Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nathan zasler | + 3363 word(s) | 3363 | 2022-03-01 02:30:07 | | | |
| 2 | Bruce Ren | Meta information modification | 3363 | 2022-03-21 02:00:38 | | | | |
| 3 | Bruce Ren | Meta information modification | 3363 | 2022-03-21 02:39:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zasler, N.; Formisano, R.; Aloisi, M. Pain in Persons with Disorders of Consciousness. Encyclopedia. Available online: https://encyclopedia.pub/entry/20775 (accessed on 12 January 2026).
Zasler N, Formisano R, Aloisi M. Pain in Persons with Disorders of Consciousness. Encyclopedia. Available at: https://encyclopedia.pub/entry/20775. Accessed January 12, 2026.
Zasler, Nathan, Rita Formisano, Marta Aloisi. "Pain in Persons with Disorders of Consciousness" Encyclopedia, https://encyclopedia.pub/entry/20775 (accessed January 12, 2026).
Zasler, N., Formisano, R., & Aloisi, M. (2022, March 21). Pain in Persons with Disorders of Consciousness. In Encyclopedia. https://encyclopedia.pub/entry/20775
Zasler, Nathan, et al. "Pain in Persons with Disorders of Consciousness." Encyclopedia. Web. 21 March, 2022.
Copy Citation
In the practice of medicine, pain is often encountered consequential to disease and/or acquired brain injuries. A recent revision of the definition of pain has been proposed by the International Association for the Study of Pain (IASP), in which pain has been defined as “some unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.” Furthermore, pain is always a subjective experience, influenced to varying degrees by biological, psychological, and social factors.
disorders of consciousness
pain
suffering
severe brain injury
ethics
pathophysiology
assessment
management
1. Introduction
In the practice of medicine, pain is often encountered consequential to disease and/or acquired brain injuries. A recent revision of the definition of pain has been proposed by the International Association for the Study of Pain (IASP) [1], in which pain has been defined as “some unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.” Furthermore, pain is always a subjective experience, influenced to varying degrees by biological, psychological, and social factors.
Pain can present in many different ways, in part dependent upon the underlying peripheral versus central mechanisms of the same, as well as the acuity versus chronicity of the pain condition. Acute pain is generally treated very differently from chronic pain due to differences in pathoetiology, as well as the frequent secondary consequences of chronic pain, such as anxiety and depression. Chronic pain is typically defined as pain lasting for longer than 6 months, although the timeframe is empirical in nature and others have advocated for 3 months or more, while some pain practitioners have opined that chronic pain is any pain that lasts beyond the timeframe for the normal resolution of the original pain generator [2]. Chronic pain syndromes occur when the pain disorder impacts quality of life as far as mood, sleep, fatigue, libido, and participation in vocational and avocational activities. Pain can persist following the acute and subacute periods, even when the pathologic condition causing the pain has resolved. Pain chronicity can be due to both peripheral and central sensitization mechanisms and/or the non-resolution of the pain generator, such as that which might occur with a chronic physical condition/impairment.
Pain is an inherent part of the human experience. Pain disorders may cause impairment in human function as well as disability-related consequences. When pain is experienced as an unpleasant physical sensation or emotion, it is typically expressed through the conscious experience of the same or externalized in the form of verbalizations, vocalizations, and/or behavioral responses, such as grimacing, crying, or agitation. In neurologically impaired patients, pain, whether consciously perceived or not, may be accompanied by increased spasticity as well as autonomic activation (i.e., hypertension, tachycardia, tachypnea, sweating, pupillary changes, etc.), including the worsening of dysautonomic symptoms [3][4][5][6].
Pain and suffering are distinct entities. Pain is the physiologic manifestation of nociception (which refers to the detection of noxious stimuli by nociceptors), although pain itself may not always be linked to a stimulus and does not necessarily correlate with the degree of injury severity [7]. Nociception, or the neural process of encoding noxious stimuli, refers to the perception (conscious or not) of such stimuli [8], eliciting the activation of an extensive cortical network. Non-nociceptive pain does not require a noxious stimulus as that which might occur in allodynia or neuropathy [9]. Additionally, pain is not necessarily a direct consequence of nociception and involves the interaction of multiple inputs [10]. Pain mediation requires a complex multidimensional neuromatrix that integrates pain sensations with complex functions involving affect, memory, and autonomic self-regulation [11][12][13][14]. Suffering entails an individual’s emotional reaction to the perception of pain and may involve an array of different responses, including frustration, denial, indignation, and self-indignation, among other responses. The relationship between pain and suffering is complex, as pain is not necessarily always associated with suffering, and suffering is certainly not always associated with pain, whether physical or emotional. Most suffering is induced by negative emotions or situations, and endorsing that a given patient is suffering would implicitly require the clinician to have evidence that such behavioral responses were in fact occurring. There remains debate regarding the boundaries of pain and suffering that the practitioner should be cognizant of in clinical practice [9]. As clinicians, avoiding or modulating both pain and suffering should be one of the primary goals for patients, regardless of their clinical condition.
Other factors must be considered when assessing pain, including sex differences in pain perception and cultural influences on the subjective experience of pain. In general, women tend to be at increased risk for chronic pain and may experience more severe pain. Women have greater pain sensitivity, reduced pain inhibition, and enhanced pain facilitation compared to men; a factor to be considered in future pain studies. There are also studies that suggest differential responses between the sexes to pharmacotherapeutic treatment for pain [15] that require further study in healthy controls with findings extending accordingly to other patient populations. Cultural influences have also been shown to play a role in a person’s subjective pain experience, as well as how they manage their pain experience [16][17].
In the context of treating persons with a disorder of consciousness (DoC), including vegetative state (VS)/unresponsive wakefulness syndrome (UWS) and minimally conscious state (MCS), one of the more challenging areas of practice is that of assessing and managing pain [18][19][20][21][22]. The number of individuals with DoC has increased due in part to progress in intensive and acute neurosurgical critical care, bringing increased attention to this group of challenging patients, regardless of the etiology of their DoC. One consequence of this progress is a trend toward increased awareness of the limitations of dogmatic early prognostication and a shift to delaying decisions about withholding and/or withdrawing care in these patients early after severe brain injury [23]. As noted by Schnakers and Zasler [18], these factors have driven greater levels of introspection regarding the clinical assessment and treatment of persons with DoC in general, and more specifically regarding issues of pain assessment and management. These advances in DoC knowledge and practice have also brought to light medicolegal as well as ethical considerations in the context of ongoing controversies regarding pain and suffering in persons with DoC, leading to a multitude of clinical management challenges extending well beyond the boundaries of end–of–life decisions [24][25][26].
2. Pain Pathoanatomy and Pathophysiology
The pain neuromatrix in the intact CNS is complex and still not fully understood [27] (see Figure 1). The pathophysiology of pain following brain insult or injury is more challenging, as the assessment of the exact underlying, potentially multisite, pathoanatomy of the injury is not always known and, for the reasons noted above, the post-injury neuropathology tends to not be of major help in determining a person’s potential for perceiving pain, whether nociceptive or non-nociceptive.
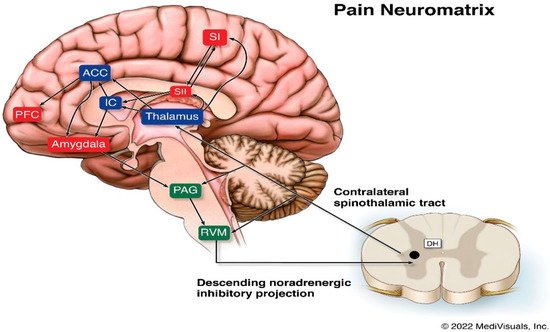
Figure 1. Legend: Schematic diagram of the pain neuromatrix involving cortical and subcortical structures. ACC, anterior cingulate cortex; DH, dorsal horn; IC, insular cortex; PAG, periaqueductal gray; PFC, prefrontal cortex; RVM, rostral ventral medulla; SI, primary somatosensory cortex; SII, secondary somatosensory cortex.
The ascending spinothalamic tract is a major pain pathway that carries nociceptive, thermal, and non-discriminative touch information to the thalamus. These axons then connect to the posterior limb of the internal capsule, and then to the postcentral gyrus and posterior paracentral lobule of the parietal lobe, where they mediate sharp pain sensations. Poorly localized pain sensations associated with the emotional features of pain are mediated via the intralaminar nuclei and end in the insular and rostral cingulate gyrus. The primary and secondary somatosensory, insular, anterior cingulate, and prefrontal cortices are the areas involved in qualitative aspects of pain, localization, intensity, and duration [28][29][30].
Suffering and the cognitive evaluative aspects of pain are mediated by the insular, anterior cingulate, and prefrontal cortices as well as subcortical limbic structures [31][32]. Spatiotemporal brain activity reorganization has been found to accompany the transition to chronic pain, with pain representation gradually shifting from sensory to emotional and limbic structures. Pain-induced limbic gamma oscillations have interestingly been shown to be related to pain perception in persons with DoC [33][34]. There is preliminary evidence that some patients in UWS may be able to perceive the affective components of pain through limbic circuit activation [33], which should give clinicians at least some pause to being dogmatic that those in a UWS cannot perceive pain.
Descending pathways transmit information from various regions of the brain, including the cortex, hypothalamus, and amygdala, to the periaqueductal gray in the midbrain, which further project to the somatosensory cortex. Spinothalamic tract damage, including to the thalamic nuclei, may be involved with persistent post-TBI pain. Dopaminergic system dysfunction due to alterations in dopamine signaling has been shown to occur after TBI and may be involved with persistent pain complaints. Hypothalamic dopamine projections to the spinal cord may augment, rather than suppress, pain, indicating that the effects of dopamine on pain are not necessarily consistent. Various other pathophysiological processes, including, but not necessarily limited to, neuroinflammation, neurodegeneration, and axonal damage have been implicated in various animal and human models of post-TBI pain, as have synaptic changes and genetic mechanisms [35][36].
Dysfunctional descending inhibition can also contribute to pain conditions resulting from brain injury. The descending system is of particular importance in the regulation of pain arising from injured peripheral tissues, such as the nerves, osseous structures, skin, and muscles. Descending inhibition is modulated by norepinephrine from the locus coeruleus and serotonin from the rostroentromedial medulla, and targets the spinal cord dorsal horn and the trigeminal nucleus. Alterations in norepinephrine neurotransmission and consequential motor and descending nociceptive inhibition have been found to play essential roles in pain modulation. Based on current knowledge, which includes neuroimaging studies, chronic pain may occur secondary to the disruption of descending pathways affecting nociceptive signaling mechanisms [35].
3. Pain Generators in Persons with DoC
The main causes of pain during early recovery post-trauma are fractures (both axial and extremity), solid organ injuries, soft tissue injuries, and inserted tubes (i.e., tracheal, nasogastric, urinary catheters, etc.). Invasive procedures, such as surgical interventions, central line insertions, and intravenous lines can all be pain generators [22][37]. In the subacute setting, similar issues are responsible for pain and may continue to serve as pain generators. Clinicians should be alert to clinical changes (vital signs, pain behaviors, including agitation, diaphoresis, and increased tone) on attempts at weaning pain medication. If pain generators persist, these reflexive responses to pain (which in and of themselves can be physiologically stressful to the patient’s system) should be addressed through appropriate pharmacological and non-pharmacological prescription. In select patients with a DoC, withdrawal of pain medications can lead to a conscious pain experience and potentially suffering.
Transition to lower levels of care does not imply that there is no longer a need for due diligence with regard to pain surveillance and treatment. As patients medically stabilize and the primary issue is not “life or death,” there tends to be greater attention paid to assessing pain if there is evidence of pain responsivity. Following acute care, common causes of pain may include spasticity, rigidity, dystonia, contractures, musculoskeletal pain, complex regional pain syndrome, shoulder subluxation, scoliosis, thalamic (central) pain, and/or skin breakdown [38][39]. Clinicians should always think about therapy interventions, such as range of motion exercises, as potentially painful experiences for individuals with a DoC [40], which has clinical implications as to whether the researchers should be pharmacologically prophylaxing for pain preemptory to such procedures.
Evidence strongly supports the mechanisms of central sensitization as being contributory to pain evolving to a chronic state [41]. Various neural areas have been found to be associated with both structural and functional changes (including in corticolimbic networks) as related to pain chronification [42][43]. Such mechanisms are generally not found to contribute to acute and subacute pain. There are now a number of different approaches utilized to treat chronic pain conditions that are theorized to be due to central sensitization phenomena, including potentially neuromodulatory techniques [39]. There are also a number of conditions that occur in persons with DoC that are unrelated to central sensitization, but are common causes of pain, including intracranial pressure changes, immobility, contractures, spasticity, skin breakdown, and urinary tract infections (with dysuria), among other conditions. Obviously, each of these conditions can be treated through appropriate surgical and/or non-surgical interventions that must be within the treatment armamentarium of any physician involved with the management of persons with DoC. Please refer to Table 1 for a list of common pain generators in persons with DoC.
Table 1. Common Pain Generators in Persons with DoC.
| Central/thalamic pain |
| Complex regional pain syndrome |
| Constipation |
| Dystonias |
| Indwelling devices |
| Infectious processes—pneumonia, urinary tract infections |
| Invasive procedures |
| Low or high intracranial pressure |
| Myofascial pain |
| Neuralgic pain |
| Neuropathic pain |
| Neurogenic heterotopic ossification |
| Neuromusculoskeletal scoliosis |
| Post-fracture pain |
| Range of motion attempts |
| Shoulder subluxation |
| Skin breakdown/pressure sores |
| Soft tissue contractures |
| Soft tissue injuries |
| Solid organ injuries |
| Spasticity, rigidity, dystonia |
4. Pain Assessment in Persons with DoC
Historically, accurate bedside assessment and the determination of consciousness of patients with a DoC has a high error rate [44][45][46]. Misinterpretation of behavioral signs may lead to errors that have ethical and clinical, as well as clinicolegal, implications relative to decisions regarding prognosis, treatment, and end–of–life decision making. In the assessment of non-communicative patients, it is essential to discriminate between reflex and higher-order behavioral responses [30]. Bedside examination of patients with DoC may determine their awareness/consciousness based on indirect clinical indicators, such as mimic reactions, crying, shouting, groaning, and grimacing. Newer evidence suggests that there may be additional clinical indicators of consciousness in the post-acute period, including the rate of spontaneous eye blinking [47].
Several scales have been validated to assess the presence of nociception in patients unable to communicate, such as newborns, infants, adolescents [48][49][50], and older and demented persons [51] (see Table 2). Since self-report pain evaluations, such as the Visual Analogic Scale (VAS) [52], are not possible to administer to non-communicative patients, the aforementioned scales are based on specific behavioral observations, such as noxious stimulus localization, restlessness or agitation, body movements, facial expressions, emotional reactions (grimaces, crying, screaming, and shouting), verbalizations, vocalizations, verbal complaints, and other non-verbal pain indicators [53]. Specific recommendations have been proposed by an American Task Force for all patients not able to communicate verbally (non-verbal patients) to receive appropriate pain management interventions [54].
Table 2. Pain Assessment Tools in Non-Communicative Patients.
| Scale | Patient Group |
|---|---|
| Children and Infants Post-operative Pain Scale (CHIPPS) | Newborns, Infants, and Adolescents |
| Face, legs, activity, cry and consolability (FLACC) | Newborns, Infants, and Adolescents |
| Pain Assessment in Advanced Dementia (PAINAD) | Geriatric/Dementia |
| Nociception Coma Scale (NCS) | DoC |
| Nociception Coma Scale–Revised (NCS–R) | DoC |
| Nociception Coma Scale–Revised–Personalized Stimulation (NCS–R–PS) | DoC |
| Brain Injury Nociception Assessment Measure (BINAM) | Severe Traumatic Brain Injury |
The variability of the pain sources in persons with prolonged DoC (PDoC) may lead to different individual reactions to the same noxious stimulation (pressure on the fingernail bed); thus, individualized pain stimulation in such patients should be used [54]. However, assessing nociception and behavioral responses to pain stimulation in individuals with severe brain injury and disorders of consciousness (DoC) still represents a challenge to any clinician charged with caring for such patients [18].
Several behavioral scales have been authored in an attempt to allow a more objective assessment of pain in this challenging population [54]. The Nociception Coma Scale (NCS) [55][56][57] is a tool that was designed to assist in the objective assessment of pain in persons with a DoC. The measure relies on the observation of the motor response (non-flaccid, abnormal posturing, flexion withdrawal, and localization), verbal response (non-verbalization, groaning, vocalization, and intelligible verbalization), visual response (none, startle response, eyes movement, and fixation), and facial expression (non-oral reflexive/startle response, grimace, and crying), following a defined and standardized noxious stimulation (i.e., pressure on the fingernail bed using an algometer). The visual item was subsequently removed from the NCS, and a revised version of the scale, the Nociception Coma Scale–Revised (NCS–R), was proposed by Chatelle [56] as it was found that the exclusion of the visual item did not change the overall assessment findings.
Sattin et al. [58] found lower pain pressure thresholds in participants with DoC compared to healthy controls. Pathologic pain responses, such as allodynia, have the potential to alter responses to stimuli, even though they may not be considered as painful. Personalized stimuli (e.g., hand opening, upper limb abduction, and head mobilization) have been proposed for pain stimuli response assessment on the NCS and NCS–R due to the alterations in pain pathway functions that might affect the response to standard pressure on the fingernail bed. Indeed, a preliminary study revealed that personalized, tailored painful stimulation was able to elicit more behavioral responses than standard stimuli [59]. An adapted version of the NCS–R using personalized stimulation (PS) may produce more intentional and specific responses to pain-inducing maneuvers. There is a clear and apparent need to conduct further research examining individual thresholds and the nature of the pain stimulus applied in patients with a DoC.
An integrated patient-centered approach for the assessment of physical pain in which clinical measures and bedside behavioral observations are assessed may improve treatment and rehabilitation outcomes. Furthermore, investigating pain perception in such patients through the use of a personalized source of nociception and pain may avoid non-specific, unreliable, and potentially harmful noxious assessments (as with standard pain scales) [22][60] and may provide tools for revealing nociception, even in the absence of any response to the standard clinical bedside exam. Through the use of data generated from the most involved caregivers (nurses, physiotherapists, physicians, and relatives), along with clinician-performed qualitative analysis, may provide more reliable data to assess for otherwise occult pain perception and potentially suffering in those who cannot otherwise convey their discomfort [54][59].
The Brain Injury Nociception Assessment Measure (BINAM), a specific measure of nociception, was developed for non-communicative patients with severe TBI. The BINAM consists of ten behavioral and physiological items and has been found to be reliable and feasible to administer, with accurate scores obtainable in about 10 min [61]. One of the advantages of BINAM in comparison with NCS–R is that the use of the latter scale may be less sensitive in the assessment of pain in patients with VS/UWS due to their low functional level [62]. Moreover, the subscores in the motor and verbal response categories of the NCS–R may be problematic to score in patients who are intubated, trached, paretic, and/or aphasic [63][64]. The effects of activity and analgesia with acetaminophen on BINAM have also been evaluated, demonstrating that the resulting score is largely independent of the level of consciousness or agitation [61][62][63][64].
Pain perception in persons with DoC remains a controversial issue, particularly due to the fact that there is evolving evidence of the presence of residual pain experience in some patients in VS/UWS [65][66][67][68][69][70]. Managing and treating pain may improve a patient’s ability to participate in assessments as well as therapies, reduce psychomotor agitation, improve sleep, and potentially even global outcomes. In this vein, the Italian Ministry of Health passed a law [71] that patients must receive proper pain treatment, and that pain assessment must be included in the patient’s evaluation. Bagnato et al. [72] found that admission NCS–R and CRS scores paralleled the levels of consciousness, but that, unlike the CRS–R, the NCS–R scoring was not related to the 6-month outcomes in UWS patients. The prior finding is seemingly somewhat paradoxical, given that prior studies showed similar correlations between higher NCS–R scores and CRS scores and the historical correlation of early higher CRS scores with better longer-term outcomes.
Proper assessment and treatment of pain in patients with DoC who may experience pain at a conscious level and potentially even suffer, yet be unable to demonstrate to the outside world their experience, should be a goal of all practitioners working with this challenging group of patients. A patient-centered and multidisciplinary approach is advocated to facilitate the assessment of pain in patients with a DoC to improve the diagnostic accuracy and ultimately the quality of management of a given patient’s pain experience [59]. It should also be noted that there is currently no consensus on which pain assessment scale is preferred in persons with a DoC based on either consensus or blinded prospective controlled studies, although there is a trend toward providing “personalized” stimulation.
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982.
- Medcape. Available online: https://emedicine.medscape.com/article/310834-overview (accessed on 14 January 2021).
- Scherer, K.R.; Schorr, A.; Johnstone, T. (Eds.) Appraisal Processes in Emotion: Theory, Methods, Research; Oxford University Press: Oxford, UK, 2001.
- Kyle, B.; McNeil, D.W. Autonomic arousal, and experimentally induced pain: A critical review of the literature. Pain Res. Manag. 2014, 19, 159–167.
- Bartolo, M.; Chiò, A.; Ferrari, S.; Tassorelli, C.; Tamburin, S.; Avenali, M.; Azicnuda, E.; Calvo, A.; Caraceni, A.T.; Defazio, G.; et al. Assessing and treating pain in movement disorders, amyotrophic lateral sclerosis, severe acquired brain injury, disorders of consciousness, dementia, oncology and neuroinfectivology. Evidence and recommendations from the Italian Consensus Conference on Pain in Neurorehabilitation. Eur. J. Phys. Rehabil. Med. 2016, 31, 841–854.
- Mischkowski, D.; Palacios-Barrios, E.E.; Banker, L.; Dildine, T.C.; Atlas, L.Y. Pain, or nociception? Subjective experience mediates the effects of acute noxious heat on autonomic responses. Pain 2018, 159, 699.
- Lee, G.I.; Neumeister, M.W. Pain: Pathways and physiology. Clin. Plast. Surg. 2020, 47, 173–180.
- Loeser, J.D.; Treede, R.D. The Kyoto protocol of IASP basic pain terminology. Pain 2008, 137, 473–477.
- Duffy, C.M. Pain versus suffering: A distinction currently without a difference. J. Med. Ethics 2021, 47, 175–178.
- Chen, J.; Kandle, P.; Murray, I.; Fitzgerald, L.A.; Sehdev, J.S. Pain, Physiology. In StatPearls; 2021; Volume 26. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539789/ (accessed on 14 January 2021).
- Duncan, G. Mind-body dualism and the biopsychosocialmodel of pain: What did descartes really say? J. Med. Philos. 2000, 25, 485–513.
- Sarno, J.E. The Mind Body Prescription: Healing the Body, Healing the Pain; Warner Books: New York, NY, USA; Hachette: Paris, French, 2001.
- Lee, M.C.; Tracey, I. Unravelling the mystery of pain, suffering, and relief with brain imaging. Curr. Pain Headache Rep. 2010, 14, 124–131.
- Frediani, F.; Bussone, G. When does the brain choose pain? J. Neurol. Sci. 2019, 40, S27–S29.
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58.
- Peacock, S.; Patel, S. Cultural influences on pain. Rev. Pain 2008, 1, 6–9.
- Miller, E.T.; Abu-Alhaija, D.M. Cultural influences on pain perception and management. Pain Manag. Nurs. 2019, 20, 183–184.
- Schnakers, C.; Zasler, N.D. Pain assessment and management in disorders of consciousness. Curr. Opin. Neurol. 2007, 20, 620–626.
- Pistoia, F.; Cassso, S.; Sarà, M.; Carolei, A. The perception of pain and management in disorders of consciousness. Curr. Pain Headache Rep. 2013, 17, 374.
- Demertzi, A.; Racine, E.; Bruno, M.A.; Ledoux, D.; Gosseries, O.; Vanhaudenhuyse, A.; Thonnard, M.; Soddu, A.; Moonen, G.; Laureys, S. Pain perception in disorders of consciousness: Neuroscience, clinical care, and ethics in dialogue. Neuroethics 2013, 6, 37–50.
- Chatelle, C.; Thibaut, A.; Whyte, J.; De Val, M.D.; Laureys, S.; Schnakers, C. Pain issues in disorders of consciousness. Brain Inj. 2014, 28, 1202–1208.
- Schnakers, C.; Zasler, N. Assessment, and management of pain in patients with disorders of consciousness. PM&R 2015, 7, 270–277.
- Kowalski, R.G.; Hammond, F.M.; Weintraub, A.H.; Nakase-Richardson, R.; Zafonte, R.D.; Whyte, J.; Giacino, J.T. Recovery of consciousness and functional outcome in moderate and severe traumatic brain injury. JAMA Neurol. 2021, 78, 548–557.
- Miller-Smith, L.; Finnsdóttir Wagner, Á.; Lantos, J.D. (Eds.) The difficulty with determining whether someone is dead. In Bioethics in the Pediatric ICU: Ethical Dilemmas Encountered in the Care of Critically Ill Children International Library of Ethics, Law, and the New Medicine; Springer International Publishing: Cham, Switzerland, 2019; pp. 45–68.
- Wolf-Meyer, M. Neurological disorders, affective bioethics, and the nervous system: Reconsidering the Schiavo case from a materialist perspective. Med. Humanity 2020, 46, 166–175.
- Formisano, R.; Zasler, N. Discontinuation of artificial nutrition and hydration and covert cognition. Brain Inj. 2020, 34, 1135.
- Khalid, S.; Tubbs, R.S. Neuroanatomy and Neuropsychology of Pain. Cureus 2017, 9, 1754.
- Coghill, R.C.; McHaffie, J.G.; Yen, Y.F. Neural correlates of interindividual differences in the subjective experience of pain. Proc. Natl. Acad. Sci. USA 2003, 14, 8538–8542.
- Chatelle, C.; Thibaut, A.; Bruno, M.A.; Boly, M.; Bernard, C.; Hustinx, R.; Schnakers, C.; Laureys, S. Nociception coma scale–revised scores correlate with metabolism in the anterior cingulate cortex. Neurorehabilit. Neural Repair 2014, 28, 149–152.
- Riganello, F.; Soddu, A.; Tonin, P. Addressing pain for a proper rehabilitation process in patients with severe disorders of consciousness. Front. Pharmacol. 2021, 12, 55.
- Bruel, B.M.; Ogidan, C.; McDeavitt, J. Chronic pain. In Textbook of Traumatic Brain Injury, 3rd ed.; Silver, J., McAllister, T., Arciniegas, D., Eds.; American Psychiatric Association Publishing: Washington, DC, USA, 2019; pp. 525–534.
- Ong, W.; Stohler, C.S.; Herr, D.R. Role of the prefrontal cortex in pain processing. Mol. Neurobiol. 2019, 56, 1137–1166.
- Calabro, R.S.; Naro, A.; Manuli, A.; Leo, A.; De Luca, R.; Buono, V.L.; Russo, M.; Bramanti, A.; Bramanti, P. Pain perception in patients with chronic disorders of consciousness: What can limbic system tell us? J. Clin. Neurophysiol. 2016, 128, 454–462.
- McCarbert, B.; Peppin, J. Pain pathways and nervous system plasticity: Learning and memory in pain. Pain Med. 2019, 20, 2421–2437.
- Irvine, K.A.; Clark, J.D. Chronic pain after traumatic brain injury: Pathophysiology and pain mechanisms. Pain Med. 2018, 19, 1315–1333.
- Walker, W.C. Pain pathoetiology after TBI: Neural and non-neural mechanisms. JHTR 2004, 19, 72–81.
- Barr, J.; Fraser, G.L.; Puntillo, K.; Ely, E.W.; Gélinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit. Care Med. 2013, 41, 263–306.
- Thibaut, A.; Chatelle, C.; Wannez, S.; Deltombe, T.; Stender, J.; Schnakers, C. Spasticity in disorders of consciousness: A behavioral study. Eur. J. Phys. Rehabil. Med. 2015, 51, 389–397.
- Zasler, N.D.; Martelli, M.F.; Clanton, S.T. Posttraumatic pain disorders: Medical assessment and management. In Brain Injury Medicine: Principles and Practice, 3rd ed.; Zasler, N.D., Katz, D., Zafonte, R., Eds.; Demos Publishers: New York, NY, USA, 2021; pp. 885–909.
- Bonin, E.A.; Fossati, M.L.B.; Filippini, M.M.; Bornheim, S.; Lejeune, N.; O’Brien, A.T.; Bodart, O.; Laureys, S.; Thibaut, A.; Chatelle, C. Evaluation of the effect of analgesic treatment on signs of nonciception-related behaviors during physiotherapy in patients with disorders of consciousness. Pain 2021, 163, e349–e356.
- Pak, D.J.; Yong, R.J.; Kaye, A.D.; Urman, R.D. Chronification of pain: Mechanisms, current understanding, and clinical implications. Curr. Pain Headache Rep. 2018, 22, 9.
- Vachon-Presseau, E.; Centeno, M.V.; Ren, W.; Berger, S.E.; Tetreault, P.; Ghantous, M.; Baria, A.; Farmer, M.; Baliki, M.N.; Schnitzer, T.J.; et al. The emotional brain as a predictor and amplifier of chronic pain. J. Den. Res. 2016, 95, 605–612.
- Yang, S.; Chang, M.C. Chronic pain: Structural and functional changes in brain structures and associated negative affective states. Int. J. Mol. Sci. 2019, 20, 3130.
- Schnakers, C.; Vanhaudenhuyse, A.; Giacino, J.; Ventura, M.; Boly, M.; Majerus, S.; Moonen, G.; Laureys, S. Diagnostic accuracy of the vegetative and minimally conscious state: Clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009, 9, 35.
- Bosco, A.; Lancioni, G.E.; Belardinelli, M.O.; Singh, N.N.; O’Reilly, M.F.; Sigafoos, J. Vegetative state: Efforts to curb misdiagnosis. Cogn. Process 2010, 11, 87–90.
- Van Erp, W.S.; Lavrijsen, J.C.; Vos, P.E.; Bor, H.; Laureys, S.; Koopmans, R.T. The vegetative state: Prevalence, misdiagnosis, and treatment limitations. J. Am. Med. Dir. Assoc. 2015, 16, 85–89.
- Magliacano, A.; Rosenfelder, M.; Hieber, N.; Bender, A.; Estraneo, A.; Trojano, L. Spontaneous eye blinking as a diagnostic marker in prolonged disorders of consciousness. Sci. Rep. 2021, 11, 22393.
- Buttner, W.; Finke, W. Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants, and young children; a comprehensive report on seven consecutive studies. Paediatr. Anaesth. 2020, 10, 303–318.
- Hummel, P.; van Dijk, M. Pain assessment: Current status and challenges. In Seminars in Fetal and Neonatal Medicine; WB Saunders: Philadelphia, PA, USA, 2006; Volume 11, pp. 237–245.
- Merkel, S.; Voepel-Lewis, T.; Malviya, S. Pain control: Pain assessment in infants and young children: The FLACC scale. Am. J. Nurs. Sci. 2002, 102, 55–58.
- Warden, V.; Hurley, A.C.; Volicer, L. Development, and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. J. Am. Med. Dir. Assoc. 2003, 4, 9–15.
- Flynn, D.; Van Schaik, P.; Van Wersch, A. A comparison of multi-item likert and visual analogue scales for the assessment of transactionally defined coping function1. Eur. J. Psychol. Assess. 2004, 20, 49–58.
- Feldt, K.S. The checklist of nonverbal pain indicators (CNPI). Pain Manag. Nurs. 2000, 1, 13–21.
- Herr, K.; Coyne, P.J.; Key, T.; Manworren, R.; McCaffery, M.; Merkel, S.; Pelosi-Kelly, J.; Wild, L. Pain assessment in the nonverbal patient: Position statement with clinical practice recommendations. American Society for Pain Management Nursing. Pain Manag. Nurs. 2006, 7, 44–52.
- Schnakers, C.; Chatelle, C.; Majerus, S.; Gosseries, O.; De Val, M.; Laureys, S. Assessment and detection of pain in non-communicative severely brain-injured patients. Expert Rev. Neurother. 2010, 10, 1725–1731.
- Chatelle, C.; Majerus, S.; Whyte, J.; Laureys, S.; Schnakers, C. A sensitive scale to assess nociceptive pain in patients with disorders of consciousness. J. Neurol. Neurosurg. Psyc. 2012, 83, 1233–1237.
- Riganello, F.; Cortese, M.D.; Arcuri, F.; Candelieri, A.; Guglielmino, F.; Dolce, G.; Sannita, W.G.; Schnakers, C. A study of the reliability of the nociception coma scale. Clin. Rehabil. 2014, 29, 388–393.
- Sattin, D.; Schnakers, C.; Pagani, M.; Arenare, F.; Devalle, G.; Giunco, F.; Guizzetti, G.; Lanfranchi, M.; Giovannetti, A.M.; Covelli, V.; et al. Evidence of altered pressure pain thresholds in persons with disorders of consciousness as measured by the Nociception Coma Scale–Italian version. Neuropsychol. Rehabil. 2018, 28, 1295–1310.
- Formisano, R.; Contrada, M.; Aloisi, M.; Ferri, G.; Schiattone, S.; Iosa, M.; Buzzi, M.G. Nociception Coma Scale with personalized painful stimulation versus standard stimulus in non-communicative patients with disorders of consciousness. Neuropsychol. Rehabil. 2020, 30, 1893–1904.
- Tsetsou, S.; Novy, J.; Oddo, M.; Rossetti, A.O. EEG reactivity to pain in comatose patients: Importance of the stimulus type. Resuscitation 2015, 97, 34–37.
- Whyte, J.; Poulsen, I.; Ni, P.; Eskildsen, M.; Guldager, R. Development of a measure of nociception for patients with severe brain injury. Clin. J. Pain 2020, 36, 281–288.
- Poulsen, I.; Balle, M.; Givard, K.L. Nociception Coma Scale–Revised: Nurses’ Experience in Clinical Practice. Pain Manag. Nurs. 2019, 20, 592–598.
- Vink, P.; Eskes, A.M.; Lindeboom, R.; van den Munckhof, P.; Vermeulen, H. Nurses assessing pain with the Nociception Coma Scale: Interrater reliability and validity. Pain Manag. Nurs. 2014, 15, 881–887.
- Vink, P.; Lucas, C.; Maaskant, J.M.; van Erp, W.S.; Lindeboom, R.; Vermeulen, H. Clinimetric properties of the Nociception Coma Scale (Revised): A systematic review. Eur. J. Pain 2017, 21, 463–1474.
- Coleman, M.R.; Davis, M.H.; Rodd, J.M.; Robson, T.; Ali, A.; Owen, A.M.; Pickard, J.D. Towards the routine use of brain imaging to aid the clinical diagnosis of disorders of consciousness. Brain 2009, 132, 2541–2552.
- de Tommaso, M.; Navarro, J.; Lanzillotti, C.; Ricci, K.; Buonocunto, F.; Livrea, P.; Lancioni, G.E. Cortical responses to salient nociceptive and not nociceptive stimuli in vegetative and minimal conscious state. Front. Hum. Neurosci. 2015, 9, 17.
- Kassubek, J.; Juengling, F.D.; Els, T.; Spreer, J.; Herpers, M.; Krause, T.; Moser, E.; Lücking, C.H. Activation of a residual cortical network during painful stimulation in long-term postanoxic vegetative state: A 15O-H2O PET study. J. Neurol. Sci. 2003, 212, 85–91.
- Laureys, S.; Faymonville, M.E.; Peigneux, P.; Damas, P.; Lambermont, B.; Del Fiore, G.; Degueldre, C.; Aerts, J.; Luxen, A.; Franck, G.; et al. Cortical processing of noxious somatosensory stimuli in the persistent vegetative state. Neuroimage 2002, 7, 732–741.
- Schnakers, C.; Chatelle, C.; Demertzi, A.; Majerus, S.; Laureys, S. What about Pain in Disorders of Consciousness? AAPS J. 2012, 14, 437–444.
- Schnakers, C.; Chatelle, C.; Vanhaudenhuyse, A.; Majerus, S.; Ledoux, D.; Boly, M.; Bruno, M.A.; Boveroux, P.; Demertzi, A.; Moonen, G.; et al. The Nociception Coma Scale: A new tool to assess nociception in disorders of consciousness. Pain 2010, 148, 215–219.
- Ministry of Health. Report to the Italian Parliament on the implementation of Law no. 38, of 15th of March 2010 on “Measures to Ensure Access to Palliative Care and Pain Therapy”. 2014. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_3046_allegato.pdf (accessed on 14 January 2021).
- Bagnato, S.; Boccagni, C.; Sant’Angelo, A.; Alito, A.; Galardi, G. Pain assessment with the revised nociception coma scale and outcomes of patients with unresponsive wakefulness syndrome: Results from a pilot study. Neurol. Sci. 2018, 39, 1073–1077.
More
Information
Subjects:
Pathology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
3 times
(View History)
Update Date:
21 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




