You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bilal Haider Abbasi | + 1942 word(s) | 1942 | 2022-02-18 09:26:03 | | | |
| 2 | Vicky Zhou | Meta information modification | 1942 | 2022-03-17 09:11:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Abbasi, B. Root Cultures in the Production of Valuable Compounds. Encyclopedia. Available online: https://encyclopedia.pub/entry/20671 (accessed on 22 December 2025).
Abbasi B. Root Cultures in the Production of Valuable Compounds. Encyclopedia. Available at: https://encyclopedia.pub/entry/20671. Accessed December 22, 2025.
Abbasi, Bilal. "Root Cultures in the Production of Valuable Compounds" Encyclopedia, https://encyclopedia.pub/entry/20671 (accessed December 22, 2025).
Abbasi, B. (2022, March 17). Root Cultures in the Production of Valuable Compounds. In Encyclopedia. https://encyclopedia.pub/entry/20671
Abbasi, Bilal. "Root Cultures in the Production of Valuable Compounds." Encyclopedia. Web. 17 March, 2022.
Copy Citation
Medicinal plants are an inevitable source of pharmaceutical drugs and most of the world population depends on these plants for health benefits. The increasing global demand for bioactive compounds from medicinal plants has posed a great threat to their existence due to overexploitation. Adventitious root and hairy root culture systems are an alternative approach to the conventional method for mass production of valuable compounds from medicinal plants owing to their rapid growth, biosynthetic and genetic stability.
adventitious root culture
hairy root culture
medicinal plants
bioreactor
1. Introduction
Medicinal plants are an inevitable source of pharmaceutical drugs and most of the world population depends on these plants for their health. Plant cell and tissue cultivation to produce pharmaceutically important components of commercial interest have gained popularity over the last few years [1]. To save lives, humans have used medicinal plants as an endless source of drugs. In recent years plant cells, tissues or organs have increasingly been cultivated to get compounds that are both pharmaceutically and economically valuable [2]. After being isolated by solvent extraction from plants (that are grown normally) these compounds are then used as pharmaceuticals, nutraceuticals, pigments, foodstuffs, and cosmetics. Increasing requirements of secondary metabolites have diverted the attention towards cell culture technology to bring possible changes in secondary metabolism to produce bioactive molecules [3]. However, secondary metabolite production is suitable neither by cell culture nor from naturally grown plants. Major hurdles are higher content of water in cells, foaming, wall growth, and unstable production of metabolites in bioreactors [4]. In comparison, metabolite biosynthesis in naturally grown plants is affected by species or genus or might be activated just during a specific growth and developmental stage or by the availability of nutrients and pesticide contamination furthermore a great danger is imposed on the existence of plant species as these are continuously been destroyed for medicines [5]. Now, it is clear that for great socio-economic value other complementary techniques for complete plant cultivation should be developed to produce biologically valuable secondary metabolites. These are the reasons why efforts have been made in past years to develop plant cell, tissue, and organ culture as an alternative method to plant cultivation to produce secondary metabolites of elite pharmacological values [3].
Recent plant research advancements depict that to produce biologically active compounds, the cultivation of adventitious roots in a bioreactor is a great alternative to other methods. A greater rate of proliferation, enormous potentialities of accumulation, and stable production of important secondary metabolites result when adventitious roots are induced in phytohormone supplemented medium under sterile conditions [6]. Considering these issues for mass scale cultivation of adventitious roots by employing bioreactor technology is utilized to produce important plant-derived secondary metabolites. Monitoring of parameters such as pH temperature and the oxygen concentration in the bioreactor can be done; therefore, bioreactor culture system is better than a traditional tissue culture system [7]. The nutrient concentration can be adjusted, and nutrient uptake can also be increased by continuous medium circulation. Moreover, cost and time can be decreased by improving cell proliferation and regeneration rates, product quality can also be controlled, the product 3 can be free of pesticide contamination, and the product can be obtained all year round to meet the increasing global requirement [8]. Despite the great advantages of secondary metabolite production by plant cell cultures, only paclitaxel, shikonin, ginsenosides, and berberine have been produced on a commercial scale, and those process plants are located in the USA, Japan, South Korea, and China, respectively [9]. To produce immense quantities of the plant-derived bioactive molecules for use in a variety of human therapy and cosmetics, adventitious root culture using automated bioreactor technology of various medicinally important plants must be established.
2. Adventitious Root Culture
Roots are regarded as a part of great secondary metabolism in the whole plant because these are a good source for bioactive molecules, proteins, and a range of metabolites [10]. Recent advances in plant biotechnology enable us to culture plant cells, tissues, and organs instead of complete plants to produce valuable secondary metabolites [11]. Because the low productivity and instability of plant cultures and non-synthesis of few important compounds in undifferentiated cells, mass production of important bioactive molecules is considered commercially difficult by plant cell culture [12]. Keeping these issues in consideration, to produce pharmaceutical and nutraceutical secondary metabolites, the adventitious root culture in a large-scale bioreactor is regarded as a great approach.
In an appropriate phytohormone supplemented medium and aseptic conditions, adventitious roots displayed a greater growth rate and a more active secondary metabolism [13]. Additionally, these are suitable biological materials for good commercial production of great secondary metabolites without foreign genes under in vitro conditions. Adventitious roots displayed greater stability in their growing environment and produced a cosmic amount of secondary metabolites in intracellular spaces and it can be more easily extracted and can be cultivated in a phytohormone amended medium with less inoculum but a greater growth rate compared to cell cultures [14].
One of the major steps of in vitro propagation is adventitious root formation which is used for the cultivation of several plant crops, including medicinal plants. As a result of new advancements in propagation technology, large amounts of biologically important compounds, such as phenols, terpenoids, and alkaloids have been produced using plant cells, tissue, as well as organ cultures [15]. Adventitious root cultures are thought to be the most promising method for biomass production because of their increased growth rate and stable metabolites production [16]. For example, adventitious roots of many important medicinal plants cultivated by micropropagation showed increased biomass production, accumulation, and biologically active compounds production. Therefore, in vitro culture of adventitious roots has enormous potential to be developed on a large scale for the production of biologically active compounds. Furthermore, plant roots are the main raw materials used for herbal drug preparations, accounting for about 60% of herbal medicinal plants used in ethnomedicine. Because of its capability for micropropagation and germplasm preservation, an adventitious root culture is highly useful [17].
3. Hairy Root Culture
Hairy root cultures hold immense potential for the biosynthesis of various classes of influential secondary metabolites along with volatile organic compounds. The production of fine roots occurs when Agrobacterium rhizogenes inserts its T-DNA into the Ri plasmid to genetically transform the plant tissue. The main advantage of the hairy root culture method is that plant growth regulators do not require media supplementation because the inserted T-DNA contains the required genes for endogenous auxin production. Hairy roots are highly branched as they lack geotropism and can, therefore, grow faster than normal roots. In addition to the typical root metabolites, they also produce metabolites that are naturally secreted by aerial parts of the plants. Moreover, like any other cell culture technology, hairy roots are physiologically and biochemically stable. They display faster growth, spontaneous shoots generation, as well as chemical and morphological similarity to the roots of a wild-type plant. These properties make hairy root cultures extremely convenient for research and industry [18][19][20][21].
4. Hairy Root vs. Adventitious Root Culture
Nowadays, studies on the production of crucial bioactive compounds in hairy root culture are increasingly popular. Agrobacterium rhizogenes is an agent which is responsible to cause hairy roots in plants characterized by a higher growth rate and genetic stability [22]. Despite the high growth rate, biochemical and genetic stability exhibited by hairy roots, the secondary metabolites produced in hairy root liquid culture remain in the root tissues which affect the cell growth and pose difficulties in extraction during the downstream process. While adventitious root cultures release secondary metabolites into the culture medium which can be easily extracted [23].
Moreover, hairy root cultures produce complex structures, i.e., opine-like substrates which are toxic to mammalian cells, and due to the complexity of these structures, hairy roots are difficult to use as a crude extract, because of the high purification cost for opine-like structures [24]. Adventitious root culture is free from opine-like complex, toxic substrates, and provides the system to study the coordination between primary and secondary metabolism [25]. Figure 1 represents a brief comparison between adventitious and hairy root cultures and the process of their induction.

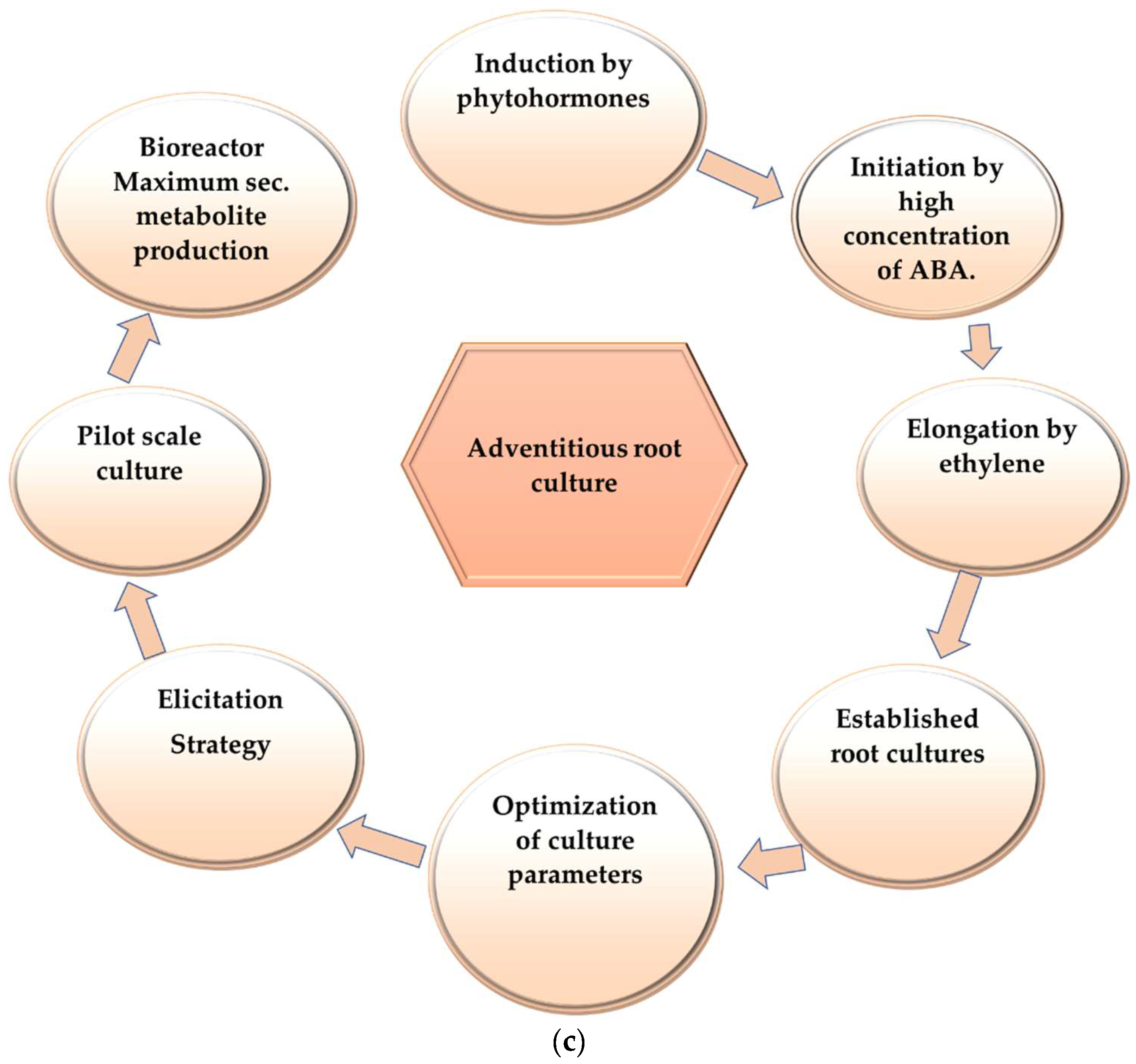 Figure 1. This figure shows a brief comparison between adventitious and hairy root culture and the process of their induction: (a) comparison between hairy root culture and adventitious root culture; (b) flowchart of hairy root induction; (c) flowchart of adventitious root culture.
Figure 1. This figure shows a brief comparison between adventitious and hairy root culture and the process of their induction: (a) comparison between hairy root culture and adventitious root culture; (b) flowchart of hairy root induction; (c) flowchart of adventitious root culture.5. Scale-Up of Culture Process
The demand for plant secondary metabolites is increasing day by day due to their vast array of applications in the health and pharmaceutical industry. To achieve this rising demand of the global market, it is necessary to produce them on large scale. The bioreactor technology is the best alternative for growing plants conventionally to scale up the production of plant secondary metabolites [26]. The cultivation of plant cell cultures in large-scale bioreactors is more profitable and feasible as scientists can control the entire process to produce high-quality yields in bulk quantities [26].
The primary challenge in commercializing plant-derived secondary metabolites is scaling up the culture in a large-scale bioreactor. Because when cultures are transferred from shake flasks to bioreactors and then scaled up from pilot to industrial size, the environment in which plant cells and roots are cultivated might be changed. Due to this shifting effect of shear stress, oxygen supply, and gas composition in bioreactors reduced productivity has been observed [26][27].
The area of plant cell fermentation and scaling-up has received a lot of attention in recent decades. Plant cells are currently being cultured in amounts up to 75,000 L, and specialized bioreactor systems and effective plant cell culture systems have been developed for growing plant cells [28]. Despite many reports, only a few commercially effective experiments for the generation of adventitious root biomass and bioactive chemicals at industrial scale reactors have been reported. Ref. [29] scaled-up ginseng adventitious root culture in a 500 L balloon-type bubble bioreactor (BTBB), while [8] scaled up ginseng adventitious root culture in a 10,000 L BTBB.
The induction of adventitious roots from selected explants, as well as the optimization of process parameters, are all involved in scaling up adventitious root culture for the generation of biomass and secondary metabolites. Studies are being carried out on the growth kinetics and exploring suitable techniques for higher metabolite accumulation without affecting root growth, and cultivation of adventitious roots in pilot-scale bioreactors. High sparge rates may remove carbon dioxide and other nutrients from the culture media, depending on the metabolic activity of the cells. In these instances, the air-lift bioreactor is the most suitable reactor choice to effectively culture the suspension cells or roots [30].
6. Conclusions
The expeditious advancement in biotechnology has offered adventitious and hairy root cultures as one of the best alternatives to whole plant cultivation for the production of valuable compounds by employing medicinal plants. Plethora of studies has been conducted with the aim to produce valuable compounds by utilizing hairy and adventitious root cultures that have made enormous advances in plant sciences. For maximum production of specific bioactive compounds in these systems, optimization of culture conditions, elicitation strategies, precursor feeding, and various other approaches have been reported in numerous studies. A promising alternative to field cultivation of medicinal plants is adventitious or hairy root culture in a bioreactor system supplied with an elicitor. The commercial exploitation of bioactive substances requires scaling up these approaches utilizing bioreactors. Optimized use of these approaches, either individually or in combination, can provide synergistic effects, resulting in increased biomass productivity and bioactive compounds accumulation. Nevertheless, both chemical and physical optimization is foremost to produce high-quality yields. Furthermore, profound knowledge of biosynthetic pathways of desired compounds in adventitious and hairy roots is still in its early stages. To improve yields metabolic engineering offers an encouraging outlook but requires the understanding of governance on secondary metabolic pathways embroiled on the levels of genes, enzymes, and products including details such as compartmentation and transport is required.
References
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 1–10.
- Wang, J.; Li, J.L.; Li, J.; Li, J.X.; Liu, S.J.; Huang, L.Q.; Gao, W.Y. Production of active compounds in medicinal plants: From plant tissue culture to biosynthesis. Chin. Herb. Med. 2017, 9, 115–125.
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult. 2018, 132, 239–265.
- Kundu, S.; Salma, U.; Gantait, S. Cryopreservation of Medicinal Herbs: Major Breakthroughs, Hurdles and Future. In Biotechnological Approaches for Medicinal and Aromatic Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 353–381.
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762.
- Yu, K.W.; Murthy, H.N.; Jeong, C.S.; Hahn, E.J.; Paek, K.Y. Organic germanium stimulates the growth of ginseng adventitious roots and ginsenoside production. Process. Biochem. 2005, 40, 2959–2961.
- Sui, Q.; Jiang, C.; Yu, D.; Chen, M.; Zhang, J.; Wang, Y.; Wei, Y. Performance of a sequencing-batch membrane bioreactor (SMBR) with an automatic control strategy treating high-strength swine wastewater. J. Hazard. Mater. 2018, 342, 210–219.
- Paek, K.Y.; Murthy, H.N.; Hahn, E.J.; Zhong, J.J. Large scale culture of ginseng adventitious roots for production of ginsenosides. Adv. Biochem. Eng. Biotechnol. 2009, 113, 151–176.
- Sivakumar, G.; Yu, K.; Paek, K. Production of biomass and ginsenosides from adventitious roots of Panax ginseng in bioreactor cultures. Eng. Life Sci. 2005, 5, 333–342.
- Habibi, P.; De Sa, M.F.G.; Makhzoum, A.; Malik, S.; da Silva, A.L.L.; Hefferon, K.; Soccol, C.R. Bioengineering hairy roots: Phytoremediation, secondary metabolism, molecular pharming, plant-plant interactions and biofuels. Sustain. Agric. Rev. 2017, 22, 213.
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590.
- Ncube, E.N.; Steenkamp, P.A.; Madala, N.E.; Dubery, I.A. Metabolite profiling of the undifferentiated cultured cells and differentiated leaf tissues of Centella asiatica. Plant Cell Tissue Organ Cult. 2017, 129, 431–443.
- Deepthi, S.; Satheeshkumar, K. Effects of major nutrients, growth regulators and inoculum size on enhanced growth and camptothecin production in adventitious root cultures of Ophiorrhiza mungos L. Biochem. Eng. J. 2017, 117, 198–209.
- Murthy, H.N.; Dandin, V.S.; Paek, K.Y. Tools for biotechnological production of useful phytochemicals from adventitious root cultures. Phytochem. Rev. 2016, 15, 129–145.
- Gantet, P.; Imbault, N.; Thiersault, M.; Doireau, P. Necessity of a functional octadecanoic pathway for indole alkaloid synthesis by Catharanthus roseus cell suspensions cultured in an auxin-starved medium. Plant Cell Physiol. 1998, 39, 220–225.
- Carvalho, E.B.; Curtis, W.R. Characterization of fluid-flow resistance in root cultures with a convective flow tubular bioreactor. Biotechnol. Bioeng. 1998, 60, 375–384.
- Sudha, C.G.; Seeni, S. Establishment and analysis of fast-growing normal root culture of Decalepis arayalpathra, a rare endemic medicinal plant. Curr. Sci. 2001, 81, 371–374.
- Guillon, S.; Trémouillaux-Guiller, J.; Pati, P.K.; Rideau, M.; Gantet, P. Harnessing the potential of hairy roots: Dawn of a new era. TRENDS Biotechnol. 2006, 24, 403–409.
- Liu, C.Z.; Abbasi, B.H.; Gao, M.; Murch, S.J.; Saxena, P.K. Caffeic acid derivatives production by hairy root cultures of Echinacea purpurea. J. Agric. Food Chem. 2006, 54, 8456–8460.
- Pistelli, L.; Giovannini, A.; Ruffoni, B.; Bertoli, A.; Pistelli, L. Hairy root cultures for secondary metabolites production. Adv. Exp. Med. Biol. 2010, 698, 167–184.
- Parsons, J.L.; Cameron, S.I.; Harris, C.S.; Smith, M.L. Echinacea biotechnology: Advances, commercialization and future considerations. Pharm. Biol. 2018, 56, 485–494.
- Roy, A. Hairy Root Culture an Alternative for Bioactive Compound Production from Medicinal Plants. Curr. Pharm. Biotechnol. 2021, 22, 136–149.
- Miao, G.P.; Han, J.; Zhang, J.F.; Zhu, C.S.; Zhang, X. A MDR transporter contributes to the different extracellular production of sesquiterpene pyridine alkaloids between adventitious root and hairy root liquid cultures of Tripterygium wilfordii Hook. f. Plant Mol. Biol. 2017, 95, 51–62.
- Choi, S.M.; Son, S.H.; Yun, S.R.; Kwon, O.W.; Seon, J.H.; Paek, K.Y. Pilot-scale culture of adventitious roots of ginseng in a bioreactor system. Plant Cell Tissue Organ Cult. 2000, 62, 187–193.
- Baque, M.A.; Moh, S.H.; Lee, E.J.; Zhong, J.J.; Paek, K.Y. Production of biomass and useful compounds from adventitious roots of high-value added medicinal plants using bioreactor. Biotechnol. Adv. 2012, 30, 1255–1267.
- Zhong, J.J. Biochemical engineering of the production of plant-specific secondary metabolites by cell suspension cultures. Adv. Biochem. Eng. Biotechnol. 2001, 72, 1–26.
- Zhong, J.J.; Pan, Z.W.; Wang, Z.Y.; Wu, J.; Chen, F.; Takagi, M.; Yoshida, T. Effect of mixing time on taxoid production using suspension cultures of Taxus chinensis in a centrifugal impeller bioreactor. J. Biosci. Bioeng. 2002, 94, 244–250.
- Dörnenburg, H.; Knorr, D. Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzym. Microb. Technol. 1995, 17, 674–684.
- Chiou, S.Y.; Sung, J.M.; Huang, P.W.; Lin, S.D. Antioxidant, antidiabetic, and antihypertensive properties of Echinacea purpurea flower extract and caffeic acid derivatives using in vitro models. J. Med. Food 2017, 20, 171–179.
- Smart, N.J.; Fowler, M.W. Effect of aeration on large-scale cultures of plant cells. Biotechnol. Lett. 1981, 3, 171–176.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
17 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




