The high-valued utilization of Ulva (previously known as Enteromorpha) bioresources has drawn increasing attention due to the periodic blooms of world-wide green tide. The polysaccharide is the main functional component of Ulva and exhibits various physiological activities. The Ulva oligosaccharide as the degradation product of polysaccharide not only possesses some obvious activities, but also possesses excellent solubility and bioavailability. Both Ulva polysaccharides and oligosaccharides hold promising potential in the food industry as new functional foods or food additives. Studies on Ulva polysaccharides and oligosaccharides are increasing and have been the focus of the marine bioresources field.
- Ulva
- polysaccharide
- oligosaccharide
- activity
1. Introduction
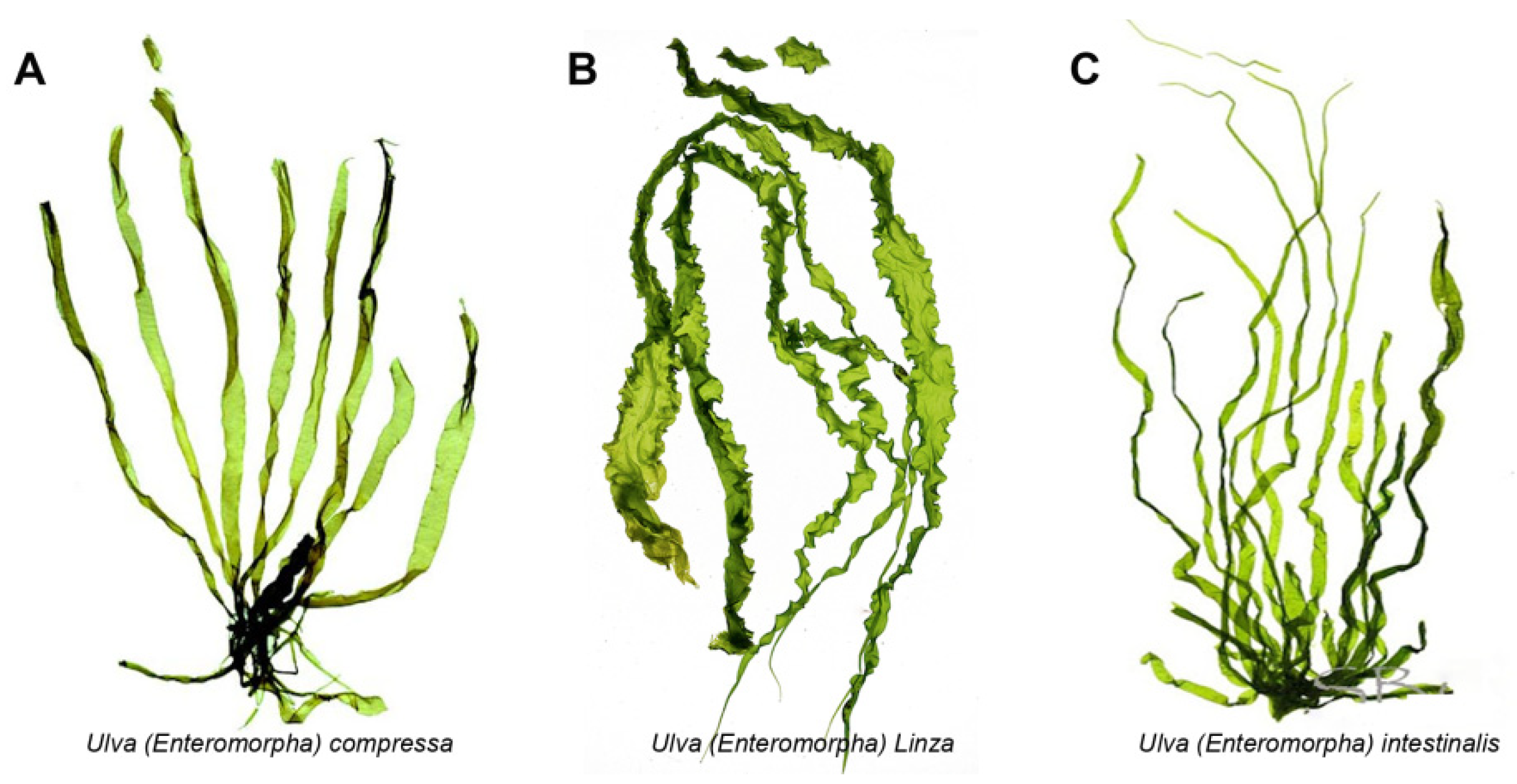
2. Activity of Ulva Polysaccharide
2.1. Antioxidant Activity
2.2. Antitumor Activity
2.3. Immune Regulatory Activity
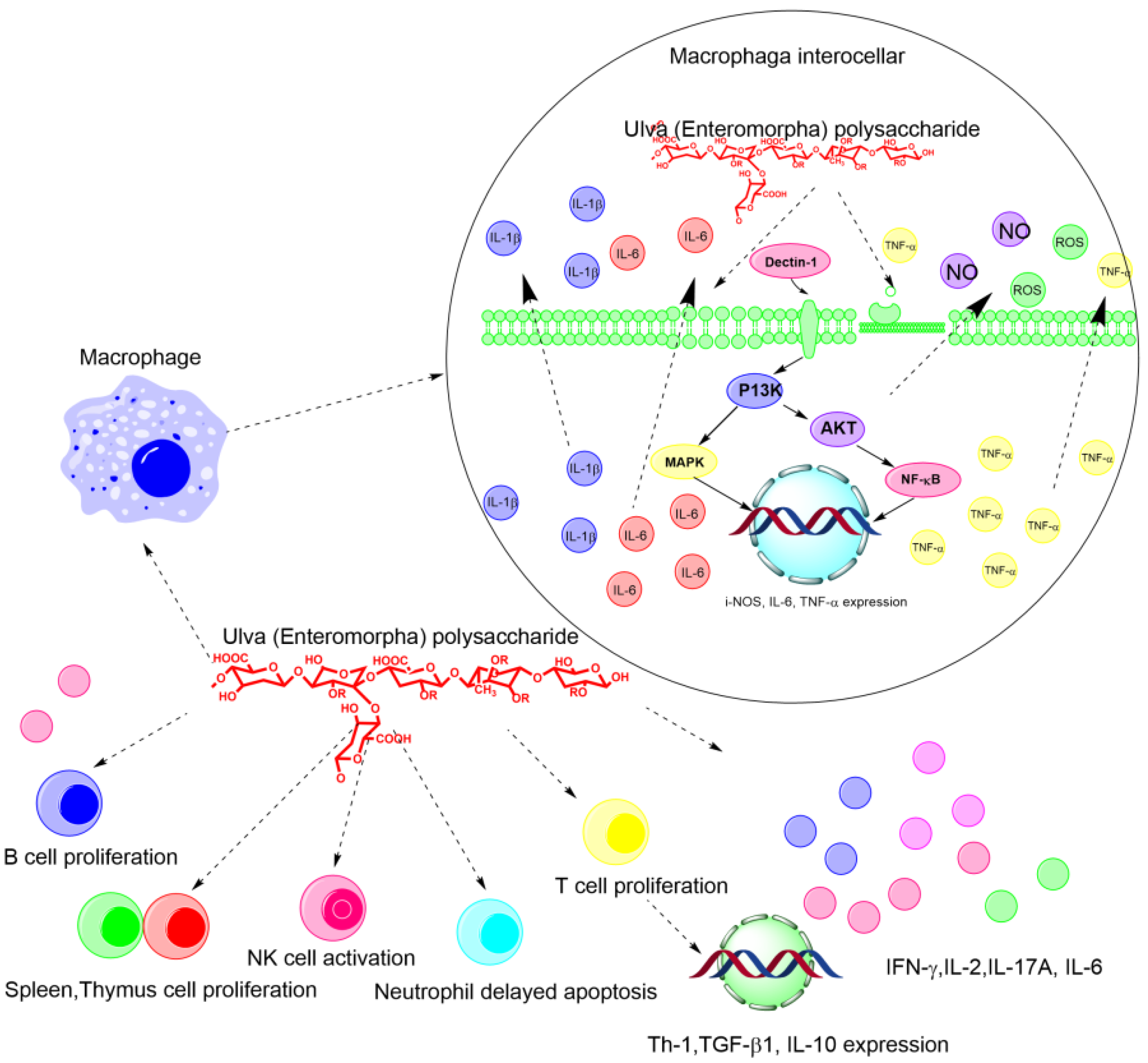
2.4. Anticoagulant Activity
2.5. Hypolipidemic Activity
3. Activity of Ulva Oligosaccharides
3. Conclusions and Future Perspective
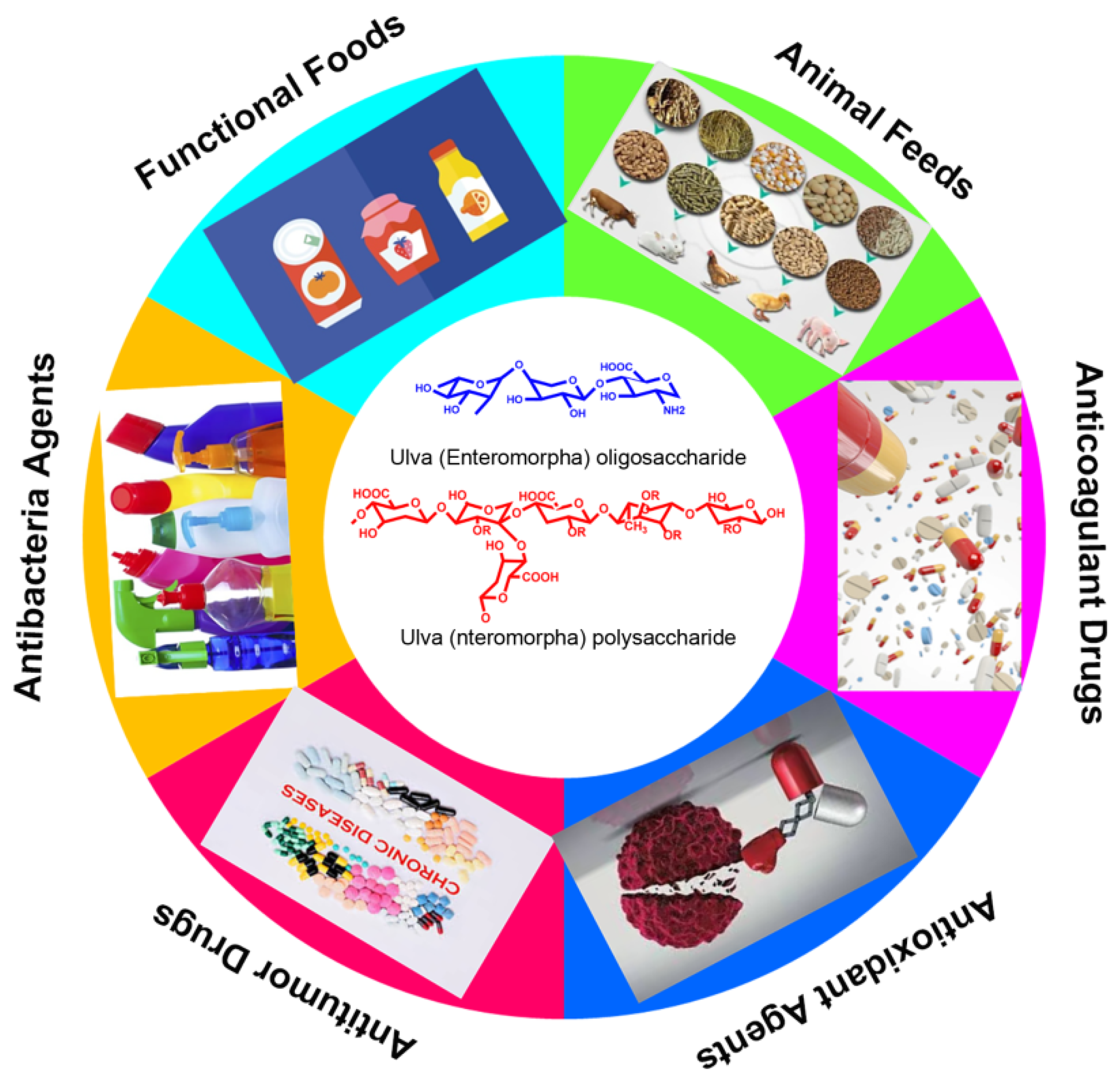 Figure 3. The potential and promising applications of Ulva polysaccharide and oligosaccharides.
Figure 3. The potential and promising applications of Ulva polysaccharide and oligosaccharides.This entry is adapted from the peer-reviewed paper 10.3390/md20030202
References
- Blomster, J.; Bäck, S.; Fewer, D.P.; Kiirikki, M.; Lehvo, A.; Maggs, C.A.; Stanhope, M.J. Novel morphology in Enteromorpha (Ulvophyceae) forming green tides. Am. J. Bot. 2002, 89, 1756–1763.
- Zhong, L.; Zhang, J.; Ding, Y. Energy Utilization of Algae Biomass Waste Enteromorpha Resulting in Green Tide in China: Pyrolysis Kinetic Parameters Estimation Based on Shuffled Complex Evolution. Sustainability 2020, 12, 2086.
- Silveira Coelho, M.; da Silva Menezes, B.; Rivero Meza, S.L.; Lainetti Gianasi, B.; de las Mercedes Salas-Mellado, M.; Copertino, M.; da Rosa Andrade Zimmermann de Souza, M. Potential Utilization of Green Tide-Forming Macroalgae from Patos Lagoon, Rio Grande-RS, Brazil. J. Aquat. Food Prod. Technol. 2016, 25, 1096–1106.
- Van Alstyne, K.L.; Nelson, T.A.; Ridgway, R.L. Environmental Chemistry and Chemical Ecology of “Green Tide” Seaweed Blooms. Integr. Comp. Biol. 2015, 55, 518–532.
- Wang, Z.; Xiao, J.; Fan, S.; Li, Y.; Liu, X.; Liu, D. Who made the world’s largest green tide in China?—An integrated study on the initiation and early development of the green tide in Yellow Sea. Limnol. Oceanogr. 2015, 60, 1105–1117.
- Yabe, T.; Ishii, Y.; Amano, Y.; Koga, T.; Hayashi, S.; Nohara, S.; Tatsumoto, H. Green tide formed by free-floating Ulva spp. at Yatsu tidal flat, Japan. Limnology 2009, 10, 239–245.
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.-O. Fucoidan from Macrocystis pyrifera Has Powerful Immune-Modulatory Effects Compared to Three Other Fucoidans. Mar. Drugs 2015, 13, 1084–1104.
- Xiao, J.; Wang, Z.; Liu, D.; Fu, M.; Yuan, C.; Yan, T. Harmful macroalgal blooms (HMBs) in China’s coastal water: Green and golden tides. Harmful Algae 2021, 107, 102061.
- Hayden, H.S.; Blomster, J.; Maggs, C.A.; Silva, P.C.; Stanhope, M.J.; Waaland, J.R. Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. Eur. J. Phycol. 2003, 38, 277–294.
- Jiao, L.; Jiang, P.; Zhang, L.; Wu, M. Antitumor and immunomodulating activity of polysaccharides from Enteromorpha intestinalis. Biotechnol. Bioprocess Eng. 2010, 15, 421–428.
- Tang, Z.; Gao, H.; Wang, S.; Wen, S.; Qin, S. Hypolipidemic and antioxidant properties of a polysaccharide fraction from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 58, 186–189.
- Chattopadhyay, K.; Mandal, P.; Lerouge, P.; Driouich, A.; Ghosal, P.; Ray, B. Sulphated polysaccharides from Indian samples of Enteromorpha compressa (Ulvales, Chlorophyta): Isolation and structural features. Food Chem. 2007, 104, 928–935.
- Jiang, F.; Chi, Z.; Ding, Y.; Quan, M.; Tian, Y.; Shi, J.; Liu, C. Wound Dressing Hydrogel of Enteromorpha prolifera Polysaccharide–Polyacrylamide Composite: A Facile Transformation of Marine Blooming into Biomedical Material. ACS Appl. Mater. Interfaces 2021, 13, 14530–14542.
- Zhong, R.; Wan, X.; Wang, D.; Zhao, C.; Liu, D.; Gao, L.; Cao, H. Polysaccharides from Marine Enteromorpha: Structure and function. Trends Food Sci. Technol. 2020, 99, 11–20.
- Tang, Z.; Yu, Z.; Zhao, W.; Guo, J.; Gao, L.; Qin, S. Ultrasonic extraction of polysaccharides from Enteromorpha. Mod. Food Sci. Technol. 2011, 27, 56–59.
- Liu, W.; Zhou, S.; Balasubramanian, B.; Zeng, F.; Sun, C.; Pang, H. Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish. Immunol. 2020, 104, 202–212.
- Shang, Q.; Wang, Y.; Pan, L.; Niu, Q.; Li, C.; Jiang, H.; Cai CHao, J.; Li, G.; Yu, G. Dietary Polysaccharide from Enteromorpha Clathrata Modulates Gut Microbiota and Promotes the Growth of Akkermansia muciniphila, Bifidobacterium spp. and Lactobacillus spp. Mar. Drugs 2018, 16, 167.
- Xie, C.; Zhang, Y.; Niu, K.; Liang, X.; Wang, H.; Shan, J.; Wu, X. Enteromorpha polysaccharide-zinc replacing prophylactic antibiotics contributes to improving gut health of weaned piglets. Anim. Nutr. 2021, 7, 641–649.
- Guo, F.; Han, M.; Lin, S.; Ye, H.; Chen, J.; Zhu, H.; Lin, W. Enteromorpha prolifera polysaccharide prevents high-fat diet-induced obesity in hamsters: A NMR-based metabolomic evaluation. J. Food Sci. 2021, 86, 3672–3685.
- Guo, F.; Zhuang, X.; Han, M.; Lin, W. Polysaccharides from Enteromorpha prolifera protect against carbon tetrachloride-induced acute liver injury in mice via activation of Nrf2/HO-1 signaling, and suppression of oxidative stress, inflammation and apoptosis. Food Funct. 2020, 11, 4485–4498.
- Li, X.; Guozhu, Z.; Zhifei, L.; Yang, S.; Peijun, L.; Jing, L. Hypoglycemic activity of Enteromorpha intestinalis polysaccharide. Sci. Technol. Food Ind. 2021, 42, 321–326.
- Zhu, B.; Ni, F.; Xiong, Q.; Yao, Z. Marine oligosaccharides originated from seaweeds: Source, preparation, structure, physiological activity and applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 60–74.
- Liu, X.; Liu, D.; Lin, G.; Wu, Y.; Gao, L.; Ai, C.; Zhao, C. Anti-ageing and antioxidant effects of sulfate oligosaccharides from green algae Ulva lactuca and Enteromorpha prolifera in SAMP8 mice. Int. J. Biol. Macromol. 2019, 139, 342–351.
- Shao, L.; Xu, J.; Shi, M.; Wang, X.; Li, Y.; Kong, L.; Hider, R.; Zhou, T. Preparation, antioxidant and antimicrobial evaluation of hydroxamated degraded polysaccharides from Enteromorpha prolifera. Food Chem. 2017, 237, 481–487.
- Xu, J.; Xu, L.; Zhou, Q.; Hao, S.; Zhou, T.; Xie, H. Isolation, purification, and antioxidant activities of degraded polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2015, 81, 1026–1030.
- Zhang, Z.; Wang, X.; Mo, X.; Qi, H. Degradation and the antioxidant activity of polysaccharide from Enteromorpha linza. Carbohydr. Polym. 2013, 92, 2084–2087.
- Lin, G.; Wu, D.; Xiao, X.; Huang, Q.; Chen, H.; Liu, D.; Zhao, C. Structural characterization and antioxidant effect of green alga Enteromorpha prolifera polysaccharide in Caenorhabditis elegans via modulation of microRNAs. Int. J. Biol. Macromol. 2020, 150, 1084–1092.
- Minton, N.P. Clostridia in cancer therapy. Nat. Rev. Microbiol. 2003, 1, 237–242.
- Sawyers, C. Targeted cancer therapy. Nature 2004, 432, 294–297.
- Jiao, L.; Li, X.; Li, T.; Jiang, P.; Zhang, L.; Wu, M.; Zhang, L. Characterization and anti-tumor activity of alkali-extracted polysaccharide from Enteromorpha intestinalis. Int. Immunopharmacol. 2009, 9, 324–329.
- Kim, J.-K.; Cho, M.L.; Karnjanapratum, S.; Shin, I.-S.; You, S.G. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2011, 49, 1051–1058.
- Delves, P.J.; Roitt, I.M. The Immune System. N. Engl. J. Med. 2000, 343, 37–49.
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789.
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232.
- Ren, X.; Liu, L.; Gamallat, Y.; Zhang, B.; Xin, Y. Enteromorpha and polysaccharides from Enteromorpha ameliorate loperamide-induced constipation in mice. Biomed. Pharmacother. 2017, 96, 1075–1081.
- Zou, M.; Chen, Y.; Sun-Waterhouse, D.; Zhang, Y.; Li, F. Immunomodulatory acidic polysaccharides from Zizyphus jujuba cv. Huizao: Insights into their chemical characteristics and modes of action. Food Chem. 2018, 258, 35–42.
- Qi, X.; Mao, W.; Gao, Y.; Chen, Y.; Chen, Y.; Zhao, C.; Shan, J. Chemical characteristic of an anticoagulant-active sulfated polysaccharide from Enteromorpha clathrata. Carbohydr. Polym. 2012, 90, 1804–1810.
- Mao, W.; Zang, X.; Li, Y.; Zhang, H. Sulfated polysaccharides from marine green algae Ulva conglobata and their anticoagulant activity. J. Appl. Phycol. 2006, 18, 9–14.
- Yasantha, A.; KiWan, L.; SeKwon, K.; YouJin, J. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour. Technol. 2007, 98, 1711–1716.
- Wang, X.; Zhang, Z.; Yao, Z.; Zhao, M.; Qi, H. Sulfation, anticoagulant and antioxidant activities of polysaccharide from green algae Enteromorpha linza. Int. J. Biol. Macromol. 2013, 58, 225–230.
- Qi, X.; Mao, W.; Chen, Y.; Chen, Y.; Zhao, C.; Li, N.; Wang, C. Chemical characteristics and anticoagulant activities of two sulfated polysaccharides from Enteromorpha linza (Chlorophyta). J. Ocean. Univ. China 2013, 12, 175–182.
- Jain, K.S.; Kathiravan, M.K.; Somani, R.S.; Shishoo, C.J. The biology and chemistry of hyperlipidemia. Bioorganic Med. Chem. 2007, 15, 4674–4699.
- Ross, R.; Harker, L. Hyperlipidemia and Atherosclerosis: Chronic hyperlipidemia initiates and maintains lesions by endothelial cell desquamation and lipid accumulation. Science 1976, 193, 1094–1100.
- Teng, Z.; Qian, L.; Zhou, Y. Hypolipidemic activity of the polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 62, 254–256.
- Yuan, Y.; Xu, X.; Jing, C.; Zou, P.; Zhang, C.; Li, Y. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohydr. Polym. 2018, 181, 902–910.
- Jiang, C.; Xiong, Q.; Gan, D.; Jiao, Y.; Liu, J.; Ma, L.; Zeng, X. Antioxidant activity and potential hepatoprotective effect of polysaccharides from Cyclina sinensis. Carbohydr. Polym. 2013, 91, 262–268.
- Lü, H.; Gao, Y.; Shan, H.; Lin, Y. Preparation and antibacterial activity studies of degraded polysaccharide selenide from Enteromorpha prolifera. Carbohydr. Polym. 2014, 107, 98–102.
- Patra, J.K.; Baek, K.-H. Antibacterial Activity and Action Mechanism of the Essential Oil from Enteromorpha linza L. against Foodborne Pathogenic Bacteria. Molecules 2016, 21, 388.
- Liu, D.; Keesing, J.K.; Dong, Z.; Zhen, Y.; Di, B.; Shi, Y.; Shi, P. Recurrence of the world’s largest green-tide in 2009 in Yellow Sea, China: Porphyra yezoensis aquaculture rafts confirmed as nursery for macroalgal blooms. Mar. Pollut. Bull. 2010, 60, 1423–1432.
- Li, B.; Liu, S.; Xing, R.; Li, K.; Li, R.; Qin, Y.; Li, P. Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohydr. Polym. 2013, 92, 1991–1996.
- Cui, J.; Li, Y.; Wang, S.; Chi, Y.; Hwang, H.; Wang, P. Directional preparation of anticoagulant-active sulfated polysaccharides from Enteromorpha prolifera using artificial neural networks. Sci. Rep. 2018, 8, 3062.
- Jin, W.; He, X.; Long, L.; Fang, Q.; Wei, B.; Sun, J.; Linhardt, R.J. Structural characterization and anti-lung cancer activity of a sulfated glucurono-xylo-rhamnan from Enteromorpha prolifera. Carbohydr. Polym. 2020, 237, 116143.
- Zhang, Y.; He, P.; Li, H.; Li, G.; Liu, J.; Jiao, F.; Jiao, N. Ulva prolifera green-tide outbreaks and their environmental impact in the Yellow Sea, China. Natl. Sci. Rev. 2019, 6, 825–838.
- De Paula Silva, P.H.; McBride, S.; de Nys, R.; Paul, N.A. Integrating filamentous ‘green tide’ algae into tropical pond-based aquaculture. Aquaculture 2008, 284, 74–80.
- Song, Y.; Han, A.; Park, S.; Cho, C.; Rhee, Y.; Hong, H. Effect of enzyme-assisted extraction on the physicochemical properties and bioactive potential of lotus leaf polysaccharides. Int. J. Biol. Macromol. 2020, 153, 169–179.
- Liu, Y.; Wu, X.; Jin, W.; Guo, Y. Immunomodulatory Effects of a Low-Molecular Weight Polysaccharide from Enteromorpha prolifera on RAW 264.7 Macrophages and Cyclophosphamide-Induced Immunosuppression Mouse Models. Mar. Drugs 2020, 18, 340.
