Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alison Ramser | + 1831 word(s) | 1831 | 2022-03-09 05:22:01 | | | |
| 2 | Dean Liu | -2 word(s) | 1829 | 2022-03-17 05:43:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ramser, A. Avian Orexin. Encyclopedia. Available online: https://encyclopedia.pub/entry/20662 (accessed on 07 February 2026).
Ramser A. Avian Orexin. Encyclopedia. Available at: https://encyclopedia.pub/entry/20662. Accessed February 07, 2026.
Ramser, Alison. "Avian Orexin" Encyclopedia, https://encyclopedia.pub/entry/20662 (accessed February 07, 2026).
Ramser, A. (2022, March 16). Avian Orexin. In Encyclopedia. https://encyclopedia.pub/entry/20662
Ramser, Alison. "Avian Orexin." Encyclopedia. Web. 16 March, 2022.
Copy Citation
Originally named for its expression in the posterior hypothalamus in rats and after the Greek word for “appetite”, hypocretin, or orexin, as it is known today, gained notoriety as a neuropeptide regulating feeding behavior, energy homeostasis, and sleep. Orexin is a neuropeptide involved in both central and peripheral control of neuroendocrine functions, energy balance, and metabolism.
orexin
central regulation
peripheral regulation
metabolism
1. Overview of Orexin
Genomic research during the 1990s resulted in the characterization of numerous, previously unidentified, genes with potential biological significance. Notably, several “orphan” G-protein coupled receptors were found and shown to be putative, but without known ligands [1]. Given that G-protein coupled receptors are the most targeted molecules for drugs used in clinics, the investigation into the ligands for these orphan receptors was undertaken and revealed two peptide ligands termed “orexins”, also known as hypocretin [1][2]. It has been shown that the administration of orexins into the central nervous system (CNS) resulted in increased food intake in mice and rats, and that their production was dependent on the nutritional state. Additionally, a group of neurons located in the lateral hypothalamic area of the brain produce the neuropeptide called orexin in two forms, orexin A and orexin B [1][2]. These forms are synthesized from proteolytic cleavage of the precursor, prepro-orexin. Orexin-A is composed of 33 amino acids with an N-terminal pyroglutamyl residue and a C-terminal amidation [3][4]. Its structure consists of four Cysteine residues forming sets of intra-chain disulfide bonds. This structure is conserved across mammalian species, including rats, mice, cows, sheep, pigs, and dogs [4]. Orexin-B is made up of 28 amino acid residues, and while its C-terminal is similar to orexin-A, its N-terminal is more variable [1]. Additionally, there are more differences in orexin-B amino acid sequences across mammalian species and chicken [5].
There are two known orexin receptors, orexin-1 receptor (OX1R) and orexin-2 receptor (OX2R). These are G-protein coupled receptors with OX1R being structurally similar to neuropeptide receptors such as the Y2 neuropeptide Y (NPY) receptor and thyrotropin-releasing hormone (TRH) receptor [1][6][7]. Both receptor genes are highly conserved across species [8]. Orexin-A has a higher affinity for OX1R than orexin-B, while OX2R is a nonselective receptor for both orexin-A and orexin-B [1][3]. This difference in affinity for OX1R is attributed to the N-terminal of orexin-A, which is specific and hydrophilic [9]. Within the CNS, OX1R is the most abundant in the locus coeruleus, but is also found in the prefrontal and infralimbic cortex, hippocampus, amygdala, periventricular nucleus, anterior hypothalamus, dorsal raphe nucleus, and laterodorsal tegmental nucleus [10][11]. OX2R is expressed in the amygdala periventricular nucleus, dorsal raphe nucleus, and laterodorsal tegmental nucleus, as well as in the tuberomammillary nucleus. These regions are critical for energy homeostasis responses and arousal, which will be discussed in more detail in a later section. Furthermore, the mRNA of prepro-orexin is expressed in the lateral hypothalamus area, known as a feeding center [10][12]. The orexin system is also expressed in peripheral tissues, including the kidney, adrenal gland, thyroid, testes, ovaries, jejunum, lung, pituitary gland, brown and white adipose tissues, and muscle [13][14][5][15][16][17][18][19].
2. Central Orexin Signaling Pathways
The molecular mechanisms of central orexin’s mode of action have also been investigated. In energy restricted dairy cows, orexin A neurons are colocalized with adenosine monophosphate-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor (PPAR) gamma. Additionally, energy restriction phosphorylates AMPK, leading to AMPK’s activation and to an increased PPARγ expression, indicating that orexin-A’s control of energy homeostasis in dairy cows involves AMPK [20]. AMPK is an enzyme that acts as a sensor for cellular energy status through changes in the ATP-to-AMP ratio and activating downstream targets to induce metabolic shifts [21][22]. AMPK is known to affect fat metabolism and glucose utilization, as well as impact the balance between catabolic and anabolic pathways within the cell [22]. PPARγ is a receptor whose ligands are known as potent insulin sensitizers, and it is known for its involvement in the mobilization of lipids and glucose metabolism [23]. It was later shown that orexin-A activated hypothalamic AMPK signaling in a calcium-dependent manner via a voltage-gated L-type calcium channel (Figure 1) [24]. This research points to orexins’ ability to directly activate AMPK signaling within the central nervous system, providing a means in which feeding behavior and energy homeostasis are linked. A link between central regulation and energy homeostasis was also established via hypothalamic orexin expression and brown adipose tissue thermogenesis. AMPK inhibition in the ventromedial nucleus of the hypothalamus, followed by increased orexin signaling in the lateral hypothalamic area, was seen under thermogenic effects induced by bone morphogenetic protein (BMP) 8B in mice. The thermogenic effect of BMP8B is due to its impact on the browning of white adipose tissue, and both its thermogenic effect and the effects on orexin expression were reduced by the knockout of glutamate vesicular transporter 2 (VGLUT2) [25]. These findings show the central control of energy homeostasis via orexin’s relationship to AMPK within the hypothalamus.
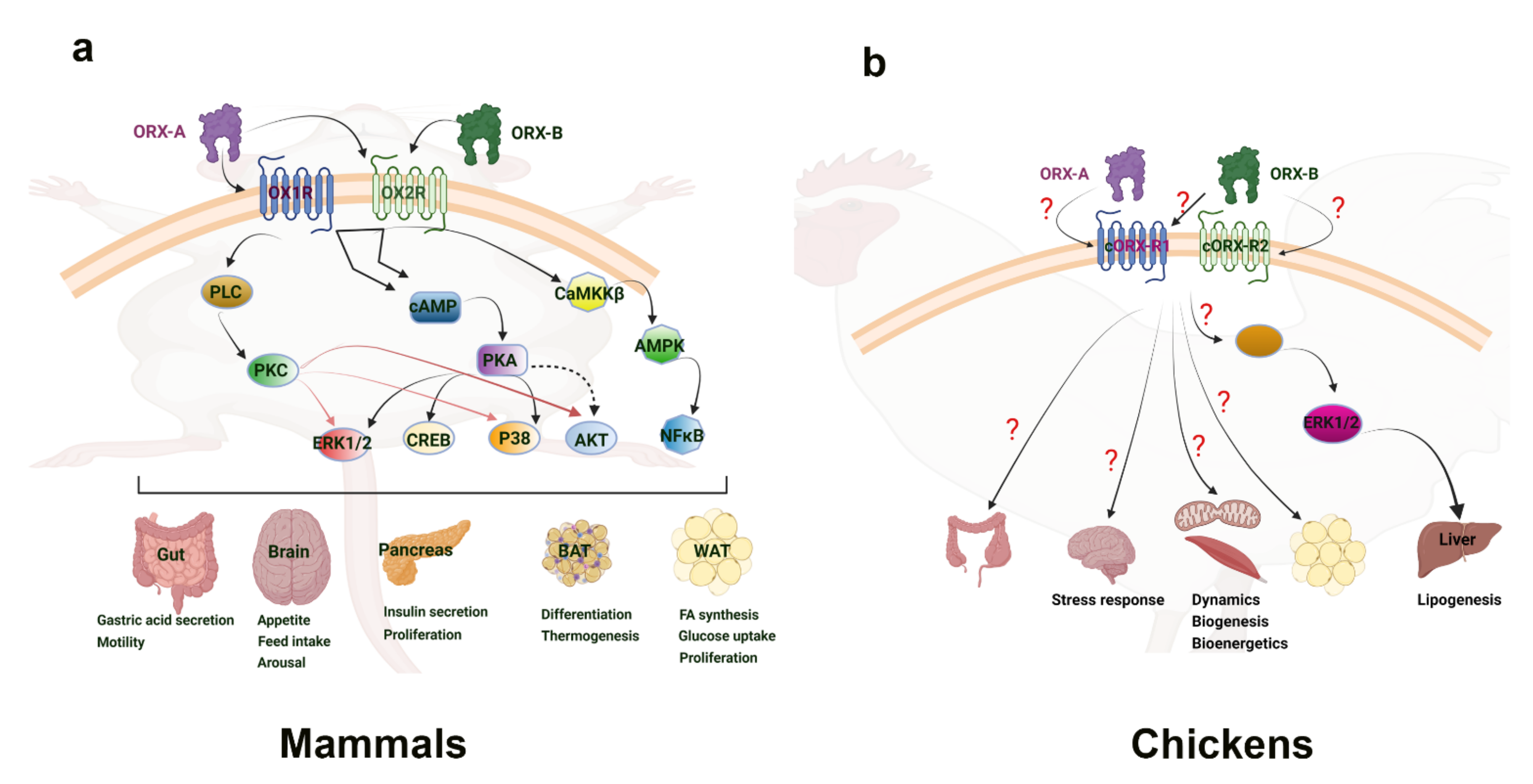
Figure 1. Orexin signaling pathways in mammalian (a) and avian species (b). “?” means that the downstream mediators are still not known or not well defined. Clear connections have been made to the link between orexin and the activation of energy sensing AMPK within the central nervous system of mammals. This provides a basis for connecting feeding behavior with orexin. Additionally, the ERK1/2 and Akt signaling pathways have been seen in hepatic response to orexin, inducing diverse cellular responses. While the main mechanism of action has yet to be determined, adipose tissue is shown to increase cytokines and other factors in response to orexin. In avian species, the ERK1/2 pathway has also been implicated in the liver response to orexin. Several mitochondrial related genes were shown to respond to orexin in avian muscle, but the direct mechanism of impact has yet to be elucidated. Culpable molecular signaling pathways have yet to be discovered in the avian central nervous system and adipose tissue response to orexin. AKT—Ak strain transforming kinase; AMPK—adenosine monophosphate-activated protein kinase; BAT—brown adipose tissue; CaMKKβ—Calcium/Calmodulin dependent protein kinase; cAMP—cyclic adenosine monophosphate; cORX-R1; chicken orexin receptor 1; cORX-R2—chicken orexin receptor 2; CREB—cAMP-response element binding protein; ERK1/2—extracellular-signal-regulated kinase 1/2; NFκB—nuclear factor kappa light chain enhancer of activated B cells; ORX-A—orexin-A; ORX-B—orexin-B; OXR1—orexin receptor 1; OXR2—orexin receptor 2; PKA—protein kinase A; PKC—protein kinase C; PLC—phospholipase C; P38—mitogen-associated protein kinase 38; WAT—white adipose tissue.
3. Orexin in the Avian Central Nervous System
The central role of orexins in avian species is a stark contrast to mammalian species. In fact, i.c.v. administration of orexins in neonatal chicks failed to stimulate appetite and food intake [26]. This is despite the fact that orexin-A and -B are highly conserved among vertebrates, as evidenced by the predicted amino-acid sequence of chicken prepro-orexin [27]. Additionally, while orexin-positive cell bodies were found in the periventricular hypothalamic nucleus and extending into the lateral hypothalamic area in chicken, fasting had no effect on the orexin mRNA expression [27]. These phenomena were further investigated upon research into the chicken orexin receptor (cOXR). It was found that cOXR corresponds more closely to OX2R in mammals, with approximately 80% similarity [5]. cOXR was found to be widely expressed throughout the bird brain, and particularly abundant in the cerebrum, hypothalamus, and optic tectum [5]. It was later found that orexin-A and -B do not seem to be involved in the wake-sleep cycle, as their expression levels did not change in the brains of sleeping vs. awake laying hens [28]. Within the avian brain, orexin-A and -B neurons have been found central on the paraventricular nucleus and extending into the lateral hypothalamic area in several birds [29][30]. The highest density of orexin neurons in the house finch was found within the preoptic area, the hypothalamus, and the thalamus, with projections also found in the third ventricle caudally [30]. The distribution of orexin neurons in varying regions within the avian brain point to a diverse involvement of orexin in numerous behavioral and centrally-regulated functions. A study by Wei and colleagues showed that keel fracture induced stress and inflammation, along with a reduced expression of the orexin system in the hypothalamus of laying hens [31]. This would implicate a central expression of orexin in stress responses, similar to what was seen in mammalian species. On the other hand, Lei et al. reported that acute heat stress did not elicit any change to the hypothalamic expression of orexin mRNA in broiler chickens [32]. Taken together, these studies suggest a potential CNS role for the avian orexin system in certain stress responses, but not in energy balance, and this role may be strain-dependent (layers vs. broilers). Therefore, further in-depth investigations are needed to elucidate the mechanisms in which orexin operates within the avian CNS and its downstream effects.
4. Orexin in Avian Peripheral Tissues
Research into orexin’s peripheral effects is still limited in avian species, despite it looking more promising than central regulation. In chickens, immunohistochemical localization and distribution of orexin-A and -B was found in the endocrine cells, nerve fibers, and neurons of the stomach and intestines, along with the protein expression of prepro-orexin and both receptors [33]. It is indicated that a potentially similar role of orexin in avian intestinal tract secretions and function as is seen in mammals.
In avian hepatoma cells, orexin-A decreased the visfatin expression while orexin-B had no significant effect [34]. Additionally, both oxidative and heat stress were shown to alter the expression of orexin and its receptors in avian species, with oxidative stress appearing to modulate post-transcriptional mechanisms of orexin regulation [35]. These results provide a basis for orexin influencing the peripheral mechanisms of metabolism in avian species, specifically through regulating secretions and functions in the liver.
The muscle was also investigated in the avian species as a main area of selection in modern broilers, leading to high metabolic demands within the muscle. To that end, the orexin system was shown to be expressed in the muscle and to regulate mitochondrial dynamics via fission- and fusion-related genes and their associated transcription factors [17]. In addition to the regulation of the orexin system within avian muscle, orexin treatment was also shown to impact mitochondrial biogenesis and functional genes, while also significantly decreasing the proton leak within a quail muscle cell line [17]. In quail muscle, acute heat stress decreased the expression of orexin and its receptor [36]. In contrast to lack of central effects, these studies showed that orexin exerts peripheral effects on metabolism, energy homeostasis, and inflammatory factors. Further research is needed to elucidate the extent to which the orexin system works within avian physiology.
In addition to physiological implications, the impact of nutrition on the orexin system has limited investigation. A recent has shown that dietary chromium increased orexin and GLUT expression levels and reduced levels of NF-κB and HSPs in the ovaries of heat-stressed laying hens, indicating nutritional interventions capable of modulating the orexin and inflammatory systems [37]. Further research into the impacts of nutrition on the orexin system within the peripheral tissues of chickens is warranted, and could provide insight into the mechanisms of improved stress response and energy homeostasis.
References
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors that Regulate Feeding Behavior. Cell 1998, 92, 573–585.
- de Lecea, L.; Kilduff, T.S.; Peyron, C.; Gao, X.; Foye, P.E.; Danielson, P.E.; Fukuhara, C.; Battenberg, E.L.; Gautvik, V.T.; Bartlett, F.S., II; et al. The Hypocretins: Hypothalamus-Specific Peptides with Neuroexcitatory Activity. Proc. Natl. Acad. Sci. USA 1998, 95, 322–327.
- Sakurai, T. Orexins and orexin receptors: Implication in feeding behavior. Regul. Pept. 1999, 85, 25–30.
- Sakurai, T.; Moriguchi, T.; Furuya, K.; Kajiwara, N.; Nakamura, T.; Yanagisawa, M.; Goto, K. Structure and Function of Human Prepro-orexin Gene. J. Biol. Chem. 1999, 274, 17771–17776.
- Ohkubo, T.; Tsukada, A.; Shamoto, K. cDNA cloning of chicken orexin receptor and tIssue distribution: Sexually dimorphic expression in chicken gonads. J. Mol. Endocrinol. 2003, 31, 499–508.
- Leonard, C.S.; Kukkonen, J.P. Orexin/hypocretin receptor signalling: A functional perspective. Br. J. Pharmacol. 2014, 171, 294–313.
- Kukkonen, J.P.; Leonard, C.S. Orexin/hypocretin receptor signalling cascades: Orexin receptor signalling cascades. Br. J. Pharmacol. 2014, 171, 314–331.
- Yin, J.; Babaoglu, K.; Brautigam, C.A.; Clark, L.; Shao, Z.; Scheuermann, T.H.; Harrell, C.M.; Gotter, A.L.; Roecker, A.J.; Winrow, C.J.; et al. Structure and ligand-binding mechanism of the human OX1 and OX2 orexin receptors. Nat. Struct. Mol. Biol. 2016, 23, 293–299.
- Takai, T.; Takaya, T.; Nakano, M.; Akutsu, H.; Nakagawa, A.; Aimoto, S.; Nagai, K.; Ikegami, T. Orexin-A is composed of a highly conserved C-terminal and a specific, hydrophilic N-terminal region, revealing the structural basis of specific recognition by the orexin-1 receptor. J. Pept. Sci. 2006, 12, 443–454.
- Tsunematsu, T.; Yamanaka, A. The role of orexin/hypocretin in the central nervous system and peripheral tissues. Vitam. Horm. 2012, 89, 19.
- Matsuki, T.; Sakurai, T. Orexins and orexin receptors: From molecules to integrative physiology. Results Probl. Cell Differ. 2008, 46, 27.
- Li, J.; Hu, Z.; Lecea, L. The hypocretins/orexins: Integrators of multiple physiological functions. Br. J. Pharmacol. 2014, 171, 332–350.
- Levanti, M.; Germanà, A.; Abbate, F. Orexin A Expression in the Ovary of Dog and Cat. Reprod. Domest. Anim. 2015, 50, 247–250.
- Li, M.; Zu, N.; Zhang, C.S.; Xie, M.Y.; Liu, Y.Z.; Xu, X.J. Orexin A promotes granulosa cell secretion of progesterone in sheep. Iran. J. Vet. Res. 2019, 20, 136–142.
- Heinonen, M.V.; Purhonen, A.K.; Makela, K.A.; Herzig, K.H. Functions of orexins in peripheral tissues. Acta Physiol. 2008, 192, 471–485.
- Madden, C.J.; Tupone, D.; Morrison, S.F. Orexin modulates brown adipose tissue thermogenesis. Biomol. Concepts 2012, 3, 381–386.
- Lassiter, K.; Dridi, S. Orexin System and Avian Muscle Mitochondria. In Muscle Cells-Recent Advances and Future Perspectives; IntechOpen: London, UK, 2020.
- Wojciechowicz, T.; Skrzypski, M.; Szczepankiewicz, D.; Hertig, I.; Kołodziejski, P.A.; Billert, M.; Strowski, M.Z.; Nowak, K.W. Original Research: Orexins A and B stimulate proliferation and differentiation of porcine preadipocytes. Exp. Biol. Med. 2016, 241, 1786–1795.
- Skrzypski, M.; Billert, M.; Nowak, K.W.; Strowski, M.Z. The role of orexin in controlling the activity of the adipo-pancreatic axis. J. Endocrinol. 2018, 238, R95–R108.
- Kuhla, B.; Goers, S.; Metges, C.C. Hypothalamic orexin A expression and the involvement of AMPK and PPAR-gamma signalling in energy restricted dairy cows. Arch. Tierzucht. 2011, 54, 567–579.
- O’neill, H.M. Review: AMPK and Exercise: Glucose Uptake and Insulin Sensitivity. Diabetes Metab. J. 2013, 37, 1.
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135.
- Janani, C.; Ranjitha Kumari, B.D. PPAR gamma gene—A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2014, 9, 46–50.
- Wu, W.-N.; Wu, P.-F.; Zhou, J.; Guan, X.-L.; Zhang, Z.; Yang, Y.-J.; Long, L.-H.; Xie, N.; Chen, J.-G.; Wang, F. Orexin-A Activates Hypothalamic AMP-Activated Protein Kinase Signaling through a Ca2+-Dependent Mechanism Involving Voltage-Gated L-Type Calcium Channel. Mol. Pharmacol. 2013, 84, 876–887.
- Martins, L.; Seoane-Collazo, P.; Contreras, C.; González-García, I.; Martínez-Sánchez, N.; González, F.; Zalvide, J.; Gallego, R.; Diéguez, C.; Nogueiras, R.; et al. A Functional Link between AMPK and Orexin Mediates the Effect of BMP8B on Energy Balance. Cell Rep. 2016, 16, 2231–2242.
- Furuse, M.; Ando, R.; Bungo, T.; Shimojo, M.; Masuda, Y. Intracerebroventricular injection of orexins does not stimulate food intake in neonatal chicks. Br. Poult. Sci. 1999, 40, 698–700.
- Ohkubo, T.; Boswell, T.; Lumineau, S. Molecular cloning of chicken prepro-orexin cDNA and preferential expression in the chicken hypothalamus. Biochim. Biophys. Acta Gene Struct. Expr. 2002, 1577, 476–480.
- Miranda, B.; Esposito, V.; de Girolamo, P.; Sharp, P.J.; Wilson, P.W.; Dunn, I.C. Orexin in the chicken hypothalamus: Immunocytochemical localisation and comparison of mRNA concentrations during the day and night, and after chronic food restriction. Brain Res. 2013, 1513, 34–40.
- Soya, S.; Sakurai, T. Evolution of Orexin Neuropeptide System: Structure and Function. Front. Neurosci. 2020, 14, 691.
- Singletary, K.G.; Deviche, P.; Strand, C.; Delville, Y. Distribution of orexin/hypocretin immunoreactivity in the brain of a male songbird, the house finch, Carpodacus mexicanus. J. Chem. Neuroanat. 2007, 33, 101–109.
- Wei, H.; Li, C.; Xin, H.; Li, S.; Bi, Y.; Li, X.; Li, J.; Zhang, R.; Bao, J. Keel Fracture Causes Stress and Inflammatory Responses and Inhibits the Expression of the Orexin System in Laying Hens. Animals 2019, 9, 804.
- Lei, L.; Hepeng, L.; Xianlei, L.; Hongchao, J.; Hai, L.; Sheikhahmadi, A.; Yufeng, W.; Zhigang, S. Effects of acute heat stress on gene expression of brain-gut neuropeptides in broiler chickens. J. Anim. Sci. 2013, 91, 5194–5201.
- Arcamone, N.; D’Angelo, L.; de Girolamo, P.; Lucini, C.; Pelagalli, A.; Castaldo, L. Orexin and orexin receptor like peptides in the gastroenteric tract of Gallus domesticus: An immunohistochemical survey on presence and distribution. Res. Vet. Sci. 2014, 96, 234–240.
- Ferver, A.; Greene, E.; Dridi, S. Hormonal regulation of visfatin gene in avian Leghorn male hepatoma (LMH) cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 240, 110592.
- Greene, E.; Khaldi, S.; Ishola, P.; Bottje, W.; Ohkubo, T.; Anthony, N.; Dridi, S. Heat and oxidative stress alter the expression of orexin and its related receptors in avian liver cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 191, 18–24.
- Nguyen, P.H.; Greene, E.; Kong, B.W.; Bottje, W.; Anthony, N.; Dridi, S. Acute Heat Stress Alters the Expression of Orexin System in Quail Muscle. Front. Physiol. 2017, 8, 1079.
- Ozdemir, O.; Tuzcu, M.; Sahin, N.; Orhan, C.; Tuzcu, Z.; Sahin, K. Organic chromium modifies the expression of orexin and glucose transporters of ovarian in heat-stressed laying hens. Cell. Mol. Biol. 2017, 63, 93–98.
More
Information
Subjects:
Endocrinology & Metabolism
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
17 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




