Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mario Iván Peñas | + 2726 word(s) | 2726 | 2022-03-07 09:52:38 | | | |
| 2 | Jason Zhu | -143 word(s) | 2583 | 2022-03-22 06:52:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Peñas, M.I. Poly (Butylene Succinate) and PBS Copolyesters Degradation. Encyclopedia. Available online: https://encyclopedia.pub/entry/20789 (accessed on 07 February 2026).
Peñas MI. Poly (Butylene Succinate) and PBS Copolyesters Degradation. Encyclopedia. Available at: https://encyclopedia.pub/entry/20789. Accessed February 07, 2026.
Peñas, Mario Iván. "Poly (Butylene Succinate) and PBS Copolyesters Degradation" Encyclopedia, https://encyclopedia.pub/entry/20789 (accessed February 07, 2026).
Peñas, M.I. (2022, March 21). Poly (Butylene Succinate) and PBS Copolyesters Degradation. In Encyclopedia. https://encyclopedia.pub/entry/20789
Peñas, Mario Iván. "Poly (Butylene Succinate) and PBS Copolyesters Degradation." Encyclopedia. Web. 21 March, 2022.
Copy Citation
The impact of plastics on the environment can be mitigated by employing biobased and/or biodegradable materials (i.e., bioplastics) instead of the traditional “commodities”. Poly (butylene succinate) (PBS) emerges as one of the most promising alternatives due to its good mechanical, thermal, and barrier properties, making it suitable for use in a wide range of applications. Nevertheless, less information regarding PBS biodegradation is available, as research is still ongoing. PBS degradation methods include hydrolytic degradation, enzymatic degradation, and biodegradation in environmental conditions, such as burial, activated sludge, and compost.
Degradation
Poly (butylene succinate)
Hydrolytic Degradation
Enzymatic Degradation
Environmental Degradation
1. Hydrolytic Degradation
One of the most common mechanisms of polymer degradation is hydrolytic degradation. In this case (and in the case of enzymatic degradation), the degradation rate depends on poly (butylene succinate) (PBS) crystallinity. Hydrolytic degradation occurs faster in the lower density amorphous regions, facilitating water penetration. This phenomenon causes an increase in the overall degree of crystallinity due to the faster degradation of amorphous domains (that can crystallize once degraded) compared to the more crystalline ones [1]. Some researchers report no variation in weight for PBS when exposed to hydrolytic degradation [2], while others report low weight loss [3][4]. One study reported a ~31% weight loss for PBS after 24 weeks of hydrolytic degradation at 37 °C. This result could be explained due to the relatively low crystallinity (~56%, as determined by DSC) of the PBS used [5]. The pH of the media is also an important parameter that must be taken into account. Morales-Huerta et al. reported a 10% weight loss for hydrolytic degradation at pH = 7.4 after 30 days, whereas the weight loss increased to values higher than 25% for a pH = 2.0 media [6].
As can be deduced from different studies, PBS can be effectively degraded by the hydrolysis of the ester bonds, achieving different results depending on many different parameters involved, such as the synthesis method, MW, crystallinity, or the experimental conditions of the biodegradation assays.
2. Enzymatic Degradation
So far, enzymatic degradation is regarded as one of the most attractive and effective methods for the biodegradation of biopolyesters. The main reason is the presence of labile ester bonds in the chemical structures of biopolyesters, where enzymes can attack [7]. Then, the enzymatic degradation process usually starts with the attachment of the enzyme on the surface, and hydrolysis proceeds via surface erosion. Among all the different types and families of enzymes that can effectively biodegrade PBS and its copolymers. Table 1 shows various enzymes and different experimental conditions for PBS enzymatic biodegradation.
Table 1. Classification of enzymes by families for different PBS enzymatic degradation studies.
| Family | Enzyme | Substrate | Experimental Conditions | Results | Reference |
|---|---|---|---|---|---|
| Cutinase | Fusarium solani | PBS films 30 × 10 × 0.1 mm3 | pH = 8.0 at 40 °C, 20 µg/mL | 100% weight loss in 6 h | [8] |
| Cutinase | Pichia pastoris | PBS films 30 × 10 × 0.5 mm3 | pH = 7.4 at 37 °C, 0.15 mg/mL | 100% weight loss in 12 h | [9] |
| Cutinase | Fusarium solani | PBS films 30 × 10 × 0.1 mm3 | pH = 7.4 at 37 °C, 10 mg/mL | 98.4% weight loss in 12 h | [10] |
| Cutinase | Fusarium solani | PBS films 30 × 10 × 0.5 mm3 | pH = 7.2 at 37 °C, 18 U/mL | ~100% weight loss in 26 h | [11] |
| Lipase | Candida antarctica (CALB) | PBS films 30 × 10 × 0.5 mm3 | pH = 7.2 at 45 °C, 18 U/mL | 95.1% weight loss in 26 h | [11] |
| Lipase | Candida rugosa | PBS films 10 × 10 × 0.5 mm3 | pH = 7.4 at 30 °C, 0.1 mg/mL | 2% weight loss after 7 weeks | [12] |
| Lipase | Pseudomonas cepacia | PBS films 20 × 30 × 0.3 mm3 | pH = 8.0 at 40 °C, 0.06 mg/mL | 2% weight loss after 90 h | [13] |
| Lipase | Candida antarctica (CALB) N435 | PBS films 30 × 10 mm2 | pH = 7.4 at 37 °C, 1.2 mg/mL | 1.8% weight loss after 90 h | [14] |
| Lipase | Porcine pancreas | PBS films 30 × 10 mm2 | pH = 7.4 at 37 °C, 0.8 mg/mL | 0.9% weight loss after 90 h | [14] |
| Lipase | Pseudomonas cepacia | PBS films 10 × 10 × 0.1 mm3 | pH = 6.86 at 45 °C, 0.22 mg/mL | 4.6% weight loss after 50 h | [15] |
| Lipase | Porcine pancreas | PBS film discs 10 × 10 × 0.2 mm3 | pH = 7.4 at 37 °C, 1 mg/mL | 21% weight loss after 30 days | [6] |
| Lipase | Pseudomonas fluorescens | PBS films 10 × 10 × 0.2 mm3 | pH = 7.3 at 37 °C, 2 mg/mL | No visible degradation after 300 h | [16] |
| Lipase | Pseudomonas cepacia | PBS films 10 × 10 × 0.1 mm3 | pH = 6.86 at 45 °C, 0.53 mg/mL | 100% weight loss after 288 h | [17] |
| Lipase | Pseudomonas cepacia | PBS film discs 20 × 20 × 0.05 mm3 | pH = 7.4 at 37 °C, 1 mg/mL | 6% weight loss after 50 h | [18] |
| Lipase | Rhizopus delemar and Pseudomonas cepacia | PBS film discs 50 × 50 × 2 mm3 | pH = 7.2 at 30 °C, 0.09 & 0.01 mg/mL | 2% weight loss after 360 h | [19] |
Enzymatic degradation assays for biopolyesters and PBS are commonly carried out at physiological temperature (i.e., 37 °C) [6][10][13]. However, it has been demonstrated that this degradation method is favored at a temperature close to Tm (the PBS melting temperature is above 100 °C) [20]. Some researchers have reported low weight loss values for PBS homopolymer at different experimental conditions, reaching a 3.5% weight loss after 12 days in the presence of Pseudomonas cepacia lipase [20], or even lower [12][14]. The low degree of degradation obtained could be attributed to the high crystallinity of this polymer compared to other aliphatic polyesters [20]. Other studies carried out under different experimental conditions report much higher degradation rates. For example, for enzymatic degradation assays employing cutinases, weight losses reach almost 100% in just 12 h [10], as seen in Table 1. An interesting study developed by Shi et al. showed the influence of two different enzymes (Fusarium solani cutinase and Candida antarctica lipase B, CALB) in the degradation rate of PBS. They found that the PBS degradation rate was much faster by the action of cutinase. PBS degraded in the presence of cutinase reached ~50% weight loss in 4 h, whereas those degraded in the presence of lipase reached ~20% weight loss over the same time. For both cases, a nearly total decomposition was achieved after 26 h [11].
Figure 1 shows the results corresponding to different biodegradation studies where the weight loss (%) of PBS in the presence of lipase from Pseudomonas cepacia has been reported. As can be observed in Figure 1, only one study shows a relatively high weight loss of PBS with this enzyme (higher than 40% in 100 h) [17]. For the rest, the weight loss reached after several hours in contact with Pseudomonas cepacia is very low (less than 6%), which could be attributed, in part, to the low concentration of the enzyme employed for some of the studies [13][15][18].
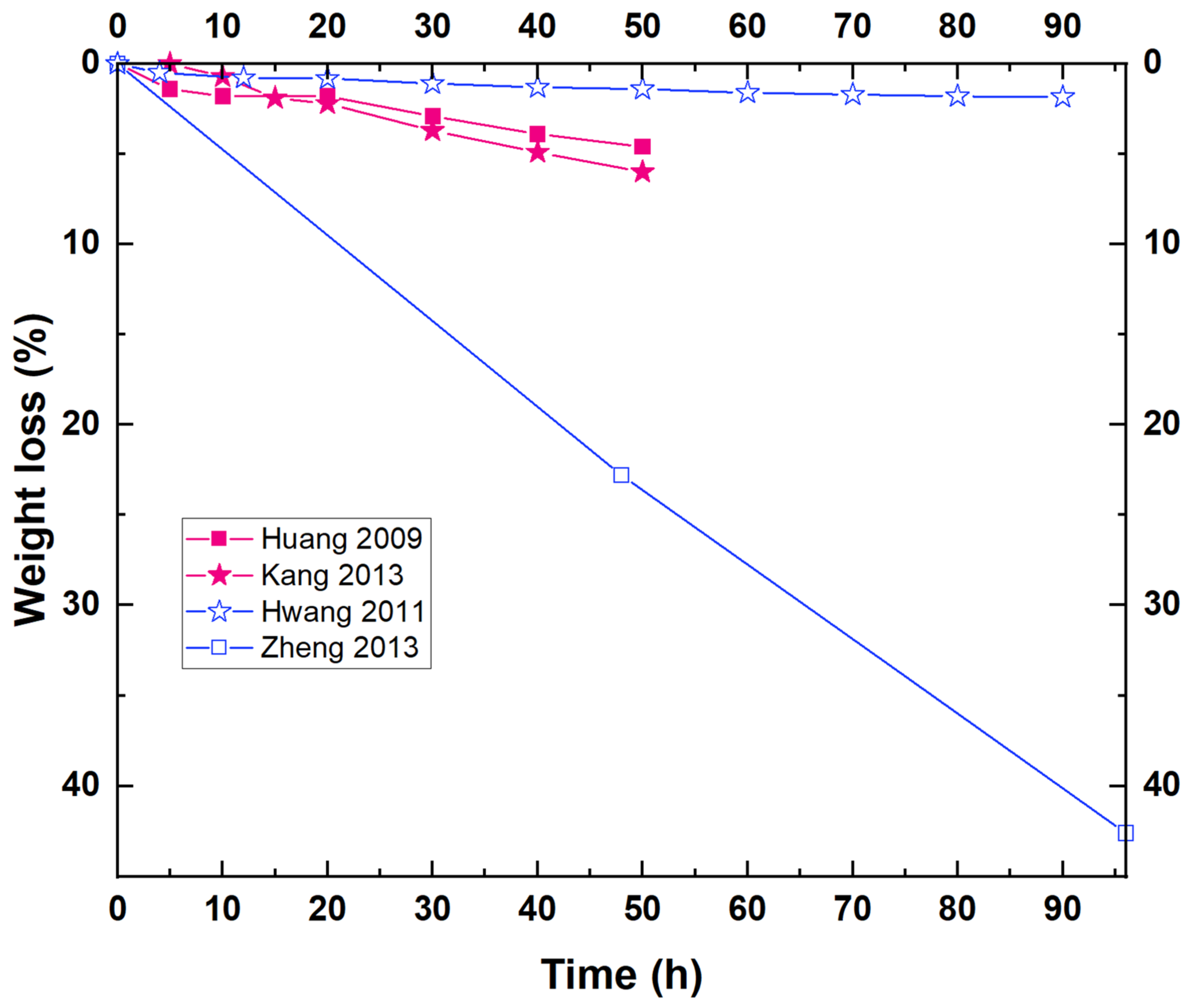
Figure 1. Weight loss curves corresponding to various enzymatic degradation studies of PBS by the action of a lipase from Pseudomonas cepacia. Experimental conditions differ from one study to another, although the same enzyme is used.
3. PBS-Based Copolymers: Hydrolytic and Enzymatic Degradation
In the case of PBS copolymers, different (and opposite) results are reported depending on the nature of the second comonomer. For instance, the biodegradability of aromatic polyesters is less favored than in the case of aliphatic polyesters such as PBS [6]. Thus, incorporating a second comonomer in the structure of PBS could favor or prevent the degradation of the polyester, attending to the nature of the second constituent.
Hydrolytic and enzymatic degradation of PBS-ran-PBFur copolyesters have been determined by placing 60:40 and 40:60 copolymers in a pH = 2.0 or pH = 7.4 medium at 37 °C [6]. Firstly, the enzymatic degradation was more effective than hydrolysis, reaching 15–20% of weight loss versus 3–5% for the latter (hydrolysis) after 30 days. Furthermore, the behavior of the copolyesters was more similar to that of PBS in the case of enzymatic degradation. Regarding the acidic medium, the results were in between enzymatic and hydrolytic degradation, but far away from those obtained for the PBS homopolymer, as the homopolymer achieved a 30% of weight loss, compared to the 10–15% weight loss of the copolymers.
Han et al. studied the enzymatic degradation behavior for different poly(butylene succinate-ran-butylene 2-methylsuccinate) (PBS-ran-PBMS) copolyesters, reporting higher degradation rates for those copolymers with a higher PBMS content. Considering the copolymer with 20% mol in PBMS, the hydrolytic degradation (without the enzyme) showed a negligible weight loss compared to that of the enzymatic degradation (amano lipase from Pseudomonas fluorescens), achieving a 30% weight loss in 300 h [16].
The copolymerization of PBS with salicylic acid was studied as an attempt to produce polymer films with potential applications in agricultural applications. Enzymatic degradation assays carried out in the presence of Candida antarctica lipase B (CALB) showed very low degradation after 20 days (~1.5 wt% for neat PBS); however, the addition of salicylic acid increased this value up to ~3.5 wt% [21].
In the case of the enzymatic hydrolysis of PBS and PBS-ran-PBA copolymers in the presence of Candida cylindracea lipase [22], the highest degradation was obtained for the copolyesters containing 25% and 50% mol of butylene succinate, reaching 20% and 30% of weight loss, respectively, after 90 h. It is necessary to remark that the enzymatic degradation is not affected by the MW; hence, similar results are obtained for low MW (i.e., Mw of 6300 g/mol) and high MW (i.e., Mw of 29,000 g/mol) [23].
4. Biodegradation in Environmental Conditions
Although enzymatic hydrolysis (laboratory conditions) has shown satisfactory results for PBS biodegradation, this biopolyester commonly degrades in environmental conditions [24]. The study of the biodegradation of PBS under environmental conditions will give an idea for the implementation of PBS in agricultural applications such as mulching films [25][26][27]. PE films are commonly employed, being an effective method for promoting plant growth during the cold seasons (i.e., spring and autumn). The problem here is the recyclability of the PE film due to the contamination caused to the soil itself, so a biodegradable film is required, and PBS is a suitable candidate to solve this issue [28].
The experiments for this type of biodegradation are usually carried out following different standards from international organizations (ISO, ASTM, and EU). Because of this, the definition of more experimental parameters is required as compared to enzymatic and hydrolytic assays. As the conditions and parameters differ from one study to another (as well as the soil employed for the tests and the microorganisms content in the soil), biodegradation in environmental conditions covers a wide range of variable results [29].
4.1. PBS Homopolymer and PBS-Based Copolymers
PBS biodegradation in environmental conditions usually takes more time as compared to enzymatic/hydrolytic PBS degradation. Kim et al. reported a low degradation of PBS when exposed to environmental degradation (below 8% weight loss after 120 days) [30]. Similar trends have been obtained by Huang et al. (below 3% weight loss in 100 days) [31] and other reports [32]. However, the study of PBS biodegradation in a controlled compost at 58 °C (based on ISO 14855-2) showed that PBS powder biodegradation reached 60% weight loss in 40 days and increased to 80% in less than 80 days. These results are highly promising, opening a path for the establishment of experimental protocols to determine the environmental biodegradation of this aliphatic polyester [33]. Kunioka et al. also reported very high biodegradation rates for PBS in powder form, reaching almost 80% weight loss in less than 80 days [33]. These outstanding results are explained as the PBS was tested in powder form, which differs from the tensile specimens commonly used to determine environmental biodegradation.
The biodegradation of PBS and PBS-ran-PBA copolymers subjected to different environments, as biodegradation in compost, soil, and artificial weathering, has been reported. For the artificial weathering, both polymers were submitted to UVA radiation and artificial rain, whereas in soil and compost experiments, no radiation was employed. For the first assay, a ~30% weight loss was achieved for PBS in 24 weeks (~50% in the case of the PBS-ran-PBA copolymer). In contrast, biodegradation in soil and artificial weathering showed negligible degradation for PBS, while PBS-ran-PBA presented a ~20% weight loss for the biodegradation in soil experiment and negligible for artificial weathering [34]. In another study, the biodegradation of PBS-ran-PBFur copolymers in compost at 58 °C showed the best results for the 20 mol% of furanoate composition, achieving an almost 100% of degradation in 80 days [35].
4.2. PBS-Based Biocomposites
The presence of fillers in PBS biocomposites has been widely studied to modulate the degradation in environmental conditions, as in the case of other types of degradation and thermomechanical and barrier properties. Special cases are natural fillers, that, in addition to being easily biodegraded, can potentially increase the degradation rate of PBS. Table 2 includes several examples of biodegradation studies carried out under environmental conditions for different PBS-based biocomposites. Among all the examples presented in the table, some interesting results will be commented on below. As a general idea to consider, the trend shows that PBS-based biocomposites degrade faster than neat PBS.
Table 2. Different biodegradation studies of PBS and PBS biocomposites carried out in environmental conditions.
| Filler | Filler Content | Experimental Conditions | Results (Weight Loss) | Reference |
|---|---|---|---|---|
| Rubberwood powders (RWP) | 0–40 wt% | 60 days, no UV radiation, water control each 48 h | <1% (PBS) 2–10% (PBS/RWP) |
[32] |
| Rice husk flour (RHF) and wood flour (WF) | 0–40 wt% | 4 months | 7% (PBS) 8–12% (PBS/RHF and PBS/WF) |
[30] |
| Sugarcane rind fiber (SRF) | 0–15 wt% | 100 days, natural soil in cropland, water control each 24 h | 2.5% (PBS) 10–20% (PBS/SRF) |
[31] |
| Microcrystalline cellulose (MCC) and nanofibrillated cellulose (NFC) | 0–40 wt% | 75 days, simulated compost, 58 °C, pH = 5.7–6.3, 50 wt% water content | 100% in 75–80 days (PBS) 100% in 65–70 days (PBS/MCC and PBS/NFC) |
[36] |
| Cotton fiber (CF) | 0–40 wt% | Based on ISO 14855-2 100 days, 58 °C, 10 mL/min air flow |
~60% (PBS) ~90% (PBS/CF) |
[37] |
| Rice husk flour (RHF) | 0–40 wt% | Based on ASTM D 6003-96 80 days, 30 °C, pH = 7, 50–60 wt% water content |
~12% (PBS) 13–18% (PBS/RHF) |
[38] |
| Jute fiber (JF) | 0–30 wt% | 180 days, compost soil, 30 °C, constant water control | 31.4% (PBS) 47.3–62.5% (PBS/JF) |
[39] |
| Abaca fiber (AF) | 10 wt% | 180 days, black soil and leaf mold for gardening, 25–30 °C, water control each 48 h | ~30% (PBS) ~50% (PBS/AF) |
[40] |
| Soy, canola, and corn gluten meals (SM, CM, CGM) and switchgrass (SG) | 25 wt% | Based on ASTM D6400 200 days, 3 month-old compost, 58 °C, pH = 7–8, 50–55 wt% water content |
~95% (PBS) ~85% (PBS/SG) 90–95% (PBS/SM, PBS/CM and PBS/CGM) |
[41] |
| Organically modified montmorillonite (OMMT) | 0–10 wt% | 180 days, natural compost, 30 °C, pH = 7.46, 60–70 wt% water content | ~9% (PBS) ~3.5–5% (PBS/OMMT) |
[42] |
| Nanofibrillated cellulose (NFC) and recycled cellulose (rCell) | 0–15 wt% (PBS/NFC) 0–50 wt% (PBS/rCell) |
80 days, 58 °C, pH = 5.7–6.5, >50 wt% water content | ~80% (PBS) ~85–92% (PBS/NFC) 100% in 70 days (PBS/rCell) |
[43][44] |
| Pistachio shell flour (PSF) | 0–30 wt% | Based on ISO 20200 112 days, compost, 58 °C, 55 % relative humidity |
~18% (PBS) ~14–17.5% (PBS/PSF) |
[45] |
Although the experimental conditions differ from one study to another, it has been observed that cellulose fillers (micro- or nano-sized) achieve one of the best results for PBS degradation. Platnieks et al. have studied many different PBS/cellulose-based composite films: microcrystalline cellulose (MCC) [36], nanofibrillated cellulose (NFC) [14][23], and recycled cellulose from TetraPak® [44]. The experimental conditions were similar for all the studies, employing a simulated compost under aerobic conditions at 58 °C, with a slightly acidic medium (pH = 5.7–6.5) and a water content of 50% or higher. The researchers found that, although almost every sample was completely disintegrated within 75–80 days, PBS-biocomposites degraded 5–10 days earlier than neat PBS films. In general, the degradation rate was faster at higher filler content. However, for the PBS/rCell biocomposite with the highest content in rCell, the degradation rate was faster in the early stages of the assay, whereas it slowed down during the course of the experiment (see Figure 2a–c). If the results were compared to corresponding to the PBS biocomposites with high filler content (i.e., 40 wt%), it was found that the PBS/MCC composite degraded faster than the other two biocomposites (i.e., PBS/NFC and PBS/rCell) which present a similar behavior, being much faster than the biodegradation of neat PBS (see Figure 2d).
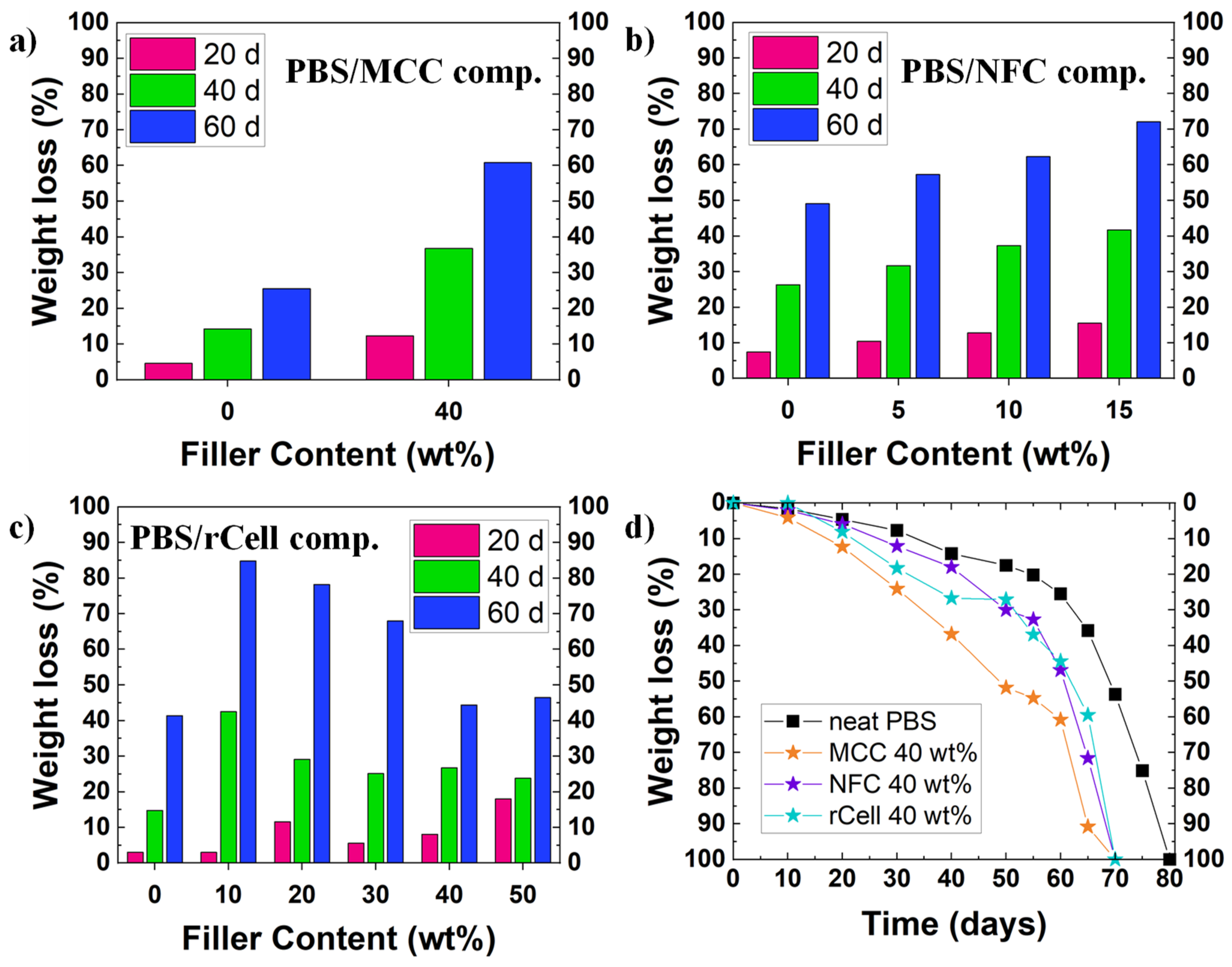
Figure 2. Biodegradation in environmental conditions for several PBS/cellulose-based biocomposites: influence of filler content in weight loss for (a) PBS/MCC composites, (b) PBS/NFC composites, and (c) PBS/rCell composites; (d) variation of biodegradation rates for PBS and the three PBS/cellulose-based biocomposites. In Figure 2a–c, weight losses at 20, 40, and 60 days are represented.
Other PBS biocomposites include rubberwood powder (RWP) from sawdust wastes as a natural filler (lignocellulosic nature). The environmental degradation of these PBS/RWP biocomposites was studied, showing a ~10 wt% weight loss after 60 days of soil burial testing. This behavior was attributed to the decrease in the crystallinity of the PBS biocomposites with the increasing content of RWP [32]. In another example, the biodegradation of the PBS biocomposites with rice husk flour (RHF) and wood flour (WF) in soil burial testing showed a 10 wt% degradation for the RHF composites after 120 days [30]. In both cases, the weight loss was directly related to the biocomposite content, increasing with the filler content.
This entry is adapted from 10.3390/polym14051025
References
- Armentano, I.; Gigli, M.; Morena, F.; Argentati, C.; Torre, L.; Martino, S. Recent advances in nanocomposites based on aliphatic polyesters: Design, synthesis, and applications in regenerative medicine. Appl. Sci. 2018, 8, 1452.
- Gualandi, C.; Soccio, M.; Govoni, M.; Valente, S.; Lotti, N.; Munari, A.; Giordano, E.; Pasquinelli, G.; Focarete, M.L. Poly(butylene/diethylene glycol succinate) multiblock copolyester as a candidate biomaterial for soft tissue engineering: Solid-state properties, degradability, and biocompatibility. J. Bioact. Compat. Polym. 2012, 27, 244–264.
- Huang, A.; Peng, X.; Geng, L.; Zhang, L.; Huang, K.; Chen, B.; Gu, Z.; Kuang, T. Electrospun poly (butylene succinate)/cellulose nanocrystals bio-nanocomposite scaffolds for tissue engineering: Preparation, characterization and in vitro evaluation. Polym. Test. 2018, 71, 101–109.
- Zhang, Y.; Yuan, W.; Liu, Y. Synthesis and characterization of bio-based poly(butylene succinate-co-10-hydroxydecanoate). J. Elastomers Plast. 2018, 50, 325–338.
- Sheikholeslami, S.N.; Rafizadeh, M.; Taromi, F.A.; Shirali, H.; Jabbari, E. Material properties of degradable Poly(butylene succinate-co-fumarate) copolymer networks synthesized by polycondensation of pre-homopolyesters. Polymer 2016, 98, 70–79.
- Morales-Huerta, J.C.; Ciulik, C.B.; De Ilarduya, A.M.; Muñoz-Guerra, S. Fully bio-based aromatic-aliphatic copolyesters: Poly(butylene furandicarboxylate-co-succinate)s obtained by ring opening polymerization. Polym. Chem. 2017, 8, 748–760.
- Satti, S.M.; Shah, A.A. Polyester-based biodegradable plastics: An approach towards sustainable development. Lett. Appl. Microbiol. 2020, 70, 413–430.
- Hu, X.; Gao, Z.; Wang, Z.; Su, T.; Yang, L.; Li, P. Enzymatic degradation of poly(butylene succinate) by cutinase cloned from Fusarium solani. Polym. Degrad. Stab. 2016, 134, 211–219.
- Pan, W.; Bai, Z.; Su, T.; Wang, Z. Enzymatic degradation of poly(butylene succinate) with different molecular weights by cutinase. Int. J. Biol. Macromol. 2018, 111, 1040–1046.
- Bai, Z.; Liu, Y.; Su, T.; Wang, Z. Effect of hydroxyl monomers on the Enzymatic degradation of poly(ethylene succinate), poly(butylene succinate), and poly(hexylene succinate). Polymers 2018, 10, 90.
- Shi, K.; Su, T.; Wang, Z. Comparison of poly(butylene succinate) biodegradation by Fusarium solani cutinase and Candida antarctica lipase. Polym. Degrad. Stab. 2019, 164, 55–60.
- Li, S.L.; Wu, F.; Wang, Y.Z.; Zeng, J.B. Biobased Thermoplastic Poly(ester urethane) Elastomers Consisting of Poly(butylene succinate) and Poly(propylene succinate). Ind. Eng. Chem. Res. 2015, 54, 6258–6268.
- Hwang, S.Y.; Jin, X.Y.; Yoo, E.S.; Im, S.S. Synthesis, physical properties and enzymatic degradation of poly (oxyethylene-b-butylene succinate) ionomers. Polymer 2011, 52, 2784–2791.
- Yang, J.; Tian, W.; Li, Q.; Li, Y.; Cao, A. Novel biodegradable aliphatic poly(butylene succinate-co-cyclic carbonate)s bearing functionalizable carbonate building blocks: II. Enzymatic biodegradation and in vitro biocompatibility assay. Biomacromolecules 2004, 5, 2258–2268.
- Huang, X.; Li, C.; Zheng, L.; Zhang, D.; Guan, G.; Xiao, Y. Synthesis, characterization and properties of biodegradable poly(butylene succinate)-block-poly(propylene glycol)segmented copolyesters. Polym. Int. 2009, 58, 893–899.
- Han, J.; Shi, J.; Xie, Z.; Xu, J.; Guo, B. Synthesis, properties of biodegradable poly(butylene succinate-co-butylene 2-methylsuccinate) and application for sustainable release. Materials 2019, 12, 1507.
- Zheng, L.; Wang, Z.; Wu, S.; Li, C.; Zhang, D.; Xiao, Y. Novel poly(butylene fumarate) and poly(butylene succinate) multiblock copolymers bearing reactive carbon-carbon double bonds: Synthesis, characterization, cocrystallization, and properties. Ind. Eng. Chem. Res. 2013, 52, 6147–6155.
- Kang, Z.H.; Wang, C.L. Novel poly(butylenes succinate-block-1,3-propylene sebacate): Synthesis and enzymatic degradation. Adv. Mater. Res. 2013, 774–776, 569–572.
- Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis, cocrystallization, and enzymatic degradation of novel poly(butylene-co-propylene succinate) copolymers. Biomacromolecules 2007, 8, 2437–2449.
- Kong, X.; Qi, H.; Curtis, J.M. Synthesis and characterization of high-molecular weight aliphatic polyesters from monomers derived from renewable resources. J. Appl. Polym. Sci. 2014, 131, 4–10.
- Wang, L.; Zhang, M.; Lawson, T.; Kanwal, A.; Miao, Z. Poly(butylene succinate-co-salicylic acid) copolymers and their effect on promoting plant growth. R. Soc. Open Sci. 2019, 6, 1–11.
- Nikolic, M.S.; Djonlagic, J. Synthesis and characterization of biodegradable poly(butylene succinate-co-butylene adipate)s. Polym. Degrad. Stab. 2001, 74, 263–270.
- Song, D.K.; Sung, Y.K. Synthesis and characterization of biodegradable poly(1,4-butanediol succinate). J. Appl. Polym. Sci. 1995, 56, 1381–1395.
- Ferreira, F.V.; Dufresne, A.; Pinheiro, I.F.; Souza, D.H.S.; Gouveia, R.F.; Mei, L.H.I.; Lona, L.M.F. How do cellulose nanocrystals affect the overall properties of biodegradable polymer nanocomposites: A comprehensive review. Eur. Polym. J. 2018, 108, 274–285.
- Sisti, L.; Totaro, G.; Marchese, P. PBS makes its entrance into the family of biobased plastics. In Biodegradable and Biobased Polymers for Environmental and Biomedical Applications; Scrivener Publishing: Berverly, MA, USA, 2016; pp. 225–286. ISBN 9781119117360.
- Ayu, R.S.; Khalina, A.; Harmaen, A.S.; Zaman, K.; Mohd Nurrazi, N.; Isma, T.; Lee, C.H. Effect of empty fruit brunch reinforcement in polybutylene-succinate/modified tapioca starch blend for agricultural mulch films. Sci. Rep. 2020, 10, 1166.
- Scott, G. “Green” polymers. Polym. Degrad. Stab. 2000, 68, 1–7.
- Nugroho, P.; Mitomo, H.; Yoshii, F.; Kume, T.; Nishimura, K. Improvement of processability of PCL and PBS blend by irradiation and its biodegradability. Macromol. Mater. Eng. 2001, 286, 316–323.
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chemie-Int. Ed. 2019, 58, 50–62.
- Kim, H.S.; Yang, H.S.; Kim, H.J. Biodegradability and mechanical properties of agro-flour-filled polybutylene succinate biocomposites. J. Appl. Polym. Sci. 2005, 97, 1513–1521.
- Huang, Z.; Qian, L.; Yin, Q.; Yu, N.; Liu, T.; Tian, D. Biodegradability studies of poly(butylene succinate) composites filled with sugarcane rind fiber. Polym. Test. 2018, 66, 319–326.
- Anankaphong, H.; Pentrakoon, D.; Junkasem, J. Effect of rubberwood content on biodegradability of poly(butylene succinate) biocomposites. Int. J. Polym. Sci. 2015, 2015, 19–21.
- Kunioka, M.; Ninomiya, F.; Funabashi, M. Biodegradation of poly(butylene succinate) powder in a controlled compost at 58 °C evaluated by naturally-occurring carbon 14 amounts in evolved CO2 based on the ISO 14855-2 method. Int. J. Mol. Sci. 2009, 10, 4267–4283.
- Puchalski, M.; Szparaga, G.; Biela, T.; Gutowska, A.; Sztajnowski, S.; Krucińska, I. Molecular and supramolecular changes in polybutylene succinate (PBS) and polybutylene succinate adipate (PBSA) copolymer during degradation in various environmental conditions. Polymers 2018, 10, 251.
- Jacquel, N.; Saint-Loup, R.; Pascault, J.P.; Rousseau, A.; Fenouillot, F. Bio-based alternatives in the synthesis of aliphatic-aromatic polyesters dedicated to biodegradable film applications. Polymer 2015, 59, 234–242.
- Platnieks, O.; Gaidukovs, S.; Barkane, A.; Sereda, A.; Gaidukova, G.; Grase, L.; Thakur, V.K.; Filipova, I.; Fridrihsone, V.; Skute, M.; et al. Bio-based poly(butylene succinate)/microcrystalline cellulose/nanofibrillated cellulose-based sustainable polymer composites: Thermo-mechanical and biodegradation studies. Polymers 2020, 12, 1472.
- Calabia, B.P.; Ninomiya, F.; Yagi, H.; Oishi, A.; Taguchi, K.; Kunioka, M.; Funabashi, M. Biodegradable poly(butylene succinate) composites reinforced by cotton fiber with silane coupling agent. Polymers 2013, 5, 128–141.
- Kim, H.S.; Kim, H.J.; Lee, J.W.; Choi, I.G. Biodegradability of bio-flour filled biodegradable poly(butylene succinate) bio-composites in natural and compost soil. Polym. Degrad. Stab. 2006, 91, 1117–1127.
- Liu, L.; Yu, J.; Cheng, L.; Yang, X. Biodegradability of poly(butylene succinate) (PBS) composite reinforced with jute fibre. Polym. Degrad. Stab. 2009, 94, 90–94.
- Teramoto, N.; Urata, K.; Ozawa, K.; Shibata, M. Biodegradation of aliphatic polyester composites reinforced by abaca fiber. Polym. Degrad. Stab. 2004, 86, 401–409.
- Anstey, A.; Muniyasamy, S.; Reddy, M.M.; Misra, M.; Mohanty, A. Processability and Biodegradability Evaluation of Composites from Poly(butylene succinate) (PBS) Bioplastic and Biofuel Co-products from Ontario. J. Polym. Environ. 2014, 22, 209–218.
- Phua, Y.J.; Lau, N.S.; Sudesh, K.; Chow, W.S.; Mohd Ishak, Z.A. Biodegradability studies of poly(butylene succinate)/organo-montmorillonite nanocomposites under controlled compost soil conditions: Effects of clay loading and compatibiliser. Polym. Degrad. Stab. 2012, 97, 1345–1354.
- Platnieks, O.; Sereda, A.; Gaidukovs, S.; Thakur, V.K.; Barkane, A.; Gaidukova, G.; Filipova, I.; Ogurcovs, A.; Fridrihsone, V. Adding value to poly(butylene succinate) and nanofibrillated cellulose-based sustainable nanocomposites by applying masterbatch process. Ind. Crops Prod. 2021, 169, 113669.
- Platnieks, O.; Barkane, A.; Ijudina, N.; Gaidukova, G.; Thakur, V.K.; Gaidukovs, S. Sustainable tetra pak recycled cellulose/poly(butylene succinate) based woody-like composites for a circular economy. J. Clean. Prod. 2020, 270, 122321.
- Rojas-Lema, S.; Arevalo, J.; Gomez-Caturla, J.; Garcia-Garcia, D.; Torres-Giner, S. Peroxide-induced synthesis of maleic anhydride-grafted poly(butylene succinate) and its compatibilizing effect on poly(butylene succinate)/pistachio shell flour composites. Molecules 2021, 26, 5927.
More
Information
Subjects:
Polymer Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.7K
Revisions:
2 times
(View History)
Update Date:
22 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




