
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Katarzyna Szwaczko | + 2467 word(s) | 2467 | 2022-02-25 07:33:43 | | | |
| 2 | Lindsay Dong | -1 word(s) | 2466 | 2022-03-23 03:20:32 | | |
Video Upload Options
Coumarin compounds are attractive organic compounds with many practical applications. Among them there are compounds with biological activity, pharmaceuticals, agrochemicals, dyes, and optoelectronic materials. For this reason, enormous and continuous attempts were made to develop new synthetic pathways and protocols to facilitate the key cyclization reaction of heterocyclic ring and its regioselective functionalization. Of the numerous proposed reactions for the preparation of coumarins, those based on transition metal catalysts have been frequently used recently. Such processes as intramolecular and intermolecular hydroarylation of alkenes or alkynes can be mentioned among the most effective reactions proceeding via the activation of the C–H bond. Knowledge about the mechanistic foundations of catalytic processes seems to be of significant importance in order to improve them and simplify the conditions. Direct functionalization of the coumarin skeleton seems to be one of the more difficult tasks in recent times.
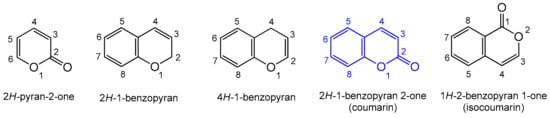
1. Introduction to Coumarins
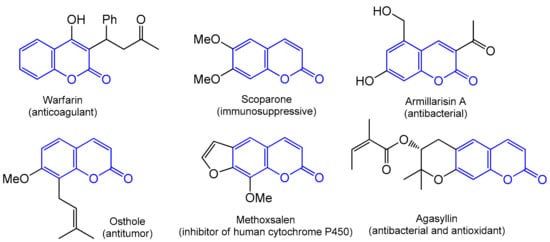
2. Methods for the Synthesis of the Coumarin Core
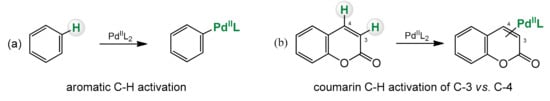
3. C–H Bond Activation in Coumarin Synthesis
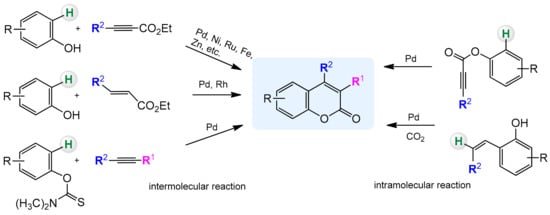
3.1. Direct Synthesis of Coumarins through Intermolecular Hydroarylation of Alkynes
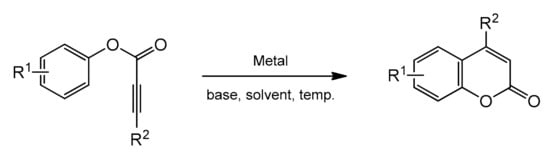
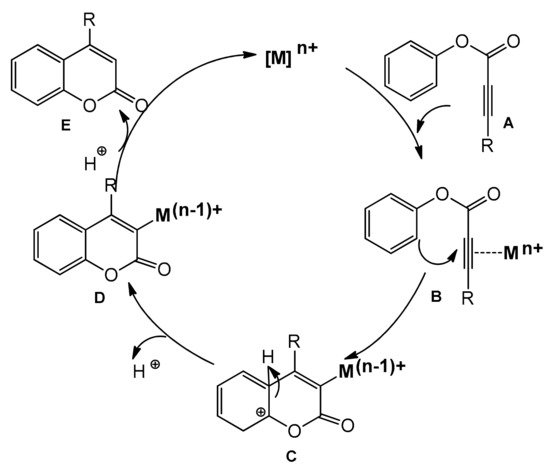
Phenol and naphthol derivatives are proper substrates for the production of 4-arylcoumarins. The reaction of their C–H functionalization takes place in the ortho position, and the metals used as catalysts in this process are, first of all, palladium, iron, and platinum.
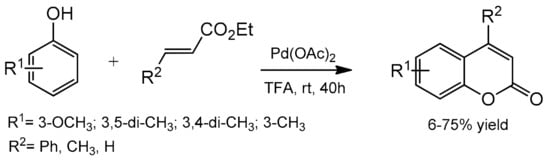
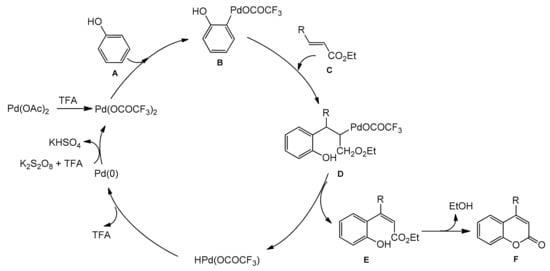
The palladium-catalyzed addition of phenols to alkynoates was initially demonstrated by Trost and co-workers [55][56]. The reactions proceeded with good yields at 35 °C with 10 mol% Pd(OAc)2 as a metal source and the addition of sodium acetate as a base and formic acid as a solvent was of key importance. Formic acid was proposed to influence the cyclization reaction by reducing PdII to Pd0. It was soon found that better results were achieved when using Pd2(dba)3 instead of Pd(OAc)2. The reaction proceeded effectively at room temperature with unsubstituted as well as alkyl- and aryl-substituted alkynoates.
The Kitamura group presented the direct hydroarylation of alkynes using a Pd(OAc)2 catalyst in TFA/DCM [57][58][59]. ron-catalyzed hydroarylation offers an interesting and improved method for the synthesis of 4-substituted coumarins [60]. The reaction proceeded efficiently in the TFA/1,2-DCE solvent system. The yields reported with the iron catalyst, in some cases, were much higher than those obtained with palladium or platinum complexes.
3.2. Direct Synthesis of Coumarins through the Intramolecular Hydroarylation of Alkynes
3.3. Direct Synthesis of Coumarins via the Intermolecular Hydroarylation of Alkenes
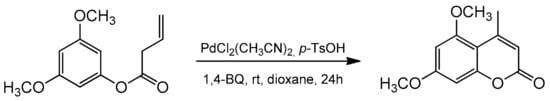
3.4. Direct Synthesis of Coumarins via Intramolecular Hydroarylation of Alkenes
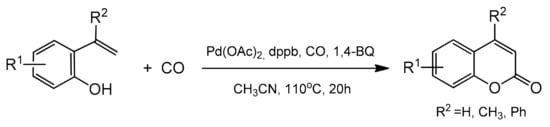
3.5. Intramolecular Cyclocarbonylation and Cyclocarboxylation
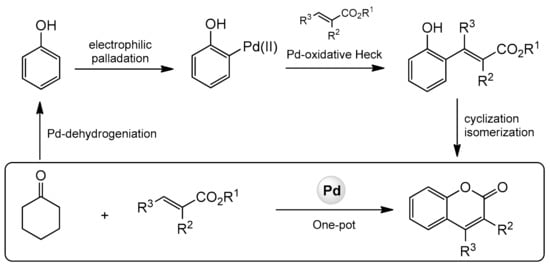
3.6. Other Methods for Direct Synthesis of Coumarins by C–H Bond Activation
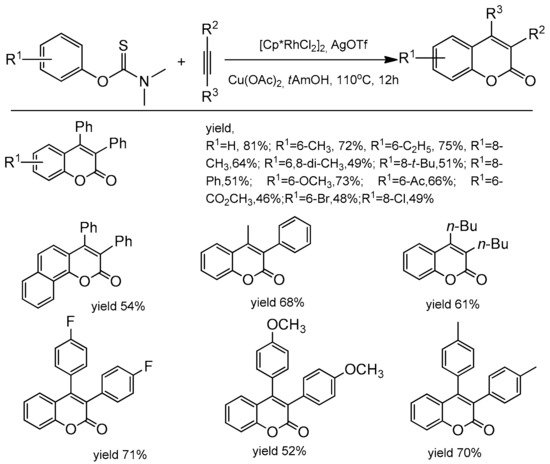
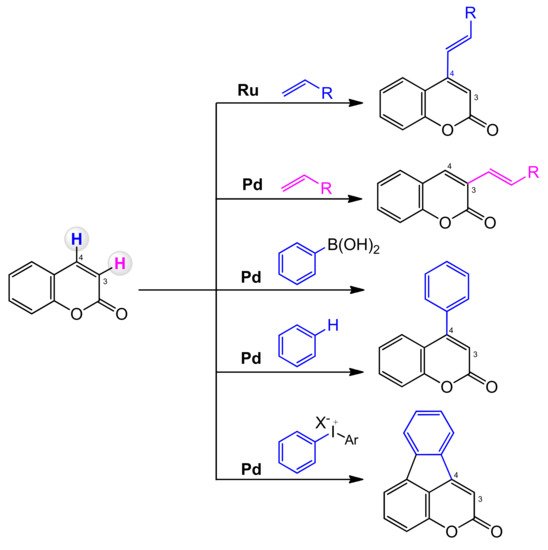
3.7. C–H bond Activation Strategy for the Synthesis of C-3 and C-4 Substituted Coumarins
3.7.1. C-3 Selective Reactions
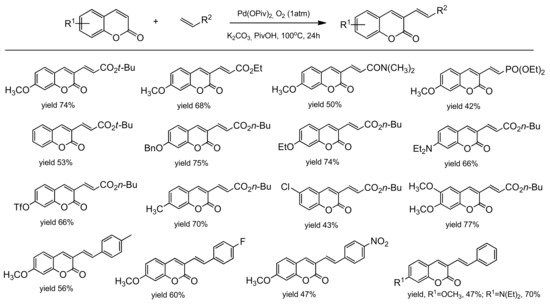
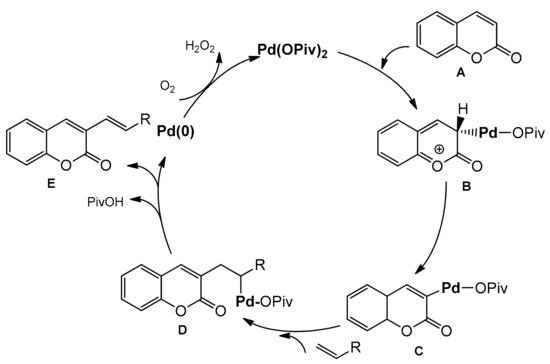
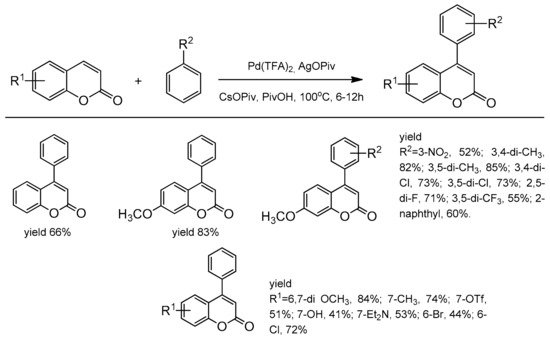
3.7.2. C-4 Selective Reactions
References
- Fairlamb, I.J.S.; Marrison, L.R.; Dickinson, J.M.; Lu, F.-J.; Schmidt, J.P. 2-Pyrones possessing antimicrobial and cytotoxic activities. Bioorg. Med. Chem. 2004, 12, 4285–4299.
- McGlacken, G.P.; Fairlamb, I.J.S. 2-Pyrone natural products and mimetics: Isolation, characterisation and biological activity. Nat. Prod. Rep. 2005, 22, 369–385.
- Favre, H.A.; Powell, W.H. Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013; Royal Society of Chemistry Publishing: London, UK, 2014.
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248.
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds-literature. Molecules 2018, 23, 250.
- Srikrishna, D.; Godugu, C.; Dubey, P.K. A Review on pharmacological properties of coumarins. Mini Rev. Med. Chem. 2018, 18, 113–141.
- Balewski, Ł.; Szulta, S.; Jalińska, A.; Kornicka, A. A Mini-review: Recent advances in coumarin-metal complexes with biological properties. Front. Chem. 2021, 9.
- Peng, X.-M.; Damu, G.L.V.; Zhou, C.-H. Current developments of coumarin compounds in medicinal chemistry. Curr. Pharm. Des. 2013, 19, 3884–3930.
- El-Naggar, A.M.; Ahmed, F.S.; Abd El-Salam, A.M.; Rady, M.A.; Latif, M.S.A. Synthesis and biological activity of some new 3-and 6-substituted coumarin amino acid derivatives. Part I. J. Heterocycl. Chem. 1981, 18, 1203–1207.
- Grover, J.; Jachak, S.M. Coumarins as privileged scaffold for anti-inflammatory drug development. RSC Adv. 2015, 5, 38892–38905.
- Emami, S.; Dadashpour, S. Current developments of coumarin based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem. 2015, 102, 611–630.
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495.
- Dai, J.; Liu, Y.; Jia, H.; Zhou, Y.-D.; Nagle, D.G. Benzochromenones from the marine crinoid comantheria rotula inhibit hypoxia-inducible factor-1 (HIF-1) in cell-based reporter assays and differentially suppress the growth of certain tumor cell lines. J. Nat. Prod. 2007, 70, 1462–1466.
- Akkol, E.K.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959.
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 2016, 123, 236–255.
- Hsieh, H.-P.; Hsu, T.-A.; Yeh, J.-Y.; Horng, J.-T.; Shih, S.-R.; Chang, S.-T.; Chao, Y.-S. Coumarin Compounds and Their Use for Treating Viral Infection. U.S. Patent Application No. 12/481,789, 17 December 2009.
- Arora, R.K.; Kaur, N.; Bansal, Y.; Bansal, G. Novel coumarin-benzimidazole derivatives as antioxidants and safer anti-inflammatory agents. Acta Pharm. Sin. B 2014, 4, 368–375.
- Pu, W.; Lin, Y.; Zhang, J.; Wang, F.; Wang, C.; Zhang, G. 3-Arylcoumarins: Synthesis and potent anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2014, 24, 5432–5434.
- Chen, L.Z.; Sun, W.; Bol, W.; Wang, J.Q.; Xiu, C.; Tang, W.J.; Shi, J.B.; Zhou, H.P.; Liu, X.H. New arylpyrazoline-coumarins: Synthesis and anti-inflammatory activity. Eur. J. Med. Chem. 2017, 138, 170–181.
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951.
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012, 20, 5678–5698.
- Satish, G. Chapter 8—Anticoagulant agents. In Advances in Structure and Activity Relationship of Coumarin Derivatives; Penta, S., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 151–159.
- Abdelhafez, O.M.; Amin, K.M.; Batran, R.Z.; Maher, T.J.; Nada, S.A.; Sethumadhavan, S. Synthesis, anticoagulant and PIVKA-II induced by new 4-hydroxycoumarin derivatives. Bioorg. Med. Chem. 2010, 18, 3371–3378.
- Yu, D.; Suzuki, M.; Xie, L.; Morris-Natschke, S.L.; Lee, K.-H. Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med. Res. Rev. 2003, 23, 322–345.
- Kostova, I. Coumarins as inhibitors of HIV reverse transcriptase. Curr. HIV Res. 2006, 4, 347–363.
- Wadhwa, P.; Priti, J.; Santosh, R.; Hemant, R.A.J. Quinoline, coumarin and other heterocyclic analogs based HIV-1 integrase inhibitors. Curr. Drug Discov. Technol. 2018, 15, 2–19.
- Dorababu, A. Pharmacological report of recently designed multifunctional coumarin and coumarin-heterocycle derivatives. Arch. Pharm. 2021, 355, e2100345.
- Edmondson, D.E.; Mattevi, A.; Binda, C.; Li, M.; Hubalek, F. Structure and mechanism of monoamine oxidases. Curr. Med. Chem. 2004, 11, 1983–1993.
- Wimbiscus, M.; Kostenko, O.; Malone, D. MAO inhibitors: Risks, benefits, and lore. Clevel. Clin. J. Med. 2010, 77, 859–882.
- Cao, D.; Liu, Z.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-based small-molecule fluorescent chemosensors. Chem. Rev. 2019, 119, 10403–10519.
- Ansary, I.; Taher, A. One-pot synthesis of coumarin derivatives. In Phytochemicals in Human Health; IntechOpen: London, UK, 2020.
- Perkin, W.H. On the artificial production of coumarin and formation of its homologues. J. Chem. Soc. 1868, 21, 53–63.
- Perkin, W.H. On the hydride of aceto-salicyl. J. Chem. Soc. 1868, 21, 181–186.
- Woods, L.L.; Sapp, J. A new one-step synthesis of substituted coumarins. J. Org. Chem. 1962, 27, 3703–3705.
- Sethna, S.M.; Shah, N.M.; Shah, R.C. Aluminium chloride, a new reagent for the condensation of β-ketonic esters with phenols. Part I. The condensations of methyl β-resorcylate, β-resorcylic acid, and resacetophenone with ethyl acetoacetate. J. Chem. Soc. 1938, 228–232.
- Corrie, J.E.T. A convenient synthesis of N-(7-dimethylamino-4-methylcoumarin-3-yl)-maleimide incorporating a novel variant of the Pechmann reaction. J. Chem. Soc. Perkin Trans. 1990, 1, 2151–2152.
- Smitha, G.; Reddy, S.C. ZrCl4- catalyzed Pechmann reaction: Synthesis of coumarins under solvent-free conditions. Synth. Commun. 2004, 34, 3997–4003.
- Valizadeh, H.; Shockravi, A. An efficient procedure for the synthesis of coumarin derivatives using TiCl4 as catalyst under solvent-free conditions. Tetrahedron Lett. 2005, 46, 3501–3503.
- John, E.; Israelstam, S. Notes. Use of cation exchange resins in organic reactions. I. The Von Pechmann reaction. J. Org. Chem. 1961, 26, 240–242.
- Laufer, M.C.; Hausmann, H.; Hölderich, W.F. Synthesis of 7-hydroxycoumarins by Pechmann reaction using nafion resin/silica nanocomposites as catalysts. J. Catal. 2003, 218, 315–320.
- Hoefnagel, A.J.; Gunnewegh, E.A.; Downing, R.S.; van Bekkum, H. Synthesis of 7-hydroxycoumarins catalysed by solid acid catalysts. J. Chem. Soc. Chem. Commun. 1995, 225–226.
- Salem, M.A.; Helal, M.H.; Gouda, M.A.; Ammar, Y.A.; El-Gaby, M. An overview on synthetic strategies to coumarins. Synth. Commun. 2018, 48, 1534–1550.
- Shilov, E.; Shul’pin, G.B. Activation of C−H bonds by metal complexes. Chem. Rev. 1997, 97, 2879–2932.
- Kuhl, N.; Hopkinson, M.N.; Wencel-Delord, J.; Glorius, F. Beyond directing groups: Transition-metal-catalyzed C-H activation of simple arenes. Angew. Chem. Int. Ed. 2012, 51, 10236–10254.
- Tasior, M.; Kim, D.; Singha, S.; Krzeszewski, M.; Ahn, K.H.; Gryko, D.T. π-Expanded coumarins: Synthesis, optical properties and applications. J. Mater. Chem. C 2015, 3, 1421–1446.
- Medina, F.G.; Marrero, J.G.; Macías-Alonso, M.; González, M.C.; Córdova-Guerrero, I.; Teissier García, A.G.; Osegueda-Robles, S. Coumarin heterocyclic derivatives: Chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507.
- Jung, J.-W.; Kim, N.-J.; Yun, H.; Han, Y.T. Recent advances in synthesis of 4-arylcoumarins. Molecules 2018, 23, 2417.
- Abdou, M.M.; Abu-Rayyanb, A.; Bedira, A.G.; Abdel-Fattaha, S.; Omara, A.M.A.; Ahmedc, A.A.; El-Desoky, E.-S.I.; Ghaithd, E.A. 3-(Bromoacetyl)coumarins: Unraveling their synthesis, chemistry, and applications. RSC Adv. 2021, 11, 38391–38433.
- Koleva, A.I.; Petkova-Yankova, N.I.; Nikolova, R.D. Synthesis and chemical properties of 3-phosphono-coumarins and 1,2-benzoxaphosphorins as precursors for bioactive compounds. Molecules 2019, 24, 2030.
- Yang, Y.; Lan, J.; You, J. Oxidative C−H/C−H coupling reactions between two (hetero)arenes. Chem. Rev. 2017, 117, 8787–8863.
- Bhatia, R.; Pathania, S.; Singh, V.; Rawal, R.K. Metal-catalyzed synthetic strategies toward coumarin derivatives. Chem. Hetero. Comp. 2018, 54, 280–291.
- Kanchana, U.S.; Diana, E.J.; Mathew, T.V.; Anilkumar, G. Palladium-catalyzed cross-coupling reactions of coumarin derivatives: An overview. Appl. Organomet. Chem. 2020, 34, e5983.
- Sharma, R.K.; Katiyar, D. Recent advances in transition-metal-catalyzed synthesis of coumarins. Synthesis 2016, 48, 2303–2322.
- Choi, H.; Min, M.; Peng, Q.; Kang, D.; Paton, R.S.; Hong, S. Unraveling innate substrate control in site-selective palladium-catalyzed C–H heterocycle functionalization. Chem. Sci. 2016, 7, 3900–3909.
- Trost, B.M.; Toste, F.D. A new palladium-catalyzed addition: A mild method for the synthesis of coumarins. J. Am. Chem. Soc. 1996, 118, 6305–6306.
- Trost, B.M.; Toste, F.D.; Greenman, K. Atom economy. Palladium-catalyzed formation of coumarins by addition of phenols and alkynoates via a net C-H insertion. J. Am. Chem. Soc. 2003, 125, 4518–4526.
- Jia, C.; Lu, W.; Oyamada, J.; Kitamura, T.; Matsuda, K.; Irie, M.; Fujiwara, Y. Novel Pd(II)- and Pt(II)-catalyzed regio- and stereoselective trans-hydroarylation of alkynes by simple arenes. J. Am. Chem. Soc. 2000, 122, 7252–7263.
- Juzo, O.; Chengguo, J.; Yuzo, F.; Tsugio, K. Direct synthesis of coumarins by Pd(II)-catalyzed reaction of alkoxyphenols and alkynoates. Chem. Lett. 2002, 380–381.
- Kotani, M.; Yamamoto, K.; Oyamada, J.; Fujiwara, Y.; Kitamura, T. A Convenient synthesis of coumarins by palladium(II)-catalyzed reaction of phenols with propiolic acids. Synthesis 2004, 1466–1470.
- Kutubi, S.; Hashimoto, T.; Kitamura, T. Improved synthesis of coumarins by iron(III)-catalyzed cascade reaction of propiolic acids and phenols. Synthesis 2011, 8, 1283–1289.
- Aoki, S.; Oyamada, J.; Kitamura, T. Formation of coumarins by palladium(II)-catalyzed reaction of phenols with ethyl acrylates. Bull. Chem. Soc. Jpn. 2005, 78, 468–472.
- Carral-Menoyo, A.; Misol, A.; Gomez-Redondo, M.; Sotomayor, N.; Lete, E. Palladium(II)-catalyzed intramolecular C-H alkenylation for the synthesis of chromanes. J. Org. Chem. 2019, 84, 2048–2060.
- Wu, X.-F.; Neumann, H.; Beller, M. Synthesis of heterocycles via palladium-catalyzed carbonylations. Chem. Rev. 2012, 113, 1–35.
- Ferguson, J.; Zeng, F.; Alper, H. Synthesis of coumarins via Pd-catalyzed oxidative cyclocarbonylation of 2-vinylphenols. Org. Lett. 2012, 14, 5602–5605.
- Kim, D.; Min, M.; Hong, S. One-pot catalysis of dehydrogenation of cyclohexanones to phenols and oxidative Heck coupling: Expedient synthesis of coumarins. Chem. Commun. 2013, 49, 4021–4022.
- Zhao, Y.; Han, F.; Yang, L.; Xia, C. Access to coumarins by rhodium-catalyzed oxidative annulation of aryl thiocarbamates with internal alkynes. Org. Lett. 2015, 17, 1477–1480.
- Min, M.; Kim, Y.; Hong, S. Regioselective palladium-catalyzed olefination of coumarins via aerobic oxidative Heck reactions. Chem. Commun. 2013, 49, 196–198.
- Min, M.; Hong, S. Regioselective palladium-catalyzed direct cross-coupling of coumarins with simple arenes. Chem. Commun. 2012, 48, 9613–9615.




