
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hiroyuki Adachi | + 1450 word(s) | 1450 | 2022-03-14 04:50:17 | | | |
| 2 | Amina Yu | + 27 word(s) | 1477 | 2022-03-16 01:59:48 | | |
Video Upload Options
Circulating tumor cells (CTCs) are dislodged from the primary tumor into the bloodstream, travel within the bloodstream to distant organs, and finally extravasate and proliferate as epithelial metastatic deposits. In surgery for malignancies, the surgical manipulation of tumors and tissues around the tumor may lead to the release of CTCs into the bloodstream. The non-touch isolation technique (NTIT) has been advocated to prevent the release of CTCs during surgery. The concept of NTIT is the prevention of intraoperative increment of CTCs from the primary tumor by the early blockade of outflow vessels, and ‘pulmonary vein (PV)-first lobectomy’ during surgery for non-small-cell lung cancer (NSCLC) corresponds to this technique.
1. Non-Touch Isolation Technique in Surgery for Other Malignancies
2. Non-Touch Isolation Technique in Surgery for Non-Small-Cell Lung Cancer
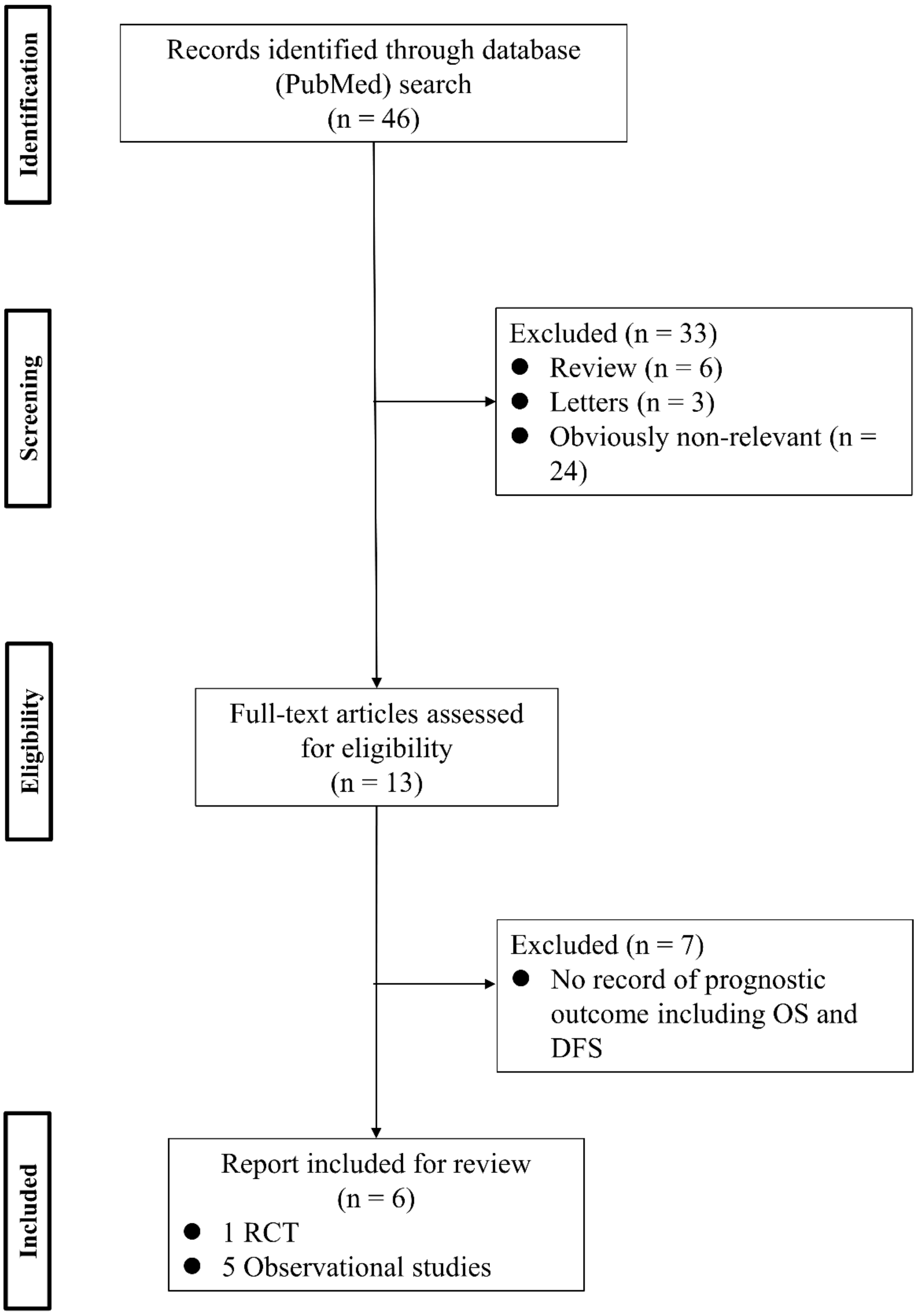
References
- Cole, W.H.; Packard, D.; Southwick, H.W. Carcinoma of the Colon with Special Reference to Prevention of Recurrence. JAMA 1954, 155, 1549–1553.
- Weitz, J.; Kienle, P.; Lacroix, J.; Willeke, F.; Benner, A.; Lehnert, T.; Herfarth, C.; von Knebel Doeberitz, M. Dissemination of Tumor Cells in Patients Undergoing Surgery for Colorectal Cancer. Clin. Cancer Res. 1998, 4, 343–348.
- Louha, M.; Nicolet, J.; Zylberberg, H.; Sabile, A.; Vons, C.; Vona, G.; Poussin, K.; Tournebize, M.; Capron, F.; Pol, S.; et al. Liver Resection and Needle Liver Biopsy Cause Hematogenous Dissemination of Liver Cells. Hepatology 1999, 29, 879–882.
- Sergeant, G.; Roskams, T.; van Pelt, J.; Houtmeyers, F.; Aerts, R.; Topal, B. Perioperative Cancer Cell Dissemination Detected with a Real-Time RT-PCR Assay for EpCAM Is Not Associated with Worse Prognosis in Pancreatic Ductal Adenocarcinoma. BMC Cancer 2011, 11, 47.
- Yao, X.; Williamson, C.; Adalsteinsson, V.A.; D’Agostino, R.S.; Fitton, T.; Smaroff, G.G.; William, R.T.; Wittrup, K.D.; Love, J.C. Tumor Cells Are Dislodged into the Pulmonary Vein During Lobectomy. J. Thorac. Cardiovasc. Surg. 2014, 148, 3224–3231.
- Matsutani, N.; Sawabata, N.; Yamaguchi, M.; Woo, T.; Kudo, Y.; Kawase, A.; Shiono, S.; Iinuma, H.; Morita, S.; Kawamura, M. Does Lung Cancer Surgery Cause Circulating Tumor Cells?—A Multicenter, Prospective Study. J. Thorac. Dis. 2017, 9, 2419–2426.
- Barnes, J.P. Physiologic Resection of the Right Colon. Surg. Gynecol. Obstet. 1952, 94, 722–726.
- Turnbull, R.B., Jr.; Kyle, K.; Watson, F.R.; Spratt, J. Cancer of the Colon: The Influence of the No-Touch Isolation Technic on Survival Rates. Ann. Surg. 1967, 166, 420–427.
- Wiggers, T.; Jeekel, J.; Arends, J.W.; Brinkhorst, A.P.; Kluck, H.M.; Luyk, C.I.; Munting, J.D.; Povel, J.A.; Rutten, A.P.; Volovics, A. No-Touch Isolation Technique in Colon Cancer: A Controlled Prospective Trial. Br. J. Surg. 1988, 75, 409–415.
- Fujita, J.; Uyama, I.; Sugioka, A.; Komori, Y.; Matsui, H.; Hasumi, A. Laparoscopic Right Hemicolectomy with Radical Lymph Node Dissection Using the No-Touch Isolation Technique for Advanced Colon Cancer. Surg. Today 2001, 31, 93–96.
- Takii, Y.; Shimada, Y.; Moriya, Y.; Nakamura, K.; Katayama, H.; Kimura, A.; Shibata, T.; Fukuda, H. A Randomized Controlled Trial of the Conventional Technique Versus the No-Touch Isolation Technique for Primary Tumor Resection in Patients with Colorectal Cancer: Japan Clinical Oncology Group Study JCOG1006. Jpn. J. Clin. Oncol. 2014, 44, 97–100.
- Nakao, A.; Takagi, H. Isolated Pancreatectomy for Pancreatic Head Carcinoma Using Catheter Bypass of the Portal Vein. Hepato-Gastroenterology 1993, 40, 426–429.
- Kobayashi, S.; Asano, T.; Ochiai, T. A Proposal of No-Touch Isolation Technique in Pancreatoduodenectomy for Periampullary Carcinomas. Hepato-Gastroenterology 2001, 48, 372–374.
- Hirota, M.; Kanemitsu, K.; Takamori, H.; Chikamoto, A.; Tanaka, H.; Sugita, H.; Sand, J.; Nordback, I.; Baba, H. Pancreatoduodenectomy Using a No-Touch Isolation Technique. Am. J. Surg. 2010, 199, e65–e68.
- Hirota, M.; Shimada, S.; Yamamoto, K.; Tanaka, E.; Sugita, H.; Egami, H.; Ogawa, M. Pancreatectomy Using the No-Touch Isolation Technique Followed by Extensive Intraoperative Peritoneal Lavage to Prevent Cancer Cell Dissemination: A Pilot Study. JOP 2005, 6, 143–151.
- Gall, T.M.H.; Jacob, J.; Frampton, A.E.; Krell, J.; Kyriakides, C.; Castellano, L.; Stebbing, J.; Jiao, L.R. Reduced Dissemination of Circulating Tumor Cells with No-Touch Isolation Surgical Technique in Patients with Pancreatic Cancer. JAMA Surg. 2014, 149, 482–485.
- Aylwin, J.A. Avoidable Vascular Spread in Resection for Bronchial Carcinoma. Thorax 1951, 6, 250–267.
- Kurusu, Y.; Yamashita, J.; Hayashi, N.; Mita, S.; Fujino, N.; Ogawa, M. The Sequence of Vessel Ligation Affects Tumor Release into the Circulation. J. Thorac. Cardiovasc. Surg. 1998, 116, 107–113.
- Song, P.P.; Zhang, W.; Zhang, B.; Liu, Q.; Du, J. Effects of Different Sequences of Pulmonary Artery and Vein Ligations During Pulmonary Lobectomy on Blood Micrometastasis of Non-Small Cell Lung Cancer. Oncol. Lett. 2013, 5, 463–468.
- Duan, X.; Zhu, Y.; Cui, Y.; Yang, Z.; Zhou, S.; Han, Y.; Yu, D.; Xiao, N.; Cao, X.; Li, Y.; et al. Circulating Tumor Cells in the Pulmonary Vein Increase Significantly After Lobectomy: A Prospective Observational Study. Thorac. Cancer 2019, 10, 163–169.
- Wei, S.; Guo, C.; He, J.; Tan, Q.; Mei, J.; Yang, Z.; Liu, C.; Pu, Q.; Ma, L.; Yuan, Y.; et al. Effect of Vein-First vs Artery-First Surgical Technique on Circulating Tumor Cells and Survival in Patients with Non-Small Cell Lung Cancer: A Randomized Clinical Trial and Registry-Based Propensity Score Matching Analysis. JAMA Surg. 2019, 154, e190972.
- Ge, M.J.; Shi, D.; Wu, Q.C.; Wang, M.; Li, L.B. Observation of Circulating Tumour Cells in Patients with Non-Small Cell Lung Cancer by Real-Time Fluorescent Quantitative Reverse Transcriptase-Polymerase Chain Reaction in Peroperative Period. J. Cancer Res. Clin. Oncol. 2006, 132, 248–256.
- Hashimoto, M.; Tanaka, F.; Yoneda, K.; Takuwa, T.; Matsumoto, S.; Okumura, Y.; Kondo, N.; Tsubota, N.; Tsujimura, T.; Tabata, C.; et al. Significant Increase in Circulating Tumour Cells in Pulmonary Venous Blood During Surgical Manipulation in Patients with Primary Lung Cancer. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 775–783.
- Refaely, Y.; Sadetzki, S.; Chetrit, A.; Simansky, D.A.; Paley, M.; Modan, B.; Yellin, A. The Sequence of Vessel Interruption During Lobectomy for Non-Small Cell Lung Cancer: Is It Indeed Important? J. Thorac. Cardiovasc. Surg. 2003, 125, 1313–1320.
- Kozak, A.; Alchimowicz, J.; Safranow, K.; Wójcik, J.; Kochanowski, L.; Kubisa, B.; Pieróg, J.; Grodzki, T. The Impact of the Sequence of Pulmonary Vessel Ligation during Anatomic Resection for Lung Cancer on Long-Term Survival—A Prospective Randomized Trial. Adv. Med. Sci. 2013, 58, 156–163.
- Li, F.; Jiang, G.; Chen, Y.; Wang, J. Curative Effects of Different Sequences of Vessel Interruption During the Completely Thoracoscopic Lobectomy on Early Stage Non-Small Cell Lung Cancer. Ann. Thorac. Cardiovasc. Surg. 2015, 21, 536–543.
- Sumitomo, R.; Fukui, T.; Marumo, S.; Otake, Y.; Huang, C.L. Effects of Vessel Interruption Sequence During Thoracoscopic Lobectomy for Non-Small Cell Lung Cancer. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 464–470.
- He, H.H.; He, J.X.; Hao, Z.X.; Wang, W.; He, J.X. Association Between Different Sequences of Vessel Ligation During Video-Assisted Thoracoscopic Lobectomy and Survival in Patients with Non-Small Cell Lung Cancer. J. Thorac. Dis. 2019, 11, 686–693.
- Sawabata, N.; Funaki, S.; Hyakutake, T.; Shintani, Y.; Fujiwara, A.; Okumura, M. Perioperative Circulating Tumor Cells in Surgical Patients with Non-Small Cell Lung Cancer: Does Surgical Manipulation Dislodge Cancer Cells Thus Allowing Them to Pass into the Peripheral Blood? Surg. Today 2016, 46, 1402–1409.
- Huang, K.L.; Deng, H.Y.; Fan, M.; Zheng, Q.; Lin, S.; Zhu, D.; Zhou, Q. The Sequence of Pulmonary Vessels Ligation During Lobectomy for Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2021, 47, 1535–1540.




