 | 2022-03-25 03:50:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ceresa, B. Epidermal Growth Factor Receptor in the Corneal Epithelium. Encyclopedia. Available online: https://encyclopedia.pub/entry/20998 (accessed on 07 February 2026).
Ceresa B. Epidermal Growth Factor Receptor in the Corneal Epithelium. Encyclopedia. Available at: https://encyclopedia.pub/entry/20998. Accessed February 07, 2026.
Ceresa, Brian. "Epidermal Growth Factor Receptor in the Corneal Epithelium" Encyclopedia, https://encyclopedia.pub/entry/20998 (accessed February 07, 2026).
Ceresa, B. (2022, March 24). Epidermal Growth Factor Receptor in the Corneal Epithelium. In Encyclopedia. https://encyclopedia.pub/entry/20998
Ceresa, Brian. "Epidermal Growth Factor Receptor in the Corneal Epithelium." Encyclopedia. Web. 24 March, 2022.
Copy Citation
It has been known for some time that the addition of epidermal growth factor (EGF) promotes the restoration of the corneal epithelium and patients using EGFR inhibitors as anti-cancer therapies are at increased risk of corneal erosions. However, the use of EGF in the clinic has been limited by downregulation of the receptor. More recent advances in EGFR signaling and trafficking in corneal epithelial cells have provided new insights in how to overcome receptor desensitization.
epidermal growth factor receptor (EGFR)
cornea
epithelium
wound healing
homeostasis
1. Cornea Structure and Function
There are two major physiologic roles for the cornea. The first is to refract light onto the retina. Approximately 70% of the refractive power of the eye is from the cornea. In order for this to be done properly, the cornea must remain transparent and pliable. The second role is to serve as a barrier against foreign substances and protect the immune-privileged eye from infectious agents. To mediate these functions, all layers of the cornea must be fully formed.
The cornea is comprised of three cell layers—the epithelium, the stroma, and endothelium. In the human cornea, these layers are separated by the Bowman’s layer [1] and Descemet’s layer [2], respectively (Figure 1A).
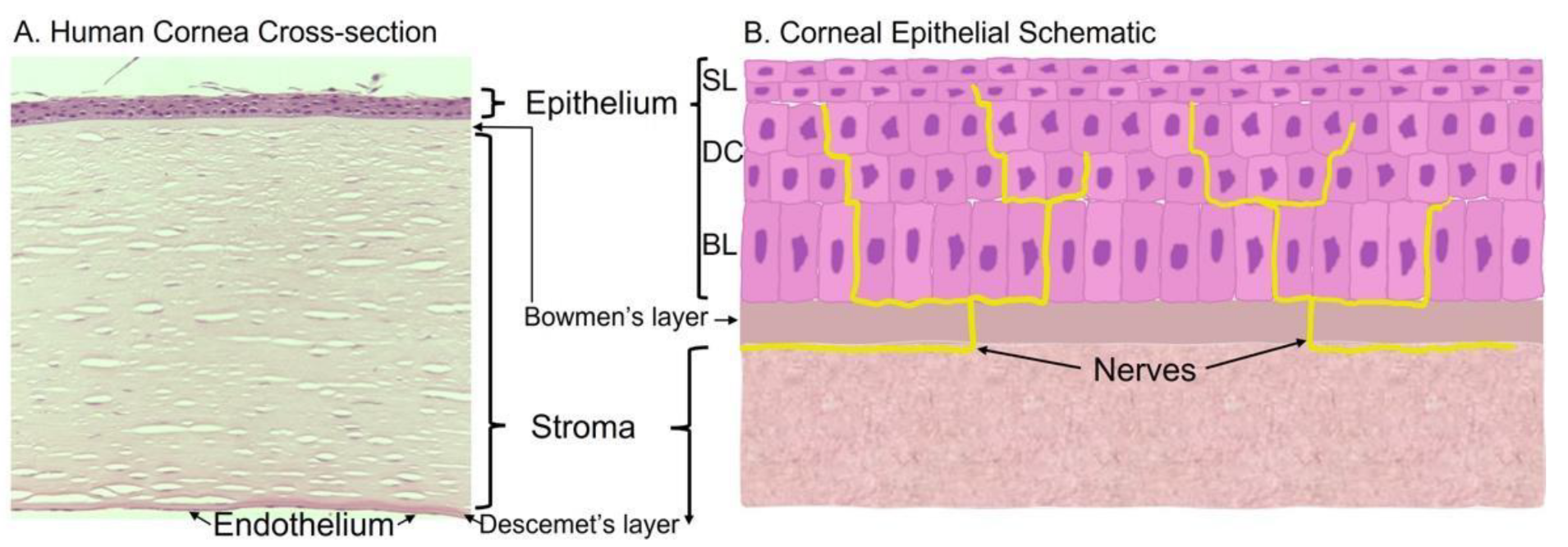
Figure 1. Histology and schematic of the cornea. (A) Human cornea obtained from cadaver donation. Corneal tissue was placed in 4% paraformaldehyde overnight, washed, and embedded in paraffin for histological sectioning and hematoxylin and eosin staining to visualize the various cell layers and membranes. (B) Schematic of the corneal epithelium that highlights the epithelial layers and innervation; BL = basal epithelial cells, DC = differentiated cells, SL = superficial layer.
2. Regulation of the Corneal Epithelial Homeostasis
There are a number of growth factor receptors and cytokine receptors whose signaling contribute to the restoration and homeostasis of the corneal epithelium [3]. This list includes receptors for platelet-derived growth factor [4], hepatocyte growth factor (HGF) [5], colony-stimulating factor 1 [6], keratinocyte growth factor [7], fibroblast growth factor [8][9], transforming growth factor-β2 [10], nerve growth factor [11], interleukin-1 [12], and interleukin-6 [13]. However, the epidermal growth factor receptor (EGFR) stands out because in rodent models, stimulation with epidermal growth factor (EGF) can accelerate corneal epithelial wound healing up to two-fold [14][15] and the addition of EGFR inhibitors (i.e., AG1478) to wounded corneal epithelium in mice prevents wound healing [16].
These experimental data are supported by reports from the clinic that patients taking EGFR inhibitors (i.e., cetuximab, erlotinib, gefitinib) for the treatment of certain cancers have an increased incidence of corneal perturbations, including punctate keratopathy, dry eye, blepharitis, conjunctivitis, and trichiasis [17][18][19][20][21]. These are typically limited to the ocular surface and are readily treatable with topical lubricants and antibiotics. The chief concerns are increased discomfort for an already ill patient and in the most severe cases, the likelihood for infection.
2.1. EGFR Expression in Corneal Epithelial Cells
The fact that corneal epithelial homeostasis is dependent on the EGFR is not entirely surprising. The relative distribution of the EGFR in corneal epithelial cells has been reported previously [15]. Indirect immunofluorescent staining of the EGFR shows the receptor is more concentrated along the plasma membrane in the basal epithelial layers, with a decrease in the intensity of receptor staining as the cells differentiate. In addition, following wounding, the receptor moves from the plasma membrane to cytosolic locations, consistent with ligand-mediated receptor internalization [22].
Immortalized human corneal epithelial (hTCEpi) cells have ~1,100,000 125I-EGF binding sites/cell and binding with negative cooperativity, as seen with the EGFR in other cell lines [23] (Figure 2A). Interestingly, the apparent Kd of 125I-EGF binding is 1.52 nM, with only modest differences between the high- and low-affinity states (1.06 nM and 2.85 nM, respectively). While the Kd for low-affinity binding was in line with reports in other cell lines, the Kd for high-affinity binding sites differs by about 10-fold [23][24][25]. Approximately ~20% of the receptor binding sites in the corneal epithelial cells are high affinity, which is higher than other cell lines (3–14% range) [23][24][26]. While the basis for these disparities is not clear, these differences may reflect cellular changes in receptor methylation [27], the presence of corneal epithelial specific effectors, or perturbations in receptor trafficking [28]. Comprehensive Scatchard analysis has not been performed in primary corneal epithelial cells due to limitations in the availability and the heterogeneity of human tissue. However, EGFR protein and mRNA are comparable in immortalized and primary cells [14][29].
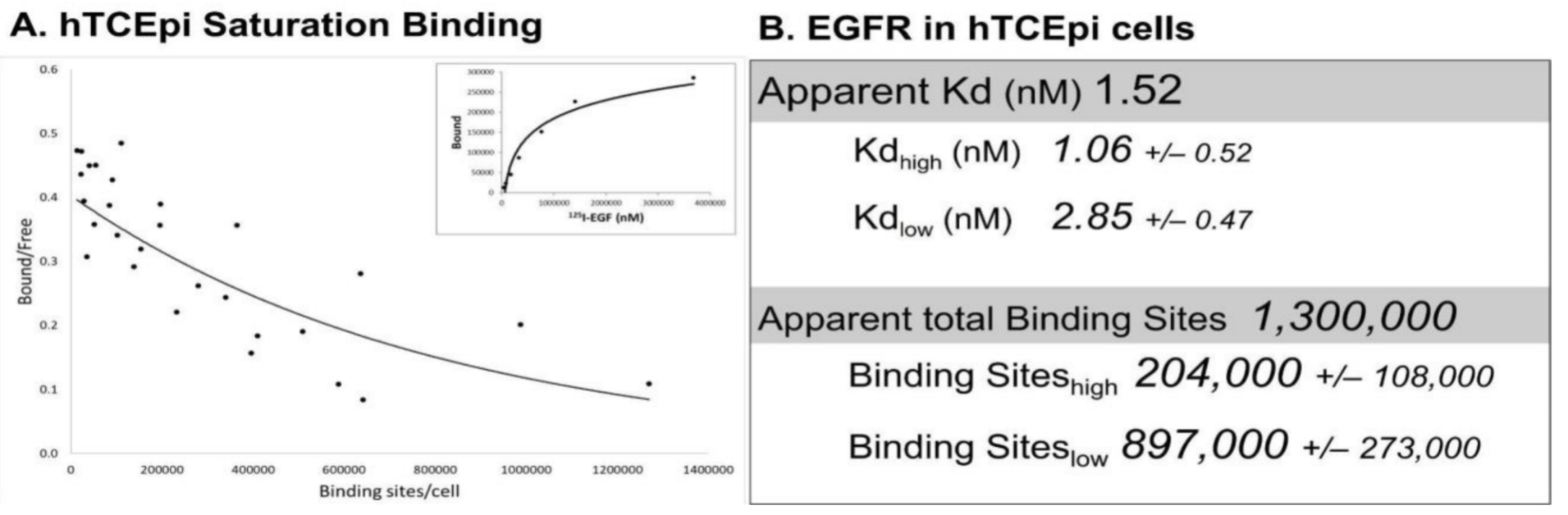
Figure 2. Saturation binding in hTCEpi cells. (A) Saturation binding assays were performed using adherent immortalized corneal epithelial (hTCEpi) cells [30] using 125I-EGF (Perkin Elmer, Waltham, MA, USA), as previously described [31][32]. Shown are cummulative data from 4 independent experiments (6–7 data points/experiment) that were subjected to Scatchard transformation. The concave-up binding curve (solid line) is consistent with two affinity sites. Saturation binding from one representative experiment is shown in the inset. (B) The apparent Kd and total binding were generated from the summative data of all four experiments. High- and low-affinity binding sites were calculated from each experiment with PRISM utilizing a non-linear, two-site binding analysis. Data are presented as the average ± S.E.M. (n = 4).
2.2. Ocular Expression of EGFR Ligands
It is worth noting that no other healthy tissue in the human body has been reported to express EGFRs at this high of a density. However, some cancer-derived cell lines express EGFRs at this density (Table 1). A representative sample of non-cancerous cell lines indicates EGFR densities are in the range of 50,000 to 70,000 receptors/cell. Importantly, those cells have many of the same EGFR-mediated responses seen in corneal epithelial cells and are associated with tissue restoration and homeostasis.
Table 1. EGFR density in selected cell lines.
| Cells | EGFRs/Cell | Reference |
|---|---|---|
| Non-cancerous cell lines | ||
| hTCEpi (human) | 1,300,000 | Figure 2 |
| Oral mucosa | 200,000 | [33] |
| Skin fibroblasts | 51,000–70,000 | [31][34][35] |
| Primary corneal endothelium | 40,000 | [36] |
| Blood cells | 7800–25,400 | [37] |
| Gastric smooth muscle | 24,000 | [38] |
| Cancer cell lines | ||
| A431 cell (epidermoid carcinoma) | 1,500,000–2,600,000 | [34][35][39] |
| MDA-MB-468 cells (mammary gland) | 1,900,000 | [34] |
| Moser-1 (colon cancer) | 295,700 | [40] |
| HepG2 (liver carcinoma) | 180,000 | [41] |
| HT29 (colon cancer) | 120,000 | [40] |
| HeLa (cervical adenocarcinoma) | 43,500 | [42] |
| MCF-7 (mammary epithelial) | 2800–10,000 | [43] |
| KM12SM (colon cancer) | 7000 | [40] |
Complementing the robust level of receptor expression are high basal levels of EGF in tear fluid. Tear fluid consists of a mixture of aqueous solutions and lipids and provides a film over the cornea to maintain hydration and lubrication of the ocular surface. In addition, it flushes away contaminating particles, protects against pathogens, nourishes the cells, and provides necessary electrolytes and metabolites [44]. Tears are produced in the lacrimal cells and the components of the tears are produced by the lacrimal gland as well as the corneal epithelial cells [15][45][46]. There is a constant basal production of tears that can increase in response to stimuli [47].
Several studies of tear fluid from healthy human volunteers (i.e., no known ocular pathologies) show that EGF levels are approximately 2 ng/mL (ranging from 0.7 to 8.4 ng/mL) under normal conditions [14][48][49][50]. It is important to note that this concentration of EGF is close to the reported Kd of the ligand for the receptor (0.32 nM) [47]. Thus, under steady-state conditions, there are sufficient levels of EGF to occupy approximately half of the EGFRs. In healthy human volunteers, the other EGFR ligands, namely transforming growth factor-α (TGFα), heparin-binding epidermal growth factor (HB-EGF), betacellulin (BTC), epiregulin (EPR), and amphiregulin (AR), were much less prevalent. These other ligands were either below the limit of detection or had average concentrations that were more than 20-fold less than their Kd for the EGFR [14].
Increases in other EGFR ligands have been documented using in vitro, ex vivo, and in vivo models of corneal epithelial wounding. For instance, cultured corneal epithelial cells subjected to mechanical wounding have an increase in EGFR-dependent cell migration that is ablated by the addition of anti-HB-EGF antibodies [51]. The addition of the matrix metalloprotease inhibitor, GM 6001, prevents activation of the EGFR, consistent with a block in processing (not production) of the HB-EGF that drives receptor activity [51]. Similar results were observed using ex vivo porcine corneas [46]. Other studies demonstrate that wounded cornea epithelial cells produce high levels of adenosine triphosphate (ATP) (~0.5 μM), which in turn activates its cognate purinergic receptor [52]. This signals the activation of Phospholipase Cβ (PLCβ), which stimulates the appropriate matrix metalloproteinase that converts the pro-HB-EGF to the soluble form [52][53].
In addition, the mRNA of other EGFR ligands is upregulated by trauma to the mouse corneal epithelium. AR, HB-EGF, and TGFα increase following debridement of the corneal epithelium [15]. In addition, EGF mRNA increases ~50% in the lacrimal gland following wounding [45]. In vivo studies indicate that the mRNA levels of hepatocyte growth factor and keratocyte growth factors increase as well, following wounding [45][54]. Further investigation will likely reveal increases in additional growth factors.
3. Physiologic Role of the EGFR and ErbB Family in Corneal Epithelial Homeostasis
3.1. EGFR in the Corneal Epithelium
Given that EGFR activity is necessary and sufficient to drive wound healing and tissue homeostasis, it seems that exogenous EGF would be an excellent therapy for treating corneal wounds. Unfortunately, this is not always the case. The success of EGF treatment is dependent on the cause of the wound. For example, patients with corneal abrasions and epithelial lesions saw marked healing with EGF treatment [55], as did corneal erosions associated with the use of cetuximab (an EGFR inhibitor) to treat cancer [19][20][56] and traumatic corneal ulcers [56]. In other studies, EGF did not promote improvement. Topical EGF does not help patients with herpes simplex dendritic ulcers, bullous keratopathies, stromal keratitis, or penetrating keratoplasty [55][57][58].
Although the exact molecular basis for the discrepancy between the laboratory and the clinic is not fully understood, several hypotheses have been put forth. The first suggests that the laboratory models of corneal wound healing are more sensitive. While some of the nuanced details of the wound healing protocols may change (i.e., wound size, method for epithelial debridement, etc.) a common feature is the use of anesthetics prior to wounding [59]. Studies have shown that the frequently used anesthetic agent, xylazine (an α2 adrenergic agonist), can decrease tear production [60]. This likely reduces the distribution of the endogenous EGF to the cornea surface and the drop in basal ligand distribution increases the response to exogenous EGF.
Alternatively, since in humans EGF concentrations are close to the Kd of the ligand for the receptor, the EGFR response may be limited due to high receptor occupancy and more EGF does not increase the response [14]. In addition, the high levels of EGF in humans may lead to desensitization of the EGFR, making more EGF counterproductive [22][29]. If mice have lower EGF levels in tear fluid, the EGFRs may be primed to be more responsive. Due to the intrinsically low tear volume, murine EGF concentrations have not been determined.
Another possible explanation is there are pharmacokinetic differences in growth factor delivery to the corneal epithelium due to proteases or binding proteins. This would limit the amount of ligand that achieves EGFR binding.
3.2. ErbB Family Members in the Corneal Epithelium
Numerous lines of evidence point to EGF and EGFR being central regulators of corneal epithelial homeostasis. However, the role of closely related ligand and receptor family members cannot be overlooked. ErbB2, ErbB3, and ErbB4 are also members of the ErbB receptor tyrosine kinase family. They are similar in size and structure, but differ in tissue distribution, enzymatic activity, and activating ligands [61].
Both ErbB2 and ErbB3 are expressed in the corneal epithelium with a similar receptor distribution as that reported for EGFR [62]. Although there were some early reports that ErbB4 was expressed in the cornea epithelium [63], a more detailed analysis of mRNA levels or antibody specificity indicate that ErbB4 is not expressed [14] (Figure 3A).
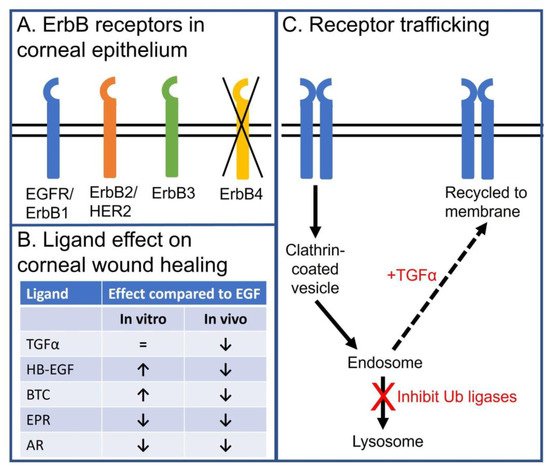
Figure 3. Schema summarizing the role of EGFR and ErbB family members in the corneal epithelium. (A) Schematic depicting expression of EGFR (ErbB1), ErbB2 (Her2), and ErbB3, but not ErbB4, in corneal epithelium. (B) Table summarizing the effects of endogenous EGFR ligand on in vitro and in vivo corneal epithelial wound healing. (C) Schematic depicting how EGFR endocytic trafficking affects receptor signaling in corneal epithelial cells. EGF stimulation promotes internalization of the EGF:EGFR complex to the early endosome. During internalization, the receptor is ubiquitylated and targeted for trafficking to lysosome for degradation. EGFR degradation can be diverted from a degradation pathway by promoting receptor recycling. This can be achieved by stimulating with TGFα, a ligand that dissociates in the mildly acidic environment of the early endosome and has reduced receptor ubiquitylation, or inhibiting the ubiquitin ligases of the EGFR in corneal epithelial cells (i.e., c-Cbl and Cbl-b).
Experiments using cultured, immortalized corneal epithelial cells have demonstrated that ErbB2 and ErbB3 activity contribute to corneal epithelial wound healing and homeostasis. Mechanical perturbation of corneal epithelial cells induces ErbB2 phosphorylation and knockdown of ErbB2 reduces cell migration in scratch and Boyden chamber cell migration assays [64]. Like the EGFR-specific inhibitors, ErbB2 inhibitors (i.e., trastuzumab) have also been associated with ulcerative keratitis [65][66].
The effects of ErbB3 are not as well described. Since ErbB3 is kinase impaired [67], the development of ErbB3 inhibitors has lagged behind those for EGFR and ErbB2. However, ErbB3-specific antibody inhibitors do exist [68]. Using these inhibitors and genetic approaches, it has been shown that ErbB3 signaling can promote corneal epithelial cell migration [69].
3.3. Specific Effects of EGFR Ligands on the Corneal Epithelial Cells
Despite the fact that EGF is the only EGFR ligand detected in the unstimulated tear fluid of healthy volunteers, other naturally occurring ligands are logical candidates for stimulating EGFR-mediated corneal epithelial homeostasis. An in vitro analysis of the other EGFR ligands demonstrates HB-EGF, BTC, and TGF-α can accelerate wound healing as well, if not better, than EGF [14] (Figure 3B).
BTC seemed particularly promising, as in vitro studies indicated BTC was ~20% better than EGF at promoting wound closure [14]. In addition, BTC-treated corneal epithelial cells were more migratory than those treated with EGF, despite lower levels of receptor phosphorylation [69]. The molecular basis for this difference was not due to ligand affinity for the receptor (BTC = 0.5 nM and EGF = 0.3 nM) [70][71], but the dimerization partner. EGF binding promotes the homodimerization of EGFRs. In corneal epithelial cells, BTC binds the EGFR, which biases the receptor to heterodimerize with ErbB3 [69]. In EGFR:ErbB3 heterodimers, the attenuated ErbB3 kinase activity reduces the amount of phosphorylated EGFR, but increases the amount of phosphorylated ErbB3. It is ErbB3’s phosphotyrosines that are thought to recruit and activate the phosphatidyl inositol 3-kinase needed for cell migration.
In addition, TGF-α was thought to be a strong therapeutic candidate. It has been well documented that TGF-α stimulation of the EGFR promotes internalization and recycling of the ligand:receptor complex [72][73]. Instead of promoting receptor downregulation (as is the case following EGF treatment), the TGF-α:EGFR complex recycles back to the plasma where it can be re-activated. Bypassing the degradative pathway makes TGF-α a more efficacious activator of the EGFR. This holds true in immortalized and primary corneal epithelial cells [22].
Despite the predicted enhanced responses from BTC and TGF-α in cell biology assays, this did not translate to in vivo wound healing using murine models (Figure 3B). Although murine models are regarded as excellent models for corneal wound healing [74], researchers cannot discount the major anatomical difference between the mouse and human cornea: mice lack a Bowman’s layer. In most studies, this has not been an issue. However, it is possible that these ligands may elicit a biological response that favors epithelial cell binding to the stroma over the Bowman’s layer. Further, researchers do not know if BTC and TGF-α are stable in the mouse tear fluid. Are there other components of the tear fluid that impact function in these animals? Does the mouse have differing levels of ErbB3 expression that might affect BTC function [69]? Does TGF-α promote EGFR recycling in the mouse cornea epithelial cells the way it does in human corneal epithelial cells? Does the production of tears in the mouse impact delivery of the ligands? In summary, work needs to be carried out to establish whether the differences between in vitro and in vivo analysis are a technical or a biological issue.
3.4. Negative Consequences of Sustained EGFR Activity
Although EGFR activity promotes corneal epithelial cell migration and proliferation, it is important to note that increasing the magnitude and duration of EGFR may not elicit only positive effects. Rodent corneal wound healing experiments indicate that daily application of EGF over the course of 35 days can be deleterious [75]. Topical EGF leads to a three-fold increase in collagen deposition immediately after wounding and EGF administration. However, 21 days post-wounding, the EGF-treated eyes had less collagen than the phosphate-buffered saline control treatment. The bimodal response to EGF treatment is possibly due to downregulation of EGFR signaling due to receptor desensitization. The levels of protease and collagenase activity were unchanged at time points (14–21 days) with reduced wound collagen.
Administration of recombinant EGF as a sustained release pellet in the cornea can lead to neovascularization [76]. In addition, studies have shown that high levels of tear EGF are associated with hypertrophy of the meibomian gland duct and cause meibomian gland hyperplasia [77]. Together, these data highlight the need for caution when treating corneal wounds with exogenous EGF.
References
- Wilson, S.E. Bowman’s layer in the cornea—Structure and function and regeneration. Exp. Eye Res. 2020, 195, 108033.
- De Oliveira, R.C.; Wilson, S.E. Descemet’s membrane development, structure, function and regeneration. Exp. Eye Res. 2020, 197, 108090.
- Yu, F.-S.; Yin, J.; Xu, K.; Huang, J. Growth factors and corneal epithelial wound healing. Brain Res. Bull. 2010, 81, 229–235.
- Hoppenreijs, V.P.; Pels, E.; Vrensen, G.F.; Felten, P.C.; Treffers, W.F. Platelet-derived growth factor: Receptor expression in corneas and effects on corneal cells. Investig. Ophthalmol. Vis. Sci. 1993, 34, 637–649.
- Wilson, S.E.; He, Y.G.; Weng, J.; Zieske, J.D.; Jester, J.V.; Schultz, G.S. Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility and differentiation of human corneal epithelial cells. Exp. Eye Res. 1994, 59, 665–678.
- Rho, C.R.; Park, M.Y.; Kang, S. Effects of granulocyte-macrophage colony-stimulating (GM-CSF) factor on corneal epithelial cells in corneal wound healing model. PLoS ONE 2015, 10, e0138020.
- Chandrasekher, G.; Kakazu, A.H.; Bazan, H.E. HGF- and KGF-induced activation of PI-3K/p70 s6 kinase pathway in corneal epithelial cells: Its relevance in wound healing. Exp. Eye Res. 2001, 73, 191–202.
- Shin, E.; Lim, D.H.; Han, J.; Nam, D.-H.; Park, K.; Ahn, M.-J.; Kang, W.K.; Lee, J.; Ahn, J.S.; Lee, S.-H.; et al. Markedly increased ocular side effect causing severe vision deterioration after chemotherapy using new or investigational epidermal or fibroblast growth factor receptor inhibitors. BMC Ophthalmol. 2020, 20, 19.
- Zhang, J.; Upadhya, D.; Lu, L.; Reneker, L.W. Fibroblast growth factor receptor 2 (FGFR2) is required for corneal epithelial cell proliferation and differentiation during embryonic development. PLoS ONE 2015, 10, e0117089.
- Carrington, L.M.; Albon, J.; Anderson, I.; Kamma, C.; Boulton, M. Differential regulation of key stages in early corneal wound healing by TGF-beta isoforms and their inhibitors. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1886–1894.
- Lambiase, A.; Manni, L.; Bonini, S.; Rama, P.; Micera, A.; Aloe, L. Nerve growth factor promotes corneal healing: Structural, biochemical, and molecular analyses of rat and human corneas. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1063–1069.
- Wilson, S.E.; Esposito, A. Focus on molecules: Interleukin-1: A master regulator of the corneal response to injury. Exp. Eye Res. 2009, 89, 124–125.
- Nishida, T.; Nakamura, M.; Mishima, H.; Otori, T.; Hikida, M. Interleukin 6 facilitates corneal epithelial wound closure in vivo. Arch. Ophthalmol. 1992, 110, 1292–1294.
- Peterson, J.L.; Phelps, E.D.; Doll, M.A.; Schaal, S.; Ceresa, B.P. The role of endogenous epidermal growth factor receptor ligands in mediating corneal epithelial homeostasis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2870–2880.
- Zieske, J.; Takahashi, H.; E Hutcheon, A.; Dalbone, A.C. Activation of epidermal growth factor receptor during corneal epithelial migration. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1346–1355.
- Rush, J.S.; Bingaman, D.P.; Chaney, P.G.; Wax, M.B.; Ceresa, B.P. Administration of menadione, vitamin K3, ameliorates off-target effects on corneal epithelial wound healing due to receptor tyrosine kinase inhibition. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5864–5871.
- Johnson, K.S.; Levin, F.; Chu, D.S. Persistent corneal epithelial defect associated with erlotinib treatment. Cornea 2009, 28, 706–707.
- Morishige, N.; Hatabe, N.; Morita, Y.; Yamada, N.; Kimura, K.; Sonoda, K.-H. Spontaneous healing of corneal perforation after temporary discontinuation of erlotinib treatment. Case Rep. Ophthalmol. 2014, 5, 6–10.
- Foerster, C.G.; Cursiefen, C.; E Kruse, F. Persisting corneal erosion under cetuximab (Erbitux) treatment (epidermal growth factor receptor antibody). Cornea 2008, 27, 612–614.
- Ibrahim, E.; Dean, W.H.; Price, N.; Gomaa, A.; Ayre, G.; Guglani, S.; Sallam, A. Perforating corneal ulceration in a patient with lung metastatic adenocarcinoma treated with gefitinib: A case report. Case Rep. Ophthalmol. Med. 2012, 2012, 379132.
- Kawakami, H.; Sugioka, K.; Yonesaka, K.; Satoh, T.; Shimomura, Y.; Nakagawa, K. Human epidermal growth factor eyedrops for cetuximab-related filamentary keratitis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, e678–e679.
- McClintock, J.L.; Ceresa, B. Transforming growth factor-α (TGF-α) enhances corneal epithelial cell migration by promoting EGFR recycling. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3455–3461.
- Macdonald, J.L.; Pike, L.J. Heterogeneity in EGF-binding affinities arises from negative cooperativity in an aggregating system. Proc. Natl. Acad. Sci. USA 2008, 105, 112–117.
- Lax, I.; Bellot, F.; Howk, R.; Ullrich, A.; Givol, D.; Schlessinger, J. Functional analysis of the ligand binding site of EGF-receptor utilizing chimeric chicken/human receptor molecules. EMBO J. 1989, 8, 421–427.
- Lemmon, M.A. Ligand-induced ErbB receptor dimerization. Exp. Cell Res. 2009, 315, 638–648.
- Pike, L.J. Negative co-operativity in the EGF receptor. Biochem. Soc. Trans. 2012, 40, 15–19.
- Liao, H.W.; Hsu, J.M.; Xia, W.; Wang, H.L.; Wang, Y.N.; Chang, W.C.; Arold, S.T.; Chou, C.K.; Tsou, P.H.; Yamaguchi, H.; et al. PRMT1-mediated methylation of the EGF receptor regulates signaling and cetuximab response. J. Clin. Investig. 2015, 125, 4529–4543.
- Ringerike, T.; Stang, E.; Johannessen, L.E.; Sandnes, D.; Levy, F.O.; Madshus, I.H. High-affinity binding of epidermal growth factor (EGF) to EGF receptor is disrupted by overexpression of mutant dynamin (K44A). J. Biol. Chem. 1998, 273, 16639–16642.
- Rush, J.S.; Boeving, M.A.; Berry, W.L.; Ceresa, B.P. Antagonizing c-Cbl enhances EGFR-dependent corneal epithelial homeostasis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4691–4699.
- Robertson, D.M.; Li, L.; Fisher, S.; Pearce, V.P.; Shay, J.W.; Wright, W.E.; Cavanagh, H.D.; Jester, J.V. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Investig. Ophthalmol. Vis. Sci. 2005, 46, 470–478.
- Carpenter, G.; Lembach, K.J.; Morrison, M.M.; Cohen, S. Characterization of the binding of 125-I-labeled epidermal growth factor to human fibroblasts. J. Biol. Chem. 1975, 250, 4297–4304.
- Limbird, L.E. A Short Course on Receptor Theory; Martinus Nyhoff/Kluwer Academic Publishing: Boston, MA, USA; Leiden, The Netherlands, 1985.
- Charoenrat, P.; Rhys-Evans, P.; Modjtahedi, H.; Court, W.; Box, G.; Eccles, S. Overexpression of epidermal growth factor receptor in human head and neck squamous carcinoma cell lines correlates with matrix metalloproteinase-9 expression and in vitro invasion. Int. J. Cancer 2000, 86, 307–317.
- Filmus, J.; Pollak, M.N.; Cailleau, R.; Buick, R.N. MDA-468, a human breast cancer cell line with a high number of epidermal growth factor (EGF) receptors, has an amplified EGF receptor gene and is growth inhibited by EGF. Biochem. Biophys. Res. Commun. 1985, 128, 898–905.
- Krupp, M.N.; Connolly, D.T.; Lane, M.D. Synthnesis, turnover, and down-regulation of epidermal growth factor receptors in human A431 epidermoid carcinoma cells and skin fibroblasts. J. Biol. Chem. 1982, 257, 11489–11496.
- Fabricant, R.N.; Alpar, A.J.; Centifanto, Y.M.; Kaufman, H.E. Epidermal growth factor receptors on corneal endothelium. Arch. Ophthalmol. 1981, 99, 305–308.
- Engelmann, B.; Schumacher, U.; Haen, E. Epidermal growth factor binding sites on human erythrocytes in donors with different ABO blood groups. Am. J. Hematol. 1992, 39, 239–241.
- Yang, S.G.; Hollenberg, M.D. Distinct receptors for epidermal growth factor-urogastrone in cultured gastric smooth muscle cells. Am. J. Physiol. Content 1991, 260, G827–G834.
- Haigler, H.; Ash, J.F.; Singer, S.J.; Cohen, S. Visualization by fluorescence of the binding and internalization of epidermal growth factor in human carcinoma cells A-431. Proc. Natl. Acad. Sci. USA 1978, 75, 3317–3321.
- Rajagopal, S.; Huang, S.; Moskal, T.L.; Lee, B.-N.; el-Naggar, A.K.; Chakrabarty, S. Epidermal growth factor expression in human colon and colon carcinomas: Anti-sense epidermal growth factor receptor RNA down-regulates the proliferation of human colon cancer cells. Int. J. Cancer 1995, 62, 661–667.
- Yang, E.B.; Wang, D.F.; Mack, P.; Cheng, L.Y. EGF receptor in Chang liver and hepatoma HepG2 cells. Biochem. Mol. Boil. Int. 1996, 38, 813–820.
- Lee, L.S.; Weinstein, I.B. Tumor-promoting phorbol esters inhibit binding of epidermal growth factor to cellular receptors. Science 1978, 202, 313–315.
- Imai, Y.; Leung, C.K.; Friesen, H.G.; Shiu, R.P. Epidermal growth factor receptors and effect of epidermal growth factor on growth of human breast cancer cells in long-term tissue culture. Cancer Res. 1982, 42, 4394–4398.
- De Souza, G.A.; Godoy, L.M.; Mann, M. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol. 2006, 7, R72.
- Wilson, S.E.; Liang, Q.; Kim, W.J. Lacrimal gland HGF, KGF, and EGF mRNA levels increase after corneal epithelial wounding. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2185–2190.
- Xu, K.-P.; Ding, Y.; Ling, J.; Dong, Z.; Yu, F.-S.X. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Investig. Opthalmology Vis. Sci. 2004, 45, 813–820.
- Wu, Z.; Begley, C.G.; Port, N.; Bradley, A.; Braun, R.; King-Smith, E. The effects of increasing ocular surface stimulation on blinking and tear secretion. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4211–4220.
- Ohashi, Y.; Motokura, M.; Kinoshita, Y.; Mano, T.; Watanabe, H.; Kinoshita, S.; Manabe, R.; Oshiden, K.; Yanaihara, C. Presence of epidermal growth factor in human tears. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1879–1882.
- Van Setten, G.B.; Schultz, G.S.; Macauley, S. Growth factors in human tear fluid and in lacrimal glands. Adv. Exp. Med. Biol. 1994, 350, 315–319.
- Van Setten, G.B.; Tervo, K.; Virtanen, I.; Tarkkanen, A.; Tervo, T. Immunohistochemical demonstration of epidermal growth factor in the lacrimal and submandibular glands of rats. Acta Ophthalmol. 1990, 68, 477–480.
- Block, E.R.; Matela, A.R.; SundarRaj, N.; Iszkula, E.R.; Klarlund, J.K. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints. J. Biol. Chem. 2004, 279, 36166.
- Boucher, I.; Yang, L.; Mayo, C.; Klepeis, V.; Trinkaus-Randall, V. Injury and nucleotides induce phosphorylation of epidermal growth factor receptor: MMP and HB-EGF dependent pathway. Exp. Eye Res. 2007, 85, 130–141.
- Block, E.R.; Klarlund, J.K. Wounding sheets of epithelial cells activates the epidermal growth factor receptor through distinct short- and long-range mechanisms. Mol. Biol. Cell 2008, 19, 4909–4917.
- Wilson, S.E.; Chen, L.; Mohanab, R.R.; Liang, Q.; Liu, J. Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp. Eye Res. 1999, 68, 377–397.
- Daniele, S.; Frati, L.; Fiore, C.; Santoni, G. The effect of the epidermal growth factor (EGF) on the corneal epithelium in humans. Albrecht Graefes Arch. Klin. Exp. Ophthalmol. 1979, 210, 159–165.
- Scardovi, C.; De Felice, G.; Gazzaniga, A. Epidermal growth factor in the topical treatment of traumatic corneal ulcers. Ophthalmologica 1993, 206, 119–124.
- Dellaert, M.M.M.J.; A Casey, T.; Wiffen, S.; Gordon, J.; Johnson, P.; Geerards, A.J.M.; Rijneveld, W.J.; Remeijer, L.; Mulder, P.G.H.; Beekhuis, W.H. Influence of topical human epidermal growth factor on postkeratoplasty re-epithelialisation. Br. J. Ophthalmol. 1997, 81, 391–395.
- Kandarakis, A.S.; Page, C.; Kaufman, H.E. The effect of epidermal growth factor on epithelial healing after penetrating keratoplasty in human eyes. Am. J. Ophthalmol. 1984, 98, 411–415.
- Koehn, D.; Meyer, K.J.; Syed, N.A.; Anderson, M.G. Ketamine/xylazine-induced corneal damage in mice. PLoS ONE 2015, 10, e0132804.
- Ghaffari, M.S.; Malmasi, A.; Bokaie, S. Effect of acepromazine or xylazine on tear production as measured by Schirmer tear test in normal cats. Vet. Ophthalmol. 2010, 13, 1–3.
- Ceresa, B.P.; Peterson, J.L. Cell and molecular biology of epidermal growth factor receptor. Int. Rev. Cell Mol. Biol. 2014, 313, 145–178.
- Liu, Z.; Carvajal, M.; Carraway, C.A.C.; Carraway, K.; Pflugfelder, S.C. Expression of the receptor tyrosine kinases, epidermal growth factor receptor, ErbB2, and ErbB3, in human ocular surface epithelia. Cornea 2001, 20, 81–85.
- Swan, J.S.; Arango, M.E.; Carothers-Carraway, C.A.C.; Carraway, K.L. An ErbB-2-Muc4 complex in rat ocular surface epithelia. Curr. Eye Res. 2002, 24, 397–402.
- Xu, K.-P.; Riggs, A.; Ding, Y.; Yu, F.-S.X. Role of ErbB2 in corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4277–4283.
- Kreps, E.O.; Derveaux, T.; Denys, H. Corneal changes in trastuzumab emtansine treatment. Clin. Breast Cancer 2018, 18, e427–e429.
- Orlandi, A.; Fasciani, R.; Cassano, A.; Agresta, A.; Calegari, M.A.; Caporossi, A.; Barone, C. Trastuzumab-induced corneal ulceration: Successful no-drug treatment of a “blind” side effect in a case report. BMC Cancer 2015, 15, 973.
- Steinkamp, M.P.; Low-Nam, S.T.; Yang, S.; Lidke, K.A.; Lidke, D.S.; Wilson, B.S. erbB3 is an active tyrosine kinase capable of homo- and heterointeractions. Mol. Cell. Biol. 2014, 34, 965–977.
- Huang, J.; Wang, S.; Lyu, H.; Cai, B.; Yang, X.; Wang, J.; Liu, B. The anti-erbB3 antibody MM-121/SAR256212 in combination with trastuzumab exerts potent antitumor activity against trastuzumab-resistant breast cancer cells. Mol. Cancer 2013, 12, 134.
- Rush, J.S.; Peterson, J.L.; Ceresa, B.P. Betacellulin (BTC) biases the EGFR to dimerize with ErbB3. Mol. Pharmacol. 2018, 94, 1382–1390.
- Schlessinger, J. Allosteric regulation of the epidermal growth factor receptor kinase. Cell Biol. 1986, 103, 2067–2072.
- Watanabe, T.; Shintani, A.; Nakata, M.; Shing, Y.; Folkman, J.; Igarashi, K.; Sasada, R. Recombinant human betacellulin. Molecular structure, biological activities, and receptor interaction. J. Biol. Chem. 1994, 269, 9966–9973.
- Korc, M.; Magun, B.E. Recycling of epidermal growth factor in human pancreatic carcinoma cell line. Proc. Natl. Acad. Sci. USA 1985, 82, 6172–6175.
- Roepstorff, K.; Grandal, M.V.; Henriksen, L.; Knudsen, S.L.J.; Lerdrup, M.; Grøvdal, L.; Willumsen, B.M.; Van Deurs, B. Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic 2009, 10, 1115–1127.
- Stepp, M.A.; Zieske, J.; Trinkaus-Randall, V.; Kyne, B.M.; Pal-Ghosh, S.; Tadvalkar, G.; Pajoohesh-Ganji, A. Wounding the cornea to learn how it heals. Exp. Eye Res. 2014, 121, 178–193.
- Hennessey, P.J.; Nirgiotis, J.G.; Shinn, M.N.; Andrassy, R.J. Continuous EGF application impairs long-term collagen accumulation during wound-healing in rats. J. Pediatr. Surg. 1991, 26, 362–366.
- Nezu, E.; Ohashi, Y.; Kinoshita, S.; Manabe, R. Recombinant human epidermal growth-factor and corneal neovascularization. Jpn. J. Ophthalmol. 1992, 36, 401–406.
- Rao, K.; Farley, W.J.; Pflugfelder, S.C. Association between high tear epidermal growth factor levels and corneal subepithelial fibrosis in dry eye conditions. Investig. Opthalmology Vis. Sci. 2010, 51, 844–849.
More
Information
Subjects:
Ophthalmology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
25 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




