
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Guomin Shen | + 4008 word(s) | 4008 | 2022-03-07 08:51:46 | | | |
| 2 | Bruce Ren | -24 word(s) | 3984 | 2022-03-25 03:37:59 | | | | |
| 3 | Bruce Ren | Meta information modification | 3984 | 2022-03-25 03:43:55 | | |
Video Upload Options
Hemophilia is an X-linked recessive bleeding disorder caused by a deficiency of coagulation factor VIII (FVIII) or factor IX (FIX), which are named hemophilia A (OMIM#306700) and hemophilia B (OMIM#306900), respectively. Coagulation factor IX (FIX) is a vitamin K dependent protein and its deficiency causes hemophilia B, an X-linked recessive bleeding disorder. More than 1000 mutations in the F9 gene have been identified in hemophilia B patients. Hemophilia is an X-linked recessive bleeding disorder caused by a deficiency of coagulation factor VIII (FVIII) or factor IX (FIX), which are named hemophilia A (OMIM#306700) and hemophilia B (OMIM#306900), respectively. Because the genes of FVIII and FIX are located in chromosome X, hemophilia has historically been considered as a “male disease".
1. Introduction
2. The Gene of FIX

3. Mechanisms of FIX Deficiency
| Regions | MTs | UMs | % of UMs | PN | % of PN | |
|---|---|---|---|---|---|---|
| Noncoding | Promoter * | Point | 23 | 2.10 | 86 | 2.32 |
| Deletion | 2 | 0.18 | 2 | 0.05 | ||
| Polymorphism | 5 | 0.46 | 5 | 0.13 | ||
| Intron | Point | 86 | 7.86 | 226 | 6.09 | |
| Deletion | 14 | 1.28 | 23 | 0.62 | ||
| Insertion | 2 | 0.18 | 8 | 0.22 | ||
| Indel | 1 | 0.09 | 1 | 0.03 | ||
| Polymorphism | 33 | 3.02 | 33 | 0.89 | ||
| 3′ UTR | Point | 2 | 0.18 | 22 | 0.59 | |
| Duplication | 1 | 0.09 | 1 | 0.03 | ||
| Polymorphism | 2 | 0.18 | 3 | 0.08 | ||
| Coding | Point | 689 | 62.98 | 2937 | 79.10 | |
| Deletion | 145 | 13.25 | 204 | 5.49 | ||
| Insertion | 33 | 3.02 | 38 | 1.02 | ||
| Indel | 15 | 1.37 | 17 | 0.46 | ||
| Duplication | 4 | 0.37 | 4 | 0.11 | ||
| Polymorphism | 11 | 1.01 | 18 | 0.48 | ||
| Multiple Regions | Deletion | 19 | 1.75 | 78 | 2.10 | |
| Insertion | 1 | 0.09 | 1 | 0.03 | ||
| Indel | 1 | 0.09 | 1 | 0.03 | ||
| Complex | 5 | 0.46 | 5 | 0.13 | ||
| Grand total | 1094 | 100 | 3713 | 100 | ||
| Regions | Mutation Effects | Unique Mutations | Patient Number | % of Total Patients |
|---|---|---|---|---|
| Promoter | Leyden/NA | 23 | 86 | 2.32 |
| Exons | Missense | 586 | 2422 | 65.23 |
| Nonsense | 87 | 469 | 12.63 | |
| Silent | 16 | 46 | 1.24 | |
| Introns | Splice | 86 | 226 | 6.09 |
| 3′ UTR | NA | 2 | 22 | 0.59 |
| Grand total | 800 | 3271 | 88.1 |
3.1. Point Mutations in Noncoding Region
3.1.1. Mutations in F9 Promoter
3.1.2. Mutations in Introns
3.1.3. Mutations in 3′UTR
3.2. Point Mutations in Coding Region
3.2.1. Silent Mutations
3.2.2. Nonsense Mutations and Ribosome Readthrough
3.2.3. Missense Mutations in Signal Peptide and Propeptide

3.2.4. Missense Mutations in Gla Domain
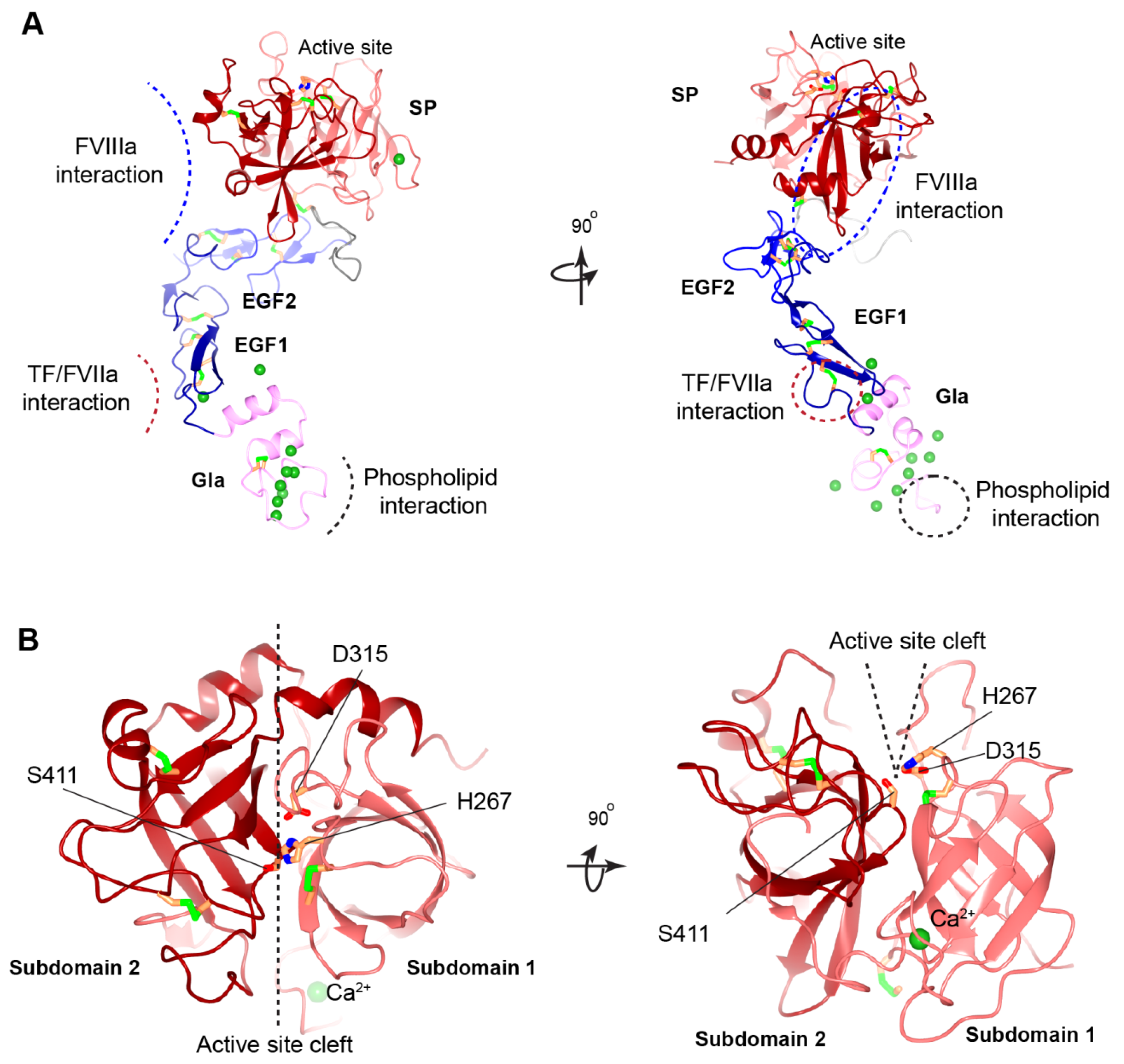
3.2.5. Missense Mutations in EGF1 and EGF2 Domains
3.2.6. Missense Mutations at Cleavage Site of Activation Peptide
3.2.7. Missense Mutations in SP Domain
References
- Staber, J.; Croteau, S.E.; Davis, J.; Grabowski, E.F.; Kouides, P.; Sidonio, R.F., Jr. The spectrum of bleeding in women and girls with haemophilia B. Haemophilia 2017, 24, 180–185.
- Biggs, R.; Douglas, A.S.; Macfarlane, R.G.; Dacie, J.V.; Pitney, W.R.; Merskey. Christmas disease: A condition previously mistaken for haemophilia. Br. Med. J. 1952, 2, 1378–1382.
- Sidonio, R.F., Jr.; Malec, L. Hemophilia B (Factor IX Deficiency). Hematol. Oncol. Clin. N. Am. 2021, 35, 1143–1155.
- Peyvandi, F.; Garagiola, I.; Young, G. The past and future of haemophilia: Diagnosis, treatments, and its complications. Lancet 2016, 388, 187–197.
- White, G.C., 2nd; Rosendaal, F.; Aledort, L.M.; Lusher, J.M.; Rothschild, C.; Ingerslev, J. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb. Haemost. 2001, 85, 560.
- Stafford, D.W. Extravascular FIX and coagulation. Thromb. J. 2016, 14, 35.
- Castaman, G.; Matino, D. Hemophilia A and B: Molecular and clinical similarities and differences. Haematologica 2019, 104, 1702–1709.
- Gao, W.; Xu, Y.; Liu, H.; Gao, M.; Cao, Q.; Wang, Y.; Cui, L.; Huang, R.; Shen, Y.; Li, S.; et al. Characterization of missense mutations in the signal peptide and propeptide of FIX in hemophilia B by a cell-based assay. Blood Adv. 2020, 4, 3659–3667.
- Goodeve, A.C. Hemophilia B: Molecular pathogenesis and mutation analysis. J. Thromb. Haemost. 2015, 13, 1184–1195.
- Choo, K.H.; Gould, K.G.; Rees, D.J.; Brownlee, G.G. Molecular cloning of the gene for human anti-haemophilic factor IX. Nature 1982, 299, 178–180.
- Kurachi, K.; Davie, E.W. Isolation and characterization of a cDNA coding for human factor IX. Proc. Natl. Acad. Sci. USA 1982, 79, 6461–6464.
- Chance, P.F.; Dyer, K.A.; Kurachi, K.; Yoshitake, S.; Ropers, H.H.; Wieacker, P.; Gartler, S.M. Regional localization of the human factor IX gene by molecular hybridization. Hum. Genet. 1983, 65, 207–208.
- Miller, C.H. The Clinical Genetics of Hemophilia B (Factor IX Deficiency). Appl. Clin. Genet. 2021, 14, 445–454.
- Yoshitake, S.; Schach, B.G.; Foster, D.C.; Davie, E.W.; Kurachi, K. Nucleotide sequence of the gene for human factor IX (antihemophilic factor B). Biochemistry 1985, 24, 3736–3750.
- Lillicrap, D. The molecular basis of haemophilia B. Haemophilia 1998, 4, 350–357.
- Rallapalli, P.M.; Kemball-Cook, G.; Tuddenham, E.G.; Gomez, K.; Perkins, S.J. An interactive mutation database for human coagulation factor IX provides novel insights into the phenotypes and genetics of hemophilia B. J. Thromb. Haemost. 2013, 11, 1329–1340.
- Harris, V.A.; Lin, W.; Perkins, S.J. Analysis of 272 Genetic Variants in the Upgraded Interactive FXI Web Database Reveals New Insights into FXI Deficiency. TH Open 2021, 5, e543–e556.
- Veltkamp, J.J.; Meilof, J.; Remmelts, H.G.; van der Vlerk, D.; Loeliger, E.A. Another genetic variant of haemophilia B: Haemophilia B Leyden. Scand. J. Haematol. 1970, 7, 82–90.
- Odaira, K.; Tamura, S.; Suzuki, N.; Kakihara, M.; Hattori, Y.; Tokoro, M.; Suzuki, S.; Takagi, A.; Katsumi, A.; Hayakawa, F.; et al. Apparent synonymous mutation F9 c.87A>G causes secretion failure by in-frame mutation with aberrant splicing. Thromb. Res. 2019, 179, 95–103.
- Green, P.M.; Bentley, D.R.; Mibashan, R.S.; Nilsson, I.M.; Giannelli, F. Molecular pathology of haemophilia B. EMBO J. 1989, 8, 1067–1072.
- Melendez-Aranda, L.; Jaloma-Cruz, A.R.; Pastor, N.; Romero-Prado, M.M.J. In silico analysis of missense mutations in exons 1-5 of the F9 gene that cause hemophilia B. BMC Bioinform. 2019, 20, 363.
- Meireles, M.R.; Bragatte, M.A.S.; Bandinelli, E.; Salzano, F.M.; Vieira, G.F. A new in silico approach to investigate molecular aspects of factor IX missense causative mutations and their impact on the hemophilia B severity. Hum. Mutat. 2019, 40, 706–715.
- Ahmed, S.Z.; O’Rourke, M.; Jenkins, V.; Regan, I.; Nolan, B. Progressive increase in FIX level in males with haemophilia B Leyden and c.35G > A mutation in early childhood not related to androgen effect. Br. J. Haematol. 2020, 189, e262–e265.
- Crossley, M.; Winship, P.R.; Austen, D.E.; Rizza, C.R.; Brownlee, G.G. A less severe form of Haemophilia B Leyden. Nucleic Acids Res. 1990, 18, 4633.
- Crossley, M.; Ludwig, M.; Stowell, K.M.; De Vos, P.; Olek, K.; Brownlee, G.G. Recovery from hemophilia B Leyden: An androgen-responsive element in the factor IX promoter. Science 1992, 257, 377–379.
- Funnell, A.P.; Crossley, M. Hemophilia B Leyden and once mysterious cis-regulatory mutations. Trends Genet. 2014, 30, 18–23.
- Funnell, A.P.; Wilson, M.D.; Ballester, B.; Mak, K.S.; Burdach, J.; Magan, N.; Pearson, R.C.; Lemaigre, F.P.; Stowell, K.M.; Odom, D.T.; et al. A CpG mutational hotspot in a ONECUT binding site accounts for the prevalent variant of hemophilia B Leyden. Am. J. Hum. Genet. 2013, 92, 460–467.
- Kurachi, S.; Huo, J.S.; Ameri, A.; Zhang, K.; Yoshizawa, A.C.; Kurachi, K. An age-related homeostasis mechanism is essential for spontaneous amelioration of hemophilia B Leyden. Proc. Natl. Acad. Sci. USA 2009, 106, 7921–7926.
- Heit, J.A.; Ketterling, R.P.; Zapata, R.E.; Ordonez, S.M.; Kasper, C.K.; Sommer, S.S. Haemophilia B Brandenberg-type promoter mutation. Haemophilia 1999, 5, 73–75.
- Vielhaber, E.; Jacobson, D.P.; Ketterling, R.P.; Liu, J.Z.; Sommer, S.S. A mutation in the 3′ untranslated region of the factor IX gene in four families with hemophilia B. Hum. Mol. Genet. 1993, 2, 1309–1310.
- Sauna, Z.E.; Kimchi-Sarfaty, C. Understanding the contribution of synonymous mutations to human disease. Nat. Rev. Genet. 2011, 12, 683–691.
- Knobe, K.E.; Sjorin, E.; Ljung, R.C. Why does the mutation G17736A/Val107Val (silent) in the F9 gene cause mild haemophilia B in five Swedish families? Haemophilia 2008, 14, 723–728.
- Simhadri, V.L.; Hamasaki-Katagiri, N.; Lin, B.C.; Hunt, R.; Jha, S.; Tseng, S.C.; Wu, A.; Bentley, A.A.; Zichel, R.; Lu, Q.; et al. Single synonymous mutation in factor IX alters protein properties and underlies haemophilia B. J. Med. Genet. 2017, 54, 338–345.
- Pinotti, M.; Caruso, P.; Canella, A.; Campioni, M.; Tagariello, G.; Castaman, G.; Giacomelli, S.; Belvini, D.; Bernardi, F. Ribosome readthrough accounts for secreted full-length factor IX in hemophilia B patients with nonsense mutations. Hum. Mutat. 2012, 33, 1373–1376.
- Branchini, A.; Ferrarese, M.; Campioni, M.; Castaman, G.; Mari, R.; Bernardi, F.; Pinotti, M. Specific factor IX mRNA and protein features favor drug-induced readthrough over recurrent nonsense mutations. Blood 2017, 129, 2303–2307.
- Hao, Z.; Jin, D.Y.; Stafford, D.W.; Tie, J.K. Vitamin K-dependent carboxylation of coagulation factors: Insights from a cell-based functional study. Haematologica 2020, 105, 2164–2173.
- Bentley, A.K.; Rees, D.J.; Rizza, C.; Brownlee, G.G. Defective propeptide processing of blood clotting factor IX caused by mutation of arginine to glutamine at position-4. Cell 1986, 45, 343–348.
- Ware, J.; Diuguid, D.L.; Liebman, H.A.; Rabiet, M.J.; Kasper, C.K.; Furie, B.C.; Furie, B.; Stafford, D.W. Factor IX San Dimas. Substitution of glutamine for Arg-4 in the propeptide leads to incomplete gamma-carboxylation and altered phospholipid binding properties. J. Biol. Chem. 1989, 264, 11401–11406.
- Huang, L.; Li, L.; Lin, S.; Chen, J.; Li, K.; Fan, D.; Jin, W.; Li, Y.; Yang, X.; Xiong, Y.; et al. Molecular analysis of 76 Chinese hemophilia B pedigrees and the identification of 10 novel mutations. Mol. Genet. Genom. Med. 2020, 8, e1482.
- Kirchhofer, D.; Lipari, M.T.; Moran, P.; Eigenbrot, C.; Kelley, R.F. The tissue factor region that interacts with substrates factor IX and Factor, X. Biochemistry 2000, 39, 7380–7387.
- Venkateswarlu, D. Structural insights into the interaction of blood coagulation co-factor VIIIa with factor IXa: A computational protein-protein docking and molecular dynamics refinement study. Biochem. Biophys. Res. Commun. 2014, 452, 408–414.
- Huang, M.; Furie, B.C.; Furie, B. Crystal structure of the calcium-stabilized human factor IX Gla domain bound to a conformation-specific anti-factor IX antibody. J. Biol. Chem. 2004, 279, 14338–14346.
- Huang, M.; Rigby, A.C.; Morelli, X.; Grant, M.A.; Huang, G.; Furie, B.; Seaton, B.; Furie, B.C. Structural basis of membrane binding by Gla domains of vitamin K-dependent proteins. Nat. Struct. Biol. 2003, 10, 751–756.
- Mann, D.M.; Stafford, K.A.; Poon, M.C.; Matino, D.; Stafford, D.W. The Function of extravascular coagulation factor IX in haemostasis. Haemophilia 2021, 27, 332–339.
- Cooley, B.; Broze, G.J., Jr.; Mann, D.M.; Lin, F.C.; Pedersen, L.G.; Stafford, D.W. Dysfunctional endogenous FIX impairs prophylaxis in a mouse hemophilia B model. Blood 2019, 133, 2445–2451.
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589.
- Rao, Z.; Handford, P.; Mayhew, M.; Knott, V.; Brownlee, G.G.; Stuart, D. The structure of a Ca(2+)-binding epidermal growth factor-like domain: Its role in protein-protein interactions. Cell 1995, 82, 131–141.
- Zogg, T.; Brandstetter, H. Structural basis of the cofactor- and substrate-assisted activation of human coagulation factor IXa. Structure 2009, 17, 1669–1678.
- Brandstetter, H.; Bauer, M.; Huber, R.; Lollar, P.; Bode, W. X-ray structure of clotting factor IXa: Active site and module structure related to Xase activity and hemophilia B. Proc. Natl. Acad. Sci. USA 1995, 92, 9796–9800.
- McNicholas, S.; Potterton, E.; Wilson, K.S.; Noble, M.E.M. Presenting your structures: The CCP4mg molecular-graphics software. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 386–394.
- Quadros, L.; Ghosh, K.; Shetty, S. Novel mutations in factor IX gene from western India with reference to their phenotypic and haplotypic attributes. J. Pediatr. Hematol. Oncol. 2009, 31, 157–160.
- Liu, J.Z.; Li, X.; Drost, J.; Thorland, E.C.; Liu, Q.; Lind, T.; Roberts, S.; Wang, H.Y.; Sommer, S.S. The human factor IX gene as germline mutagen test: Samples from Mainland China have the putatively endogenous pattern of mutation. Hum. Mutat. 2000, 16, 31–36.
- Schmidt, A.E.; Bajaj, S.P. Structure-function relationships in factor IX and factor IXa. Trends Cardiovasc. Med. 2003, 13, 39–45.
- Wu, P.C.; Hamaguchi, N.; Yu, Y.S.; Shen, M.C.; Lin, S.W. Hemophilia B with mutations at glycine-48 of factor IX exhibited delayed activation by the factor VIIa-tissue factor complex. Thromb. Haemost. 2000, 84, 626–634.
- Branchini, A.; Morfini, M.; Lunghi, B.; Belvini, D.; Radossi, P.; Bury, L.; Serino, M.L.; Giordano, P.; Cultrera, D.; Molinari, A.C.; et al. F9 missense mutations impairing factor IX activation are associated with pleiotropic plasma phenotypes. J. Thromb. Haemost. 2021, 20, 69–81.
- Zogg, T.; Brandstetter, H. Activation mechanisms of coagulation factor IX. Biol. Chem. 2009, 390, 391–400.
- Lenting, P.J.; ter Maat, H.; Clijsters, P.P.; Donath, M.J.; van Mourik, J.A.; Mertens, K. Cleavage at arginine 145 in human blood coagulation factor IX converts the zymogen into a factor VIII binding enzyme. J. Biol. Chem. 1995, 270, 14884–14890.
- Gailani, D.; Geng, Y.; Verhamme, I.; Sun, M.F.; Bajaj, S.P.; Messer, A.; Emsley, J. The mechanism underlying activation of factor IX by factor XIa. Thromb. Res. 2014, 133 (Suppl. 1), S48–S51.
- Diuguid, D.L.; Rabiet, M.J.; Furie, B.C.; Furie, B. Molecular defects of factor IX Chicago-2 (Arg 145----His) and prothrombin Madrid (Arg 271----cys): Arginine mutations that preclude zymogen activation. Blood 1989, 74, 193–200.
- Liddell, M.B.; Peake, I.R.; Taylor, S.A.; Lillicrap, D.P.; Giddings, J.C.; Bloom, A.L. Factor IX Cardiff: A variant factor IX protein that shows abnormal activation is caused by an arginine to cysteine substitution at position 145. Br. J. Haematol. 1989, 72, 556–560.
- Belvini, D.; Salviato, R.; Radossi, P.; Pierobon, F.; Mori, P.; Castaldo, G.; Tagariello, G. Molecular genotyping of the Italian cohort of patients with hemophilia B. Haematologica 2005, 90, 635–642.
- Huang, M.N.; Kasper, C.K.; Roberts, H.R.; Stafford, D.W.; High, K.A. Molecular defect in factor IXHilo, a hemophilia Bm variant: Arg----Gln at the carboxyterminal cleavage site of the activation peptide. Blood 1989, 73, 718–721.
- Usharani, P.; Warn-Cramer, B.J.; Kasper, C.K.; Bajaj, S.P. Characterization of three abnormal factor IX variants (Bm Lake Elsinore, Long Beach, and Los Angeles) of hemophilia-B. Evidence for defects affecting the latent catalytic site. J. Clin. Investig. 1985, 75, 76–83.
- Monroe, D.M.; McCord, D.M.; Huang, M.N.; High, K.A.; Lundblad, R.L.; Kasper, C.K.; Roberts, H.R. Functional consequences of an arginine180 to glutamine mutation in factor IX Hilo. Blood 1989, 73, 1540–1544.
- Radic, C.P.; Rossetti, L.C.; Abelleyro, M.M.; Candela, M.; Perez Bianco, R.; de Tezanos Pinto, M.; Larripa, I.B.; Goodeve, A.; De Brasi, C. Assessment of the F9 genotype-specific FIX inhibitor risks and characterisation of 10 novel severe F9 defects in the first molecular series of Argentinian patients with haemophilia B. Thromb. Haemost. 2013, 109, 24–33.
- Yu, T.; Dai, J.; Liu, H.; Ding, Q.; Lu, Y.; Wang, H.; Wang, X.; Fu, Q. Spectrum of F9 mutations in Chinese haemophilia B patients: Identification of 20 novel mutations. Pathology 2012, 44, 342–347.
- Weinmann, A.F.; Murphy, M.E.; Thompson, A.R. Consequences of factor IX mutations in 26 families with haemophilia B. Br. J. Haematol. 1998, 100, 58–61.
- Bajaj, S.P. Region of factor IXa protease domain that interacts with factor VIIIa: Analysis of select hemophilia B mutants. Thromb. Haemost. 1999, 82, 218–225.
- Mathur, A.; Zhong, D.; Sabharwal, A.K.; Smith, K.J.; Bajaj, S.P. Interaction of factor IXa with factor VIIIa. Effects of protease domain Ca2+ binding site, proteolysis in the autolysis loop, phospholipid, and factor X. J. Biol. Chem. 1997, 272, 23418–23426.




