Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Circulating tumor cells (CTCs) are dislodged from the primary tumor into the bloodstream, travel within the bloodstream to distant organs, and finally extravasate and proliferate as epithelial metastatic deposits.
- non-touch isolation technique
- circulating tumor cell
- non-small-cell lung cancer
- PV first lobectomy
- surgery
1. Non-Touch Isolation Technique in Surgery for Other Malignancies
The CTCs are released from the primary tumor into the bloodstream, especially in CTC clusters, which have a high metastatic potential and are released as tumorous microemboli. Theoretically, this release may be promoted by perioperative procedures, such as preoperative tumor biopsy and intraoperative manipulation. Some researchers have reported an increase in the number of CTCs after surgical manipulation in colorectal [22,23], hepatocellular [24], pancreatic [25], and lung [26,27] cancer. To avoid the release of CTCs by intraoperative surgical manipulation, a non-touch isolation technique (NTIT) has been devised for various cancer surgeries. The concept of NTIT is the prevention of the intraoperative increase in CTCs from primary tumors by the early blockade of outflow vessels.
NTIT was first described for colorectal cancer surgery in 1952. Barnes advocated the ligation of the vascular pedicles and division of the bowel prior to handling the cancer-bearing segment [28]. Thereafter, Turnbull et al. [29] reported the results of a retrospective study evaluating the long-term outcomes of patients who underwent colorectal cancer surgery with NTIT. Their study included 896 patients who underwent colorectal cancer surgery between 1950 and 1964 and showed better outcomes in patients with NTIT than in those without NTIT (5-year OS of 50.86% in patients with NTIT and 34.82% in patients without NTIT). To investigate the efficacy of NTIT, a randomized controlled trial (RCT) was conducted in the 1980s, but this trial could not show any statistical significance regarding NTIT due to the lack of statistical power [30]; therefore, NTIT is not yet a standard procedure for colorectal cancer. However, several studies have shown the efficacy of NTIT in colorectal cancer surgery [31]. An RCT with a large sample size that aims to evaluate the efficacy of NTIT for colorectal cancer surgery has been conducted in Japan [32]. The enrollment for this study has been completed, and the results will be released in the near future.
NTIT has also been adopted for surgery for pancreatic cancers and hepatocellular carcinoma. In the 1990s, Nakao et al. [33] first reported the use of NTIT for pancreatic head cancer using an antithrombogenic portal vein bypass catheter between the mesenteric and intrahepatic portal veins.
Kobayashi et al. proposed the use of NTIT for pancreatoduodenectomy without removing the portal vein for periampullary carcinoma [34]. Hirota et al. [35] proposed NTIT for pancreatoduodenectomy using a hanging-up and clamping technique. Hirota et al. [36] also reported that the 3-year OS rate after surgery for patients with NTIT was superior to that for patients without NTIT (75% and 14%, respectively). However, Gall et al. [37] showed no impact of NTIT on postoperative survival in pancreatic cancer, despite the significant decrease in the number of CTCs detected in the portal vein with NTIT. RCTs evaluating the efficacy of NTIT for pancreatic cancer have been conducted because of the rarity of surgery for pancreatic cancer, meaning that the efficacy of NTIT for pancreatic cancer is yet to be established.
2. Non-Touch Isolation Technique in Surgery for Non-Small-Cell Lung Cancer
Regarding surgery for non-small-cell lung cancer (NSCLC), “pulmonary vein (PV)-first lobectomy” is regarded as NTIT. Anatomical lung resection, including lobectomy, which is the standard procedure for the treatment of NSCLC, requires the dissection of the lobar branch of the pulmonary artery (PA), PV, and bronchus. These structures have a complex three-dimensional location; thus, lung manipulation is required for anatomical lung resection. Regarding other types of cancers, thoracic surgeons and researchers, for many years, have thought that surgical manipulation results in the spillage of tumor cells from primary tumors, and many researchers have demonstrated an increase in the number of CTCs or surrogate substances in peripheral blood and the PV after surgical procedures for NSCLC. PV-first lobectomy, which is characterized by early PV ligation, has been advocated since the 1950s [38], as it theoretically prevents the outflow of CTCs produced by surgical manipulation to the circulation. This is because the PV is the discharge canal for the central bloodstream; thus, PV-first lobectomy improves postoperative prognosis. To evaluate the efficacy of PV-first lobectomy, researchers recently attempted to demonstrate its effect on the behavior of perioperative CTCs and postoperative prognoses. For the analysis of perioperative changes in CTC after PV-first lobectomy, Kurusu et al. [39] conducted an RCT in a small cohort dividing patients into two groups (PA-first ligation and PV-first ligation) and showed that patients in the PV-first group had a lower positive conversion rate for peripheral arterial carcinoembryonic antigen (CEA) mRNA expression than patients in the PA-first group. Song et al. [40] showed in their RCT that the mRNA expression of CD44v6 and CK19 in the PA-first group increased after vessel ligation compared to before ligation, whereas the expressions in the PV-first group were similar before and after vessel ligation. Duan et al. [41] and Wei et al. [42] also reported the efficacy of the PV-first procedure in preventing CTC production. However, Ge et al. [43] conducted an RCT focused on the detection of CK19 and CEA mRNA in peripheral blood and reported no significant difference between the PV-first and PA-first groups in the trend of change of the perioperative values of CEA mRNA. Moreover, they detected circulating epithelial cells (supposed non-malignant bronchial epithelial cells entering into the bloodstream by surgical manipulation) in two patients in the control group who underwent surgery for non-malignant lesions, which suggests that CK19 mRNA might be detected even in the peripheral blood of patients without malignancies. Hashimoto et al. [44] also reported that the PV-first procedure had a significant influence on the intraoperative increase in CTCs, and the role of PV-first lobectomy in preventing the increment of CTCs is still uncertain.
To analyze the effect of PV-first lobectomy on postoperative prognosis, we systematically reviewed the literature. The selection of studies was based on the titles, abstracts, and full papers, with the following inclusion criteria: (1) comparative studies examining PV-first versus PA-first lobectomy, (2) RCTs or observational retrospective/prospective cohort and case-control studies, and (3) studies that reported postoperative prognostic outcomes, such as recurrence and survival rates. The literature search was conducted using PubMed with search terms: (“lung cancer” OR “lung carcinoma” OR “lung neoplasm”) AND (“vein-first” OR “artery-first” OR “vessel ligation” OR “vessel sequence”) AND (“surgery” OR “operat*” OR “postoperative”), and 46 studies were identified. Among them, six studies were included (Figure 1). Refaely et al. [55] reported in their retrospective study that the sequence of vessel interruption (PV-first or PA-first) had no influence on disease recurrence. Kozak et al. [56] also reported the non-efficacy of the sequence of vessel interruption regarding postoperative survival in their RCT. However, their studies included patients who underwent lobectomy through open thoracotomy, which requires more aggressive manual manipulation than VATS lobectomy and may lead to the release of large amounts of CTCs in the early phase of surgery before PV ligation. Li et al. [57] reported the non-efficacy of PV-first lobectomy for postoperative survival in their study, which included only patients who underwent lobectomy by VATS, but their analyses were conducted with patient classification into three groups (PV-first, PA-first, and PA-PV-PA sequence), and no data comparing PV-first and PA-first alone were shown. However, recent studies that included only VATS lobectomy showed the efficacy of PV-first lobectomy for postoperative survival. Sumitomo et al. [58] reported in their retrospective cohort study that PV-first was an independent prognostic factor for better disease-free survival, and He et al. [59] reported in their retrospective study that PV-first lobectomy through the VATS approach was preferred for patients with squamous cell carcinoma, which seemed to metastasize through the bloodstream rather than the lymphatic stream. Moreover, Wei et al. [47] conducted a retrospective cohort study with propensity score matching to minimize selection bias and reported that the 5-year OS rate in the PV-first group was significantly better than that in the PA-first group (73.5% in PV-first vs. 57.6% in PA-first, p = 0.002). In addition, the 5-year disease-free survival and 5-year lung cancer-specific survival in the PV-first group were significantly better than those in the PA-first group. Huang et al. [60] conducted a meta-analysis of five studies, including those by Kozak et al. [56], Li et al. [57], Sumitomo et al. [58], He et al. [59], and Wei et al. [47], and concluded that PV-first ligation is recommended during lobectomy for patients with NSCLC whenever possible. Based on these results, we consider that PV-first lobectomy through the VATS approach has the potential to improve postoperative survival in patients with surgically resectable NSCLC. However, only a few studies have evaluated the relationship between the PV-first technique and postoperative survival. Large-scale, prospective, and multicenter RCTs are needed to clarify the efficacy of PV-first lobectomy.
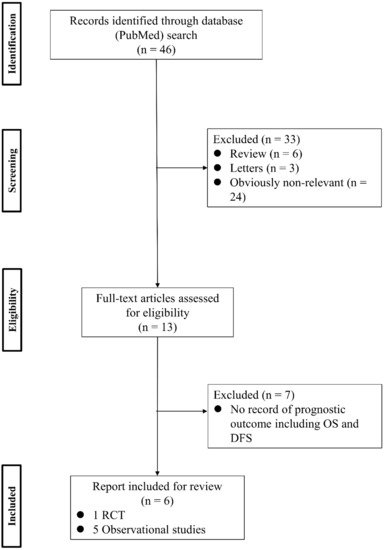
Figure 1. Flowchart of the retrieval of relevant studies. DFS: disease-free survival, OS: overall survival, RCT: randomized controlled trial.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14061448
This entry is offline, you can click here to edit this entry!
