Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Benwei Zhu | + 1960 word(s) | 1960 | 2022-03-19 04:13:58 | | | |
| 2 | Vicky Zhou | + 3 word(s) | 1963 | 2022-03-19 05:26:07 | | | | |
| 3 | Vicky Zhou | + 3 word(s) | 1963 | 2022-03-19 05:26:54 | | | | |
| 4 | Vicky Zhou | + 3 word(s) | 1963 | 2022-03-19 05:27:20 | | | | |
| 5 | Vicky Zhou | + 3 word(s) | 1963 | 2022-03-19 05:30:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhu, B. Ulva (Enteromorpha) Polysaccharides and Oligosaccharides. Encyclopedia. Available online: https://encyclopedia.pub/entry/20755 (accessed on 07 February 2026).
Zhu B. Ulva (Enteromorpha) Polysaccharides and Oligosaccharides. Encyclopedia. Available at: https://encyclopedia.pub/entry/20755. Accessed February 07, 2026.
Zhu, Benwei. "Ulva (Enteromorpha) Polysaccharides and Oligosaccharides" Encyclopedia, https://encyclopedia.pub/entry/20755 (accessed February 07, 2026).
Zhu, B. (2022, March 19). Ulva (Enteromorpha) Polysaccharides and Oligosaccharides. In Encyclopedia. https://encyclopedia.pub/entry/20755
Zhu, Benwei. "Ulva (Enteromorpha) Polysaccharides and Oligosaccharides." Encyclopedia. Web. 19 March, 2022.
Copy Citation
The high-valued utilization of Ulva (previously known as Enteromorpha) bioresources has drawn increasing attention due to the periodic blooms of world-wide green tide. The polysaccharide is the main functional component of Ulva and exhibits various physiological activities. The Ulva oligosaccharide as the degradation product of polysaccharide not only possesses some obvious activities, but also possesses excellent solubility and bioavailability. Both Ulva polysaccharides and oligosaccharides hold promising potential in the food industry as new functional foods or food additives. Studies on Ulva polysaccharides and oligosaccharides are increasing and have been the focus of the marine bioresources field.
Ulva
polysaccharide
oligosaccharide
activity
1. Introduction
The Ulva (previously known as Enteromorpha Enteromorpha), known as green-tide-forming macroalgae, has drawn increasing attention in both the marine environment protection and marine bioresources fields [1][2]. Recently, the green tide blooms more and more frequently due to the global seawater eutrophication and temperature rise [3][4][5][6][7]. The largest Ulva-forming green tide in history occurred in the Yellow Sea of China this year, and covered almost 1746 km2, producing over 24 million tons of biomass [8]. The Ulva genus belongs to the Ulvaceae family and includes nearly 40 kinds of species such as Ulva prolifera (previously known as Enteromorpha prolifera), Ulva linza (previously known as Enteromorpha linza), and Ulva intestinalis (previously known as Enteromorpha intestinalis) (as shown in Figure 1) [9]. For a long time, the Ulva and Enteromorpha were considered as two different genera, but the molecular evidence indicated that Ulva and Enteromorpha are not distinct evolutionary entities and should not be recognized as separate genera [9]. Therefore, the taxonomic name “Enteromorpha” is currently regarded as a synonym for Ulva.
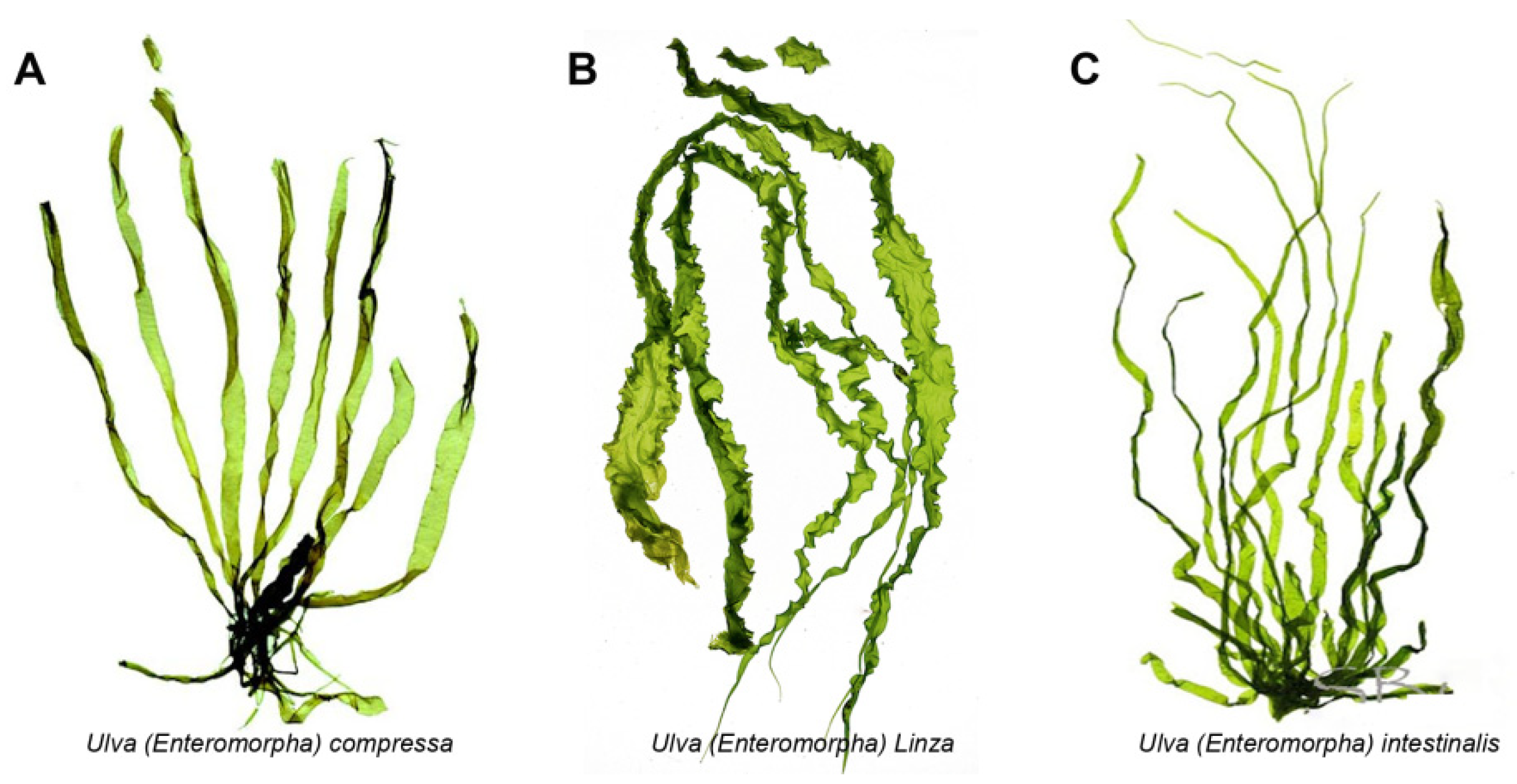
Figure 1. The morphology pictures of three kinds of Ulva species. (A). Ulva compressa; (B). Ulva linza; (C). Ulva intestinalis.
The Ulva polysaccharide constitutes the main component of the cell wall of Ulva species algae, and it accounts for nearly 18% of the dry weight. In addition, it possesses various physiological properties such as antioxidant, anticoagulant, antitumor, antiaging and immune regulatory activities [10][11][12]. Therefore, the Ulva polysaccharides could be widely used as medicine and chemical agents in the agricultural and medical fields [13][14].
It is worth noting that another green algal polysaccharide, Ulvan, has also drawn increased attention, and its structure has been well characterized. The water-soluble sulfated polysaccharide is mainly extracted from Ulva sp. and consists of a linear backbone with L-rhamnose-3-sulfate (Rha3S), D-glucuronic acid (GlcUA), L-iduronic acid (IdoA) and D-xylose (Xyl), and the sulfate group is linked to the rhamnose. The two major repeating disaccharide units of ulvan are →4)-β-D-glucuronic acid (1→4)-α-L-rhamnose-3-sulfate (1→(A3S) and →4) α-L-iduronic acid (1→4)-α-L-rhamnose-3-sulfate (1→(B3S) [15]. However, the Ulva polysaccharide possesses a more complex chemical composition and fine structure. In addition, the Ulva polysaccharides exhibit great potential as functional foods and food additives due to their obvious metabolism-regulatory activity [16][17][18]. For instance, Guo et al. discovered that the polysaccharides extracted from Ulva prolifera could prevent high-fat diet-induced obesity in hamsters [19]. They also found that polysaccharides isolated from Ulva prolifera could protect against carbon tetrachloride-induced acute liver injury in mice via the activation of Nrf2/HO-1 signaling, and the suppression of oxidative stress, inflammation and apoptosis [20]. Li et al. found that the Ulva polysaccharides could improve blood glucose regulation, blood lipid metabolism and liver oxidative stress in T2DM cells [21]. However, the applications of the Ulva polysaccharide have been greatly limited by its poor solubility and low bioavailability [22]. In order to overcome this drawback, it is feasible to degrade the polysaccharide into oligosaccharide, which also possesses the biological activities but also has much better solubility and bioavailability [23]. The methods for polysaccharide degradation mainly include physicochemical or enzymatic methods [24][25][26]. In particular, the enzymatic method has drawn increasing attention due to its advantages, such as its mild reaction conditions and specific product distributions.
2. Activity of Ulva Polysaccharide
The activity of Ulva polysaccharide has been symmetrically investigated and characterized, and this green algal polysaccharide exhibited diverse biological activities such as antioxidant, antitumor, immunomodulatory, anticoagulant and hypolipidemic activities [11].
2.1. Antioxidant Activity
Many algal polysaccharides such as alginate, carrageenan and agar possessed obvious antioxidant activity by cleaning the oxidant radicals and improving antioxidant enzymes’ activity [22]. Xu et al. evaluated the antioxidant activities of Ulva polysaccharide by determining their ability to scavenge 1, 1-diphenyl-2-picrylhydrazyl (DPPH), hydroxyl (OH•), and superoxide anion (O2•−) radicals [25]. The results suggested that the Ulva polysaccharide could clean up DPPH, OH•, and O2•− [25]. It could also improve the activities of endogenous antioxidant enzymes such as catalase, glutathione peroxidase, and superoxide dismutase, which have been viewed as the major defense system against reactive oxygen species (ROS) during oxidative stress [27]. Moreover, Tang et al. found that the polysaccharides could reduce the content of maleic dialdehyde (MDA) in serum. The low MDA levels resulted in lower oxidant stress and lipid peroxidation [11].
2.2. Antitumor Activity
The antitumor activity of Ulva polysaccharide has aroused increasing interest due to the tumor’s multiplicity worldwide [28][29]. Jiao et al. found that polysaccharides could inhibit tumor growth in S180 tumor-bearing mice, and could increase the relative spleen and thymus weight [10]. They also promoted the expression of tumor necrosis factor-alpha (TNF-α) in serum and induced lymphocyte proliferation, induced the production of TNF-α in macrophages, and stimulated macrophages to produce nitric oxide dose-dependently through the up-regulation of inducible NO synthase activity [30]. The Ulva polysaccharide could motivate modulation of the immune system to indirectly inhibit tumor cells without direct cytotoxicity [31].
2.3. Immune Regulatory Activity
The immune system includes nonspecific and specific immunity [32]. Nonspecific immunity can immediately respond to invaders without encountering previous pathogens, and gives signals to subsequently activate adaptive specific immunity [33]. Specific immunity involves B- and T-lymphocytes, and its function is activated immediately after the initial antigenic stimulus [34]. The Ulva polysaccharide can significantly increase the relative spleen and thymus weight of tumor-bearing animals, promote the secretion of tumor necrosis factor alpha (TNF-α), stimulate lymphocyte proliferation, and augment phagocytosis and secretion of NO and TNF-α in peritoneal macrophages [35]. In addition, the Ulva polysaccharide could promote the proliferation of B lymphocytes and T lymphocytes, activate the NK cell and induce the delayed apoptosis of neutrophils, as shown in Figure 2. More specifically, the polysaccharides could increase the production of reactive oxygen species (ROS), IL-6, and TNF-α through regulating the expressions of iNOS, IL-6, and TNF-α. In addition, the polysaccharides can strengthen the macrophage phagocytic activity, activate NK cells, increase thymus and spleen indices, and delay neutrophil apoptosis [7][36] (Figure 2).
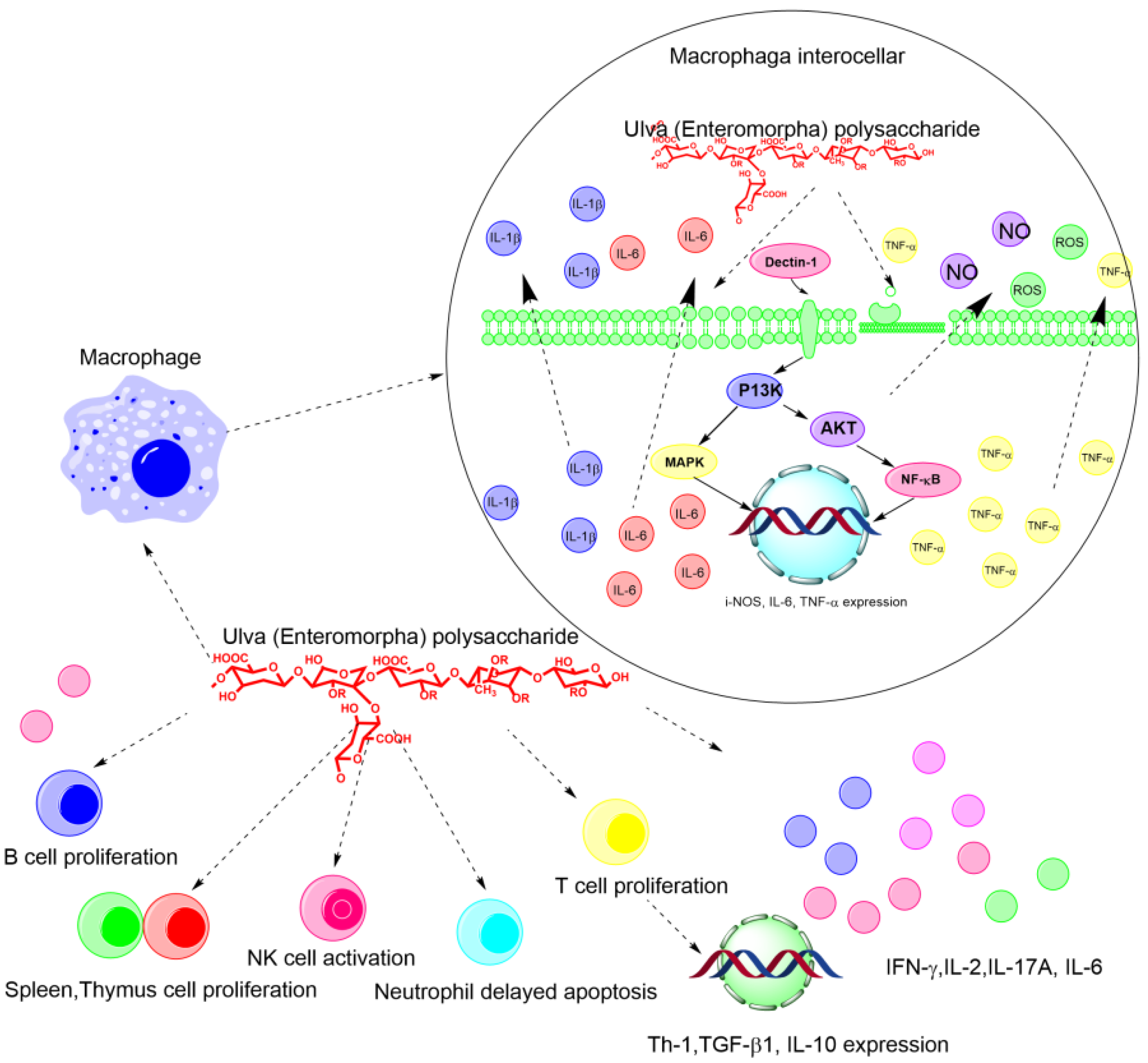
Figure 2. The schematic diagram of the immune regulatory and antitumor mechanism of Ulva polysaccharides on the molecular and cellular level.
2.4. Anticoagulant Activity
It has been reported that polysaccharides from green alga have been investigated, showing stronger anticoagulant activities than those from brown and red alga [37][38][39]. Wang et al. investigated and elucidated the anticoagulant activity of polysaccharide from green algae Ulva linza in the coagulation assays, and activated partial thromboplastin time (APTT), thrombin time (TT) and prothrombin time (PT) [40]. The results suggested that the sulfated polysaccharides could prolong APTT and TT, but not TP. These activities strongly depended on the degree of sulfation (DS), the molecular weights (MW) and the branching structure of polysaccharides [40]. Qi et al. evaluated the anticoagulant activity of polysaccharides from Ulva clathrata and an in vitro anticoagulant assay indicated that FEP effectively prolonged the activated partial thromboplastin time and thrombin time [37][41].
2.5. Hypolipidemic Activity
Hyperlipidemia, as a common endocrine disease, induces cerebrovascular and cardiovascular activity and atherosclerosis [42][43]. While hypolipidemic drugs such as statins prevent and cure hyperlipidemia, their side effects cannot be ignored [42]. Teng et al. reported that Ulva prolifera polysaccharides presented high anti-hyperlipidemic activities which inhibited the body weight gain and also decreased triacylglycerol (TG), the total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) levels of plasma and liver [44]. They also inhibited the expressions of sterol regulatory element-binding protein-1c (SREBP-1c) and hepatic acetyl-CoA carboxylase (ACC) in high-fat diet rats. SREBP-1c enhances the transcription of the required genes for fatty acid synthesis [44]. ACC, as the rate-limiting enzyme in de-novo lipogenesis, controls the β-oxidation of fatty acids in the mitochondria. Moreover, Ulva prolifera polysaccharides showed pancreatic lipase inhibition activity [45]. The polysaccharide from Ulva prolifera exhibited a stronger hypolipidemic effect than simvastatin and enhanced endogenous antioxidant enzymes and decreased MDA content and lipid peroxidation in serum [44][46].
3. Activity of Ulva Oligosaccharides
So far, more and more studies are focusing on the activity of Ulva oligosaccharides [22][23]. However, the reports are still very scattered with the mechanism of related activity and the structural-activity relationship of oligosaccharides was still undefined due to the complexity of the Ulva oligosaccharides’ structure. Lü et al. evaluated the antibacterial activity of Ulva oligosaccharides and their selenized derivatives prepared by acid method [47]. They found that the selenized Ulva oligosaccharides showed stronger inhibitory activity towards Eschetichia coli and plant pathogenic fungi than that to Staphylococcus aureus [47][48]. Liu et al. studied the anti-aging and anti-oxidation effects of Ulva oligosaccharides in SAMP8 mice [23]. They found that Ulva oligosaccharides can protect neurons in the hippocampus by significantly reducing the secretion of inflammatory factors such as IFN-γ, TNF-α and IL-6, and improving the brain-derived neurotrophic factor (BDNF) [23]. Liu et al. evaluated the immunoregulatory effect of Ulva oligosaccharides in a cyclophosphamide-induced immunosuppression mouse model [49]. It can be found that Ulva oligosaccharides can activate the immune system by promoting the secretion of NO, up-regulating the expression of cytokines such as IL-1β, IL-6 and TNF-α, and activating inflammatory bodies such as iNOS, COX2 and NLRP3 [49]. Xu et al. studied the antioxidant activities of three Ulva oligosaccharides, and found that Ulva oligosaccharides can effectively eliminate the DPPH, OH•, and O2•− [25]. Li et al. investigated the antioxidant capacity of Ulva oligosaccharides and found that the activity was closely related to molecular weight [50]. Specifically, Ulva oligosaccharides with low molecular weight can scavenge superoxide anion and hydroxyl radicals with an IC50 of 0.39 mg/mL [50]. Zhang et al. found that 2.28 mg/mL of Ulva oligosaccharides prepared by an H2O2 oxidation method can scavenge 92.2% of the hydroxyl radical, which is higher than Ulva polysaccharides with the same concentration [26]. That is probably because there were more hydroxyl groups in the oligosaccharides’ structure [26]. Cui et al. prepared complexes of Fe2+ ions and Ulva oligosaccharides, which can be used to treat iron deficiency anemia as a nutritional supplement for iron [51]. Wang et al. discovered that Ulva oligosaccharides possessed an anticoagulant activity which was closely related to the number and distribution of sulfuric acid groups in oligosaccharides [40]. Jin et al. prepared ep-3-H, a glucuronic-xylo-rhamnose-component, from Ulva prolifera, and found that EP-3-H could inhibit cell proliferation of human lung cancer cells by interacting with the fibroblast growth factors FGF1 and FGF2 [52]. In addition, the physiological activities may differ in Ulva oligosaccharides and polysaccharides, but the specific mechanism still remains unclear. However, herein could propose the possible reasons based on some experience. The active groups appeared after the linkage of the polysaccharide was broken down by physical, chemical or enzymatic hydrolysis, and therefore the activities of Ulva oligosaccharide became more obvious than the polysaccharide. To sum up, the current studies on the activity of Ulva oligosaccharides are relatively superficial since there is still no appropriate method to obtain oligosaccharides with a fine structure for studying the structure-activity relationship of oligosaccharides due to their quite complex structure.
3. Conclusions and Future Perspective
In recent years, the biomass of Ulva has increased rapidly worldwide, resulting in a large number of green tides [4][49][53]. Actually, Ulva prolifera has invaded the Yellow Sea for 15 consecutively years, which has damaged the marine ecological environment in Qingdao and the coastal cities of Shandong Province. It is therefore urgent to effectively curb the growth of Ulva prolifera and achieve the harmless and high-value utilization of Enteromorpha prolifera [54][55][56] (as shown in Figure 3). For instance, the Ulva polysaccharide and oligosaccharide could eliminate the oxidative radicals such as DPPH, OH•, and O2•−, and they could also promote the proliferation of probiotics of intestinal microbiome composition. In addition, the Ulva polysaccharide exhibited obvious hypolipidemic activity; therefore, the Ulva polysaccharide and oligosaccharide can be used as a functional food, a food additive, an antioxidant agent, and animal feed. Due to its excellent rheological properties, gelling behavior, texture characteristics and antibacterial activity, the Ulva polysaccharide could be developed as a novel medical dressing to prevent bacterial infection. More importantly, the Ulva polysaccharide and oligosaccharide both possess obvious physiological activities such as immune regulatory, antitumor, anticoagulant and hypolipidemic activities, they are important resources for developing novel marine drugs for curing various malignant tumors, and they are used in the treatment of hyperlipidemia, hypertension and other metabolic diseases. This kind of carbohydrate that originated from green algae has drawn increasing attentions and became a topic of much discussion in the marine bioresources and functional foods fields.
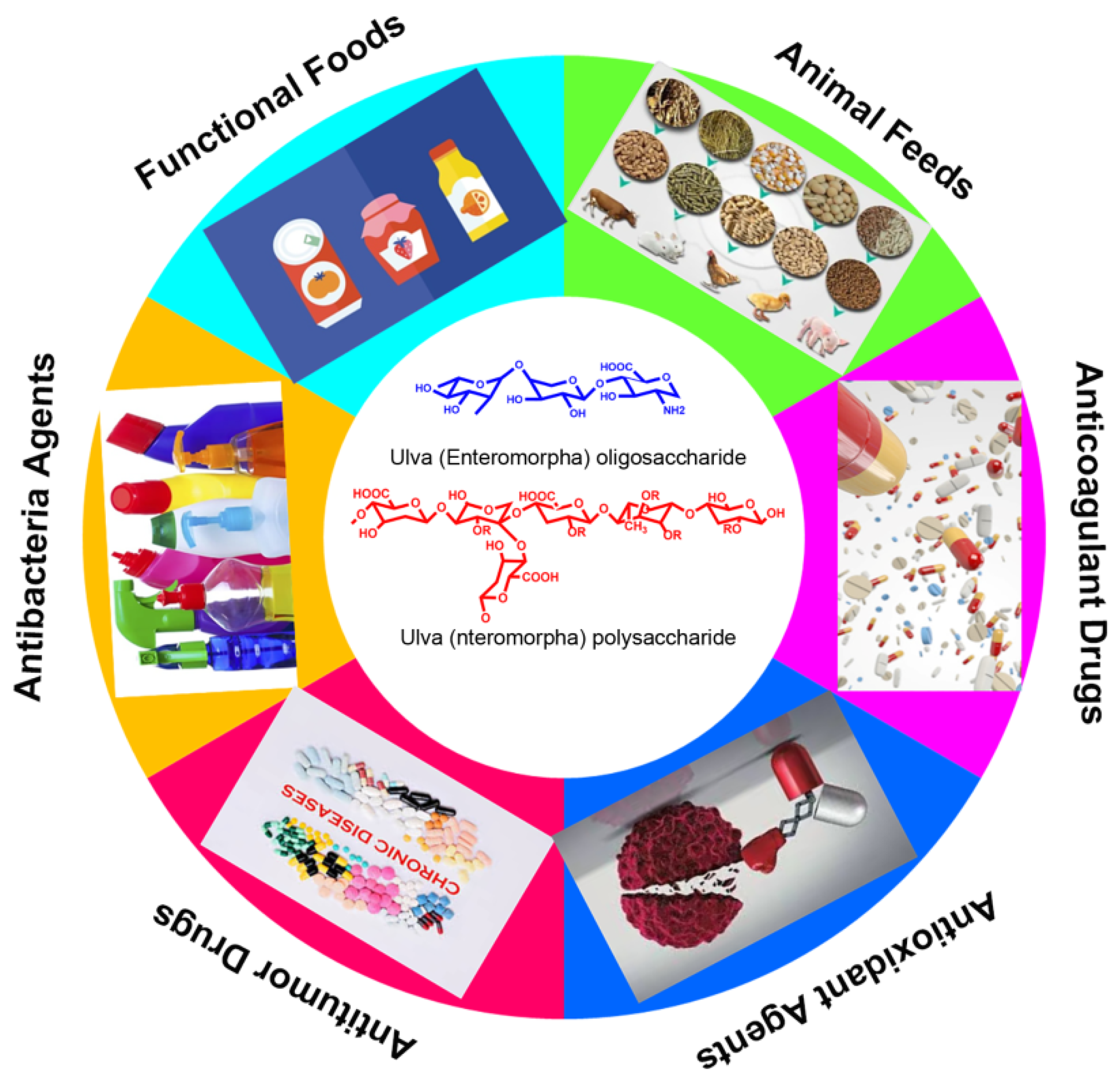 Figure 3. The potential and promising applications of Ulva polysaccharide and oligosaccharides.
Figure 3. The potential and promising applications of Ulva polysaccharide and oligosaccharides.References
- Blomster, J.; Bäck, S.; Fewer, D.P.; Kiirikki, M.; Lehvo, A.; Maggs, C.A.; Stanhope, M.J. Novel morphology in Enteromorpha (Ulvophyceae) forming green tides. Am. J. Bot. 2002, 89, 1756–1763.
- Zhong, L.; Zhang, J.; Ding, Y. Energy Utilization of Algae Biomass Waste Enteromorpha Resulting in Green Tide in China: Pyrolysis Kinetic Parameters Estimation Based on Shuffled Complex Evolution. Sustainability 2020, 12, 2086.
- Silveira Coelho, M.; da Silva Menezes, B.; Rivero Meza, S.L.; Lainetti Gianasi, B.; de las Mercedes Salas-Mellado, M.; Copertino, M.; da Rosa Andrade Zimmermann de Souza, M. Potential Utilization of Green Tide-Forming Macroalgae from Patos Lagoon, Rio Grande-RS, Brazil. J. Aquat. Food Prod. Technol. 2016, 25, 1096–1106.
- Van Alstyne, K.L.; Nelson, T.A.; Ridgway, R.L. Environmental Chemistry and Chemical Ecology of “Green Tide” Seaweed Blooms. Integr. Comp. Biol. 2015, 55, 518–532.
- Wang, Z.; Xiao, J.; Fan, S.; Li, Y.; Liu, X.; Liu, D. Who made the world’s largest green tide in China?—An integrated study on the initiation and early development of the green tide in Yellow Sea. Limnol. Oceanogr. 2015, 60, 1105–1117.
- Yabe, T.; Ishii, Y.; Amano, Y.; Koga, T.; Hayashi, S.; Nohara, S.; Tatsumoto, H. Green tide formed by free-floating Ulva spp. at Yatsu tidal flat, Japan. Limnology 2009, 10, 239–245.
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.-O. Fucoidan from Macrocystis pyrifera Has Powerful Immune-Modulatory Effects Compared to Three Other Fucoidans. Mar. Drugs 2015, 13, 1084–1104.
- Xiao, J.; Wang, Z.; Liu, D.; Fu, M.; Yuan, C.; Yan, T. Harmful macroalgal blooms (HMBs) in China’s coastal water: Green and golden tides. Harmful Algae 2021, 107, 102061.
- Hayden, H.S.; Blomster, J.; Maggs, C.A.; Silva, P.C.; Stanhope, M.J.; Waaland, J.R. Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. Eur. J. Phycol. 2003, 38, 277–294.
- Jiao, L.; Jiang, P.; Zhang, L.; Wu, M. Antitumor and immunomodulating activity of polysaccharides from Enteromorpha intestinalis. Biotechnol. Bioprocess Eng. 2010, 15, 421–428.
- Tang, Z.; Gao, H.; Wang, S.; Wen, S.; Qin, S. Hypolipidemic and antioxidant properties of a polysaccharide fraction from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 58, 186–189.
- Chattopadhyay, K.; Mandal, P.; Lerouge, P.; Driouich, A.; Ghosal, P.; Ray, B. Sulphated polysaccharides from Indian samples of Enteromorpha compressa (Ulvales, Chlorophyta): Isolation and structural features. Food Chem. 2007, 104, 928–935.
- Jiang, F.; Chi, Z.; Ding, Y.; Quan, M.; Tian, Y.; Shi, J.; Liu, C. Wound Dressing Hydrogel of Enteromorpha prolifera Polysaccharide–Polyacrylamide Composite: A Facile Transformation of Marine Blooming into Biomedical Material. ACS Appl. Mater. Interfaces 2021, 13, 14530–14542.
- Zhong, R.; Wan, X.; Wang, D.; Zhao, C.; Liu, D.; Gao, L.; Cao, H. Polysaccharides from Marine Enteromorpha: Structure and function. Trends Food Sci. Technol. 2020, 99, 11–20.
- Tang, Z.; Yu, Z.; Zhao, W.; Guo, J.; Gao, L.; Qin, S. Ultrasonic extraction of polysaccharides from Enteromorpha. Mod. Food Sci. Technol. 2011, 27, 56–59.
- Liu, W.; Zhou, S.; Balasubramanian, B.; Zeng, F.; Sun, C.; Pang, H. Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish. Immunol. 2020, 104, 202–212.
- Shang, Q.; Wang, Y.; Pan, L.; Niu, Q.; Li, C.; Jiang, H.; Cai CHao, J.; Li, G.; Yu, G. Dietary Polysaccharide from Enteromorpha Clathrata Modulates Gut Microbiota and Promotes the Growth of Akkermansia muciniphila, Bifidobacterium spp. and Lactobacillus spp. Mar. Drugs 2018, 16, 167.
- Xie, C.; Zhang, Y.; Niu, K.; Liang, X.; Wang, H.; Shan, J.; Wu, X. Enteromorpha polysaccharide-zinc replacing prophylactic antibiotics contributes to improving gut health of weaned piglets. Anim. Nutr. 2021, 7, 641–649.
- Guo, F.; Han, M.; Lin, S.; Ye, H.; Chen, J.; Zhu, H.; Lin, W. Enteromorpha prolifera polysaccharide prevents high-fat diet-induced obesity in hamsters: A NMR-based metabolomic evaluation. J. Food Sci. 2021, 86, 3672–3685.
- Guo, F.; Zhuang, X.; Han, M.; Lin, W. Polysaccharides from Enteromorpha prolifera protect against carbon tetrachloride-induced acute liver injury in mice via activation of Nrf2/HO-1 signaling, and suppression of oxidative stress, inflammation and apoptosis. Food Funct. 2020, 11, 4485–4498.
- Li, X.; Guozhu, Z.; Zhifei, L.; Yang, S.; Peijun, L.; Jing, L. Hypoglycemic activity of Enteromorpha intestinalis polysaccharide. Sci. Technol. Food Ind. 2021, 42, 321–326.
- Zhu, B.; Ni, F.; Xiong, Q.; Yao, Z. Marine oligosaccharides originated from seaweeds: Source, preparation, structure, physiological activity and applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 60–74.
- Liu, X.; Liu, D.; Lin, G.; Wu, Y.; Gao, L.; Ai, C.; Zhao, C. Anti-ageing and antioxidant effects of sulfate oligosaccharides from green algae Ulva lactuca and Enteromorpha prolifera in SAMP8 mice. Int. J. Biol. Macromol. 2019, 139, 342–351.
- Shao, L.; Xu, J.; Shi, M.; Wang, X.; Li, Y.; Kong, L.; Hider, R.; Zhou, T. Preparation, antioxidant and antimicrobial evaluation of hydroxamated degraded polysaccharides from Enteromorpha prolifera. Food Chem. 2017, 237, 481–487.
- Xu, J.; Xu, L.; Zhou, Q.; Hao, S.; Zhou, T.; Xie, H. Isolation, purification, and antioxidant activities of degraded polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2015, 81, 1026–1030.
- Zhang, Z.; Wang, X.; Mo, X.; Qi, H. Degradation and the antioxidant activity of polysaccharide from Enteromorpha linza. Carbohydr. Polym. 2013, 92, 2084–2087.
- Lin, G.; Wu, D.; Xiao, X.; Huang, Q.; Chen, H.; Liu, D.; Zhao, C. Structural characterization and antioxidant effect of green alga Enteromorpha prolifera polysaccharide in Caenorhabditis elegans via modulation of microRNAs. Int. J. Biol. Macromol. 2020, 150, 1084–1092.
- Minton, N.P. Clostridia in cancer therapy. Nat. Rev. Microbiol. 2003, 1, 237–242.
- Sawyers, C. Targeted cancer therapy. Nature 2004, 432, 294–297.
- Jiao, L.; Li, X.; Li, T.; Jiang, P.; Zhang, L.; Wu, M.; Zhang, L. Characterization and anti-tumor activity of alkali-extracted polysaccharide from Enteromorpha intestinalis. Int. Immunopharmacol. 2009, 9, 324–329.
- Kim, J.-K.; Cho, M.L.; Karnjanapratum, S.; Shin, I.-S.; You, S.G. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2011, 49, 1051–1058.
- Delves, P.J.; Roitt, I.M. The Immune System. N. Engl. J. Med. 2000, 343, 37–49.
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789.
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232.
- Ren, X.; Liu, L.; Gamallat, Y.; Zhang, B.; Xin, Y. Enteromorpha and polysaccharides from Enteromorpha ameliorate loperamide-induced constipation in mice. Biomed. Pharmacother. 2017, 96, 1075–1081.
- Zou, M.; Chen, Y.; Sun-Waterhouse, D.; Zhang, Y.; Li, F. Immunomodulatory acidic polysaccharides from Zizyphus jujuba cv. Huizao: Insights into their chemical characteristics and modes of action. Food Chem. 2018, 258, 35–42.
- Qi, X.; Mao, W.; Gao, Y.; Chen, Y.; Chen, Y.; Zhao, C.; Shan, J. Chemical characteristic of an anticoagulant-active sulfated polysaccharide from Enteromorpha clathrata. Carbohydr. Polym. 2012, 90, 1804–1810.
- Mao, W.; Zang, X.; Li, Y.; Zhang, H. Sulfated polysaccharides from marine green algae Ulva conglobata and their anticoagulant activity. J. Appl. Phycol. 2006, 18, 9–14.
- Yasantha, A.; KiWan, L.; SeKwon, K.; YouJin, J. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour. Technol. 2007, 98, 1711–1716.
- Wang, X.; Zhang, Z.; Yao, Z.; Zhao, M.; Qi, H. Sulfation, anticoagulant and antioxidant activities of polysaccharide from green algae Enteromorpha linza. Int. J. Biol. Macromol. 2013, 58, 225–230.
- Qi, X.; Mao, W.; Chen, Y.; Chen, Y.; Zhao, C.; Li, N.; Wang, C. Chemical characteristics and anticoagulant activities of two sulfated polysaccharides from Enteromorpha linza (Chlorophyta). J. Ocean. Univ. China 2013, 12, 175–182.
- Jain, K.S.; Kathiravan, M.K.; Somani, R.S.; Shishoo, C.J. The biology and chemistry of hyperlipidemia. Bioorganic Med. Chem. 2007, 15, 4674–4699.
- Ross, R.; Harker, L. Hyperlipidemia and Atherosclerosis: Chronic hyperlipidemia initiates and maintains lesions by endothelial cell desquamation and lipid accumulation. Science 1976, 193, 1094–1100.
- Teng, Z.; Qian, L.; Zhou, Y. Hypolipidemic activity of the polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 62, 254–256.
- Yuan, Y.; Xu, X.; Jing, C.; Zou, P.; Zhang, C.; Li, Y. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohydr. Polym. 2018, 181, 902–910.
- Jiang, C.; Xiong, Q.; Gan, D.; Jiao, Y.; Liu, J.; Ma, L.; Zeng, X. Antioxidant activity and potential hepatoprotective effect of polysaccharides from Cyclina sinensis. Carbohydr. Polym. 2013, 91, 262–268.
- Lü, H.; Gao, Y.; Shan, H.; Lin, Y. Preparation and antibacterial activity studies of degraded polysaccharide selenide from Enteromorpha prolifera. Carbohydr. Polym. 2014, 107, 98–102.
- Patra, J.K.; Baek, K.-H. Antibacterial Activity and Action Mechanism of the Essential Oil from Enteromorpha linza L. against Foodborne Pathogenic Bacteria. Molecules 2016, 21, 388.
- Liu, D.; Keesing, J.K.; Dong, Z.; Zhen, Y.; Di, B.; Shi, Y.; Shi, P. Recurrence of the world’s largest green-tide in 2009 in Yellow Sea, China: Porphyra yezoensis aquaculture rafts confirmed as nursery for macroalgal blooms. Mar. Pollut. Bull. 2010, 60, 1423–1432.
- Li, B.; Liu, S.; Xing, R.; Li, K.; Li, R.; Qin, Y.; Li, P. Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohydr. Polym. 2013, 92, 1991–1996.
- Cui, J.; Li, Y.; Wang, S.; Chi, Y.; Hwang, H.; Wang, P. Directional preparation of anticoagulant-active sulfated polysaccharides from Enteromorpha prolifera using artificial neural networks. Sci. Rep. 2018, 8, 3062.
- Jin, W.; He, X.; Long, L.; Fang, Q.; Wei, B.; Sun, J.; Linhardt, R.J. Structural characterization and anti-lung cancer activity of a sulfated glucurono-xylo-rhamnan from Enteromorpha prolifera. Carbohydr. Polym. 2020, 237, 116143.
- Zhang, Y.; He, P.; Li, H.; Li, G.; Liu, J.; Jiao, F.; Jiao, N. Ulva prolifera green-tide outbreaks and their environmental impact in the Yellow Sea, China. Natl. Sci. Rev. 2019, 6, 825–838.
- De Paula Silva, P.H.; McBride, S.; de Nys, R.; Paul, N.A. Integrating filamentous ‘green tide’ algae into tropical pond-based aquaculture. Aquaculture 2008, 284, 74–80.
- Song, Y.; Han, A.; Park, S.; Cho, C.; Rhee, Y.; Hong, H. Effect of enzyme-assisted extraction on the physicochemical properties and bioactive potential of lotus leaf polysaccharides. Int. J. Biol. Macromol. 2020, 153, 169–179.
- Liu, Y.; Wu, X.; Jin, W.; Guo, Y. Immunomodulatory Effects of a Low-Molecular Weight Polysaccharide from Enteromorpha prolifera on RAW 264.7 Macrophages and Cyclophosphamide-Induced Immunosuppression Mouse Models. Mar. Drugs 2020, 18, 340.
More
Information
Subjects:
Marine & Freshwater Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.3K
Revisions:
5 times
(View History)
Update Date:
19 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




