
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | sonal sonal | + 6638 word(s) | 6638 | 2021-12-13 05:00:56 | | | |
| 2 | Peter Tang | -3 word(s) | 6635 | 2022-03-15 02:36:04 | | |
Video Upload Options
Alloying has been very common practice in materials engineering to fabricate metals of desirable properties for specific applications. Traditionally, a small amount of the desired material is added to the principal metal. However, a new alloying technique emerged in 2004 with the concept of adding several principal elements in or near equi-atomic concentrations. These are popularly known as high entropy alloys (HEAs) which can have a wide composition range.
1. Introduction
1.1. The Definitions of High Entropy Alloys
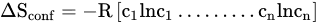
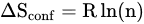
2. Manufacturing of HEAs
2.1. Background and Conventional Methods
2.2. Additive Manufacturing of HEAs
|
Source |
Alloy Composition |
Microstructure (Grain Size) |
Result UTS (MPa), YS (MPa), BS (MPa), δb (mm), ε (%), H, CS (MPa), C (%) |
|---|---|---|---|
|
Chen et al. [110] |
CoCrFeMnNi |
FCC (53.1 µm) |
UTS = 281 ± 18, YS = 12.5 ± 0.5, H = 261 ± 7 HV |
|
Niu et al. [101] |
CoCrFeMnNi |
FCC (<5 µm) |
CS = 2447.7 |
|
Li et al. [111] |
CoCrFeMnNi + TiNp nanoparticles |
FCC |
UTS = 601–1036, ε = 12–30 |
|
Li et al. [112] |
CoCrFeMnNi + Fe based metallic glass |
FCC |
UTS = 916–1517 |
|
Li et al. [113] |
CoCrFeMnNi + TiN nanoparticles |
FCC |
- |
|
Kim et al. [114] |
(CoCrFeMnNi)C |
FCC (180–330 nm) |
YS = 800–900, ε = 25–30 |
|
Li et al. [115] |
CoCrFeMnNi + 12 wt% nano-TiNp |
FCC (<2 µm) |
UTS = 1100 |
|
Piglione et al. [116] |
CoCrFeMnNi |
FCC (0.52–0.64 µm) |
H = 212 HV |
|
Zhu et al. [85] |
CoCrFeMnNi |
FCC |
- |
|
Xu et al. [117] |
CoCrFeMnNi |
FCC (1–2 µm) |
H = 2.84 ± 0.13 GPa |
|
Park et al. [86] |
CoCrFeMnNi +1 at%C |
FCC (20–35 µm) |
UTS = 829–989, YS = 741, ε = 24.3 |
|
Ren et al. [118] |
CoCrFeMnNi |
- |
- |
|
Dovgyy et al. [119] |
CoCrFeMnNi |
FCC & cubic (0.2–0.8 µm) |
- |
|
Zhou et al. [87] |
CoCrFeNi + 0.5 at%C |
FCC (40–50 µm) |
UTS = 776–797, YS = 630–656, ε = 7.7–13.5 |
|
Wu et al. [120] |
CoCrFeNi + 0.5 at%C |
FCC (40–50 µm) |
UTS = 795, YS = 638 |
|
Lin et al. [24] |
CoCrFeNi |
FCC |
|
|
Sun et al. [121] |
CoCrFeNi |
-, ~3 mm in length and ~200 μm in width |
UTS = 676.7–691, YS = 556.7–572, ε = 12.4–17.9 |
|
Song et al. [122] |
CoCrFeNi + N (1.8%) |
FCC |
UTS = 600–853, YS = 520–650, ε = 27 |
|
Zhou et al. [123] |
(CoCrFeNi)1−x (WC)x |
FCC |
H = 603–768 HV |
|
Brif et al. [88] |
CoCrFeNi |
FCC |
UTS = 480–745, YS = 402–600, ε = 8–32, H = 205–238 |
|
Niu et al. [124] |
AlCoCrFeNi |
Disordered (A2) + Ordered (B2) BCC |
H = 632.8 HV |
|
Karlsson et al. [102] |
AlCoCrFeNi |
FCC & BCC (<20 µm) |
- |
|
Peyrouzet et al. [89] |
Al0.3CoCrFeNi |
FCC (width~13 and length~70–120 µm) |
UTS = 896, YS = 730, ε = 29 |
|
Sun et al. [90] |
Al0.5CoCrFeNi |
FCC & BCC (1 µm) |
UTS = 878, YS = 609, H = 270HV |
|
Zhou et al. [92] |
Al0.5CoCrFeNi |
FCC |
UTS = 721, YS = 579, ε = 22 |
|
Luo et al. [125] |
AlCrCuFeNi |
BCC (avg. width~4 µm) |
CS = 1655.2–2052.8, C = 6.5–6.8 |
|
Luo et al. [126] |
AlCrCuFeNix (2 ≤ x ≤ 3) |
FCC (thickness~490 nm) & BCC (~140 nm) Avg. thickness of both ~ 650 nm |
UTS = 957, ε = 14.3 |
|
Li et al. [38] |
AlCoCuFeNi |
BCC |
YS = 744, ε = 13.1, CS = 1600 |
|
Yao et al. [127] |
AlCrFeNiV |
FCC (width~15 µm, length~75–200 µm) |
UTS = 1057.47, ε = 30.3 |
|
Wang et al. [128] |
AlCoCrCuFeNi |
FCC & BCC |
H = 710.4 HV |
|
Wang et al. [129] |
AlMgScZrMn |
Al3 (Sc, Zr) (1–10 nm + 7 µm) |
UTS = 394, ε = 10.5 |
|
Sarawat et al. [130] |
AlCoFeNiV0.9Sm0.1 AlCoFeNiSm0.1TiV0.9 AlCoFeNiSm0.05TiV0.95Zr, AlCoFeNiTiVZr |
FCC |
H~42.8–86.7 HV |
|
Agrawal et al. [131] |
Fe40Mn20Co20Cr15Si5 |
HCP |
UTS = 1100, YS = 530, ε = 30 |
|
NbMoTaW |
BCC (13.4 μm) |
H = 826 HV |
|
|
Ni6Cr4WFe9Ti |
FCC (300–1000 nm) + unknown phase |
UTS = 972, YS = 742, ε = 12.2 |
|
|
Chen et al. [136] |
CoCrFeNiMn |
FCC + Mn2O3 particles |
YS = 620, UTS = 730, ε~12 |
|
Litwa et al. [137] |
CoCrFeNiMn |
FCC |
H~320 HV |
|
Zhang et al. [138] |
CoCrFeNiMn |
FCC |
YS~729.6 |
|
Kim et al. [139] |
CoCrFeNiMn |
FCC |
YS = 752.6 |
|
Choi et al. [140] |
CoCrFeNiMn |
FCC |
|
|
Su et al. [141] |
CrCuFeNi2 Al0.5CrCuFeNi2 Al0.75CrCuFeNi2 AlCrCuFeNi2 |
FCC FCC FCC + BCC/B2 FCC + BCC/B2 |
|
|
Peng et al. [142] |
CoCrFeNi + Ti coated diamond CoCrFeNi + diamond |
FCC + diamond particles FCC + Cr7C3 + diamond particles |
H = 622 HV, BS = 530, δb = 0.64 H = 615 HV, BS = 925, δb = 0.48 |
|
Wang et al. [143] |
CoCrFeNiMn |
FCC |
H = 164–370 HV |
|
Sun et al. [144] |
Al0.1CrCuFeNi Al0.5CrCuFeNi AlCrCuFeNi |
FCC FCC FCC + BCC/B2 (NiAl) |
|
|
Ishimoto et al. [145] |
Ti1.4Nb0.6Ta0.6Zr1.4Mo0.6 |
BCC |
YS = 1690, |
|
Park et al. [146] |
(CoCrFeMnNi)99C1 |
FCC |
YS~741, UTS~874 |
|
Lin et al. [147] |
CoCrFeNi |
FCC |
YS = 701 ± 14, UTS = 907 ± 25 |
|
Kim et al. [148] |
CoCrFeNiMn |
FCC |
- |
|
Jin et al. [149] |
CoCrFeNiMn |
FCC |
YS = 520 ± 10, UTS = 770 ± 10, ε~25 |
|
Lin et al. [150] |
Al0.2Co1.5CrFeNi1.5Ti0.3 |
FCC + σ + L12 |
YS = 1235, UTS = 1550 |
|
Peng et al. [151] |
CoCrFeNiMn |
FCC |
- |
|
Vogiatzief et al. [152] |
AlCrFe2Ni2 Heat treatment (750–950 °C, 3 h & 6 h) |
FCC + BCC |
H = 276–483 HV |
|
Liao et al. [153] |
Al0.5FeCrNi2.5V0.2 |
FCC |
H = 220–240 HV |
|
Guo et al. [154] |
CoCrFeNiMn |
FCC |
YS = 622, UTS = 763, ε~16 |
|
Kim et al. [155] |
(CoCrFeNiMn)100−xCx |
FCC (15–22 µm) |
YS = 653–753, UTS = 766–911 |
|
Zhao et al. [156] |
CoCrFeNi |
FCC |
H = 238–525 HV |
|
Gu et al. [157] |
CoCr2.5FeNi2TiW0.5 |
FCC |
YS = 449–581, CS = 823–893, ε = 4.4–9.9, H = 436.7–499.2 HV |
|
Source |
Alloy Composition |
Microstructure (Grain Size) |
Result UTS (MPa), YS (MPa), ε (%), H, CS (MPa), C (%) |
|---|---|---|---|
|
Peng et al. [158] |
CoCrFeNiMn |
FCC |
YS = 196 |
|
Wang et al. [159] |
CoCrFeMnNi |
FCC (65) |
UTS = 497, 205, H = 157.1HV |
|
Kuwabara et al. [160] |
AlCoCrFeNi |
BCC & FCC |
UTS = 1073, YS = 769, ε = 0–1.2 YS = 944–1015, CS = 1447–1668, C = 14.5–26.4 |
|
Wang et al. [161] |
AlCoCrFeNi |
BCC |
- |
|
Fujieda et al. [162] |
CoCrFeNiTi |
FCC + Cubic |
UTS = 1178, YS = 773, ε = 25.8 |
|
Popov et al. [163] |
Al0.5CrMoNbTa0.5 |
BCC |
|
Scheme |
Alloy Composition |
Microstructure (Grain Size) |
Result UTS (MPa), YS (MPa), ε (%), H, CS (MPa), C (%) |
|---|---|---|---|
|
Guan et al. [164] |
CoCrFeMnNi |
FCC (13 μm) |
YS = 517, ε = 26 |
|
Melia et al. [165] |
CoCrFeMnNi |
FCC (~4 μm) |
UTS = 647–651, YS = 232–424 |
|
Li et al. [166] |
CoCrFeMnNi |
FCC |
|
|
Gao et al. [167] |
CoCrFeMnNi |
FCC (30–150 μm) + BCC |
UTS = 620, YS = 448 |
|
CoCrFeNiMn |
FCC |
UTS = 400–600 |
|
|
Chew et al. [170] |
CoCrFeNiMn |
FCC (3.68 ± 0.85 μm) |
UTS = 660, YS = 518 |
|
Qiu et al. [171] |
CoCrFeMnNi |
FCC |
UTS = 891, YS = 564 |
|
Li et al. [172] |
CoCrFeMnNi + WC (0–10 wt%) |
FCC |
UTS = 550–845, YS = 300–675, ε = 9 |
|
Amar et al. [173] |
CoCrFeMnNi + TiC (0–5 wt%) |
FCC |
UTS = 550–723, YS = 300–385, ε = 32 |
|
Guan et al. [174] |
CoCrFeMnNi AlCoCrFeNiTi0.5 |
FCC (24 µm) BCC (7 µm) + FCC |
YS = 888–1100, H = 197–657 HV |
|
Wang et al. [175] |
CoCrFeNiMo0.2 |
FCC |
UTS = 532–928, ε = 37 |
|
Zhou et al. [176] |
CoCrFeNiNbx (x = 0–0.2) |
FCC |
UTS = 400–820, YS = 220–750 |
|
Gwalani et al. [177] |
AlxCoCrFeNi (x = 0.3–0.7) |
FCC |
|
|
Nartu et al. [178] |
Al0.3CoCrFeNi |
FCC |
YS = 410–630, ε = 18–28 |
|
Mohanty et al. [179] |
AlxCoCrFeNi (x = 0.3–0.7) |
FCC + BCC |
H = 170–380 HV |
|
Vikram et al. [180] |
AlCoCrFeNi2.1 |
FCC & BCC |
YS = 309–711, H = 278 ± 11–316 ± 14 HV |
|
Gwalani et al. [181] |
AlCrFeMoVx (x = 0–1) |
BCC (68–165 μm) |
H = 485–581 HV |
|
Guan et al. [182] |
AlCoCrFeNiTi0.5 |
BCC (12 μm) |
- |
|
Malatji et al. [183] |
AlCrCuFeNi |
BCC & FCC |
H = 350 HV, |
|
AlCoCrFeNiCu AlTiCrFeCoNi |
H = 600 HV, H = 850 HV |
||
|
Moorehead et al. [186] |
NbMoTaW |
BCC |
- |
|
Kunce et al. [187] |
TiZrNbMoV |
BCC |
- |
|
Dobbelstein et al. [188] |
TiZrNbHfTa |
BCC |
H = 509 HV0.2 |
|
Pegues et al. [189] |
CoCrFeNiMn |
FCC |
- |
|
Li et al. [190] |
CoCrFeNiMn |
FCC |
- |
|
Tong et al. [191] |
CoCrFeNiMn Vacuum arc melting 1 impact Laser shock peening 3 impact Laser shock peening 5 impact Laser shock peening |
FCC |
YS = 320.7, UTS = 531.7 YS = 427.4, UTS = 570.7 YS~435, UTS~600 YS = 489.9, UTS = 639.9 |
|
Shen et al. [192] |
CoCrFeNi (SiC)x |
FCC + Cr7C3 (1 µm) |
UTS = 2155–2499, YS = 142–713, H = 139–310 |
|
Cai et al. [193] |
CoCrFeNi AlCoCrFeNi |
BCC (102.27 µm) BCC (18.75 µm) |
YS = 318, UTS = 440, ε = 8.56 YS = 383, UTS = 533, ε = 10.6 |
|
Zhang et al. [194] |
NbMoTa NbMoTaTi NbMoTaNi NbMoTaTi0.5Ni0.5 |
BCC BCC + α-Ti BCC BCC + Ni3Ta + β-Ti |
YS = 1252, CS = 1282, ε = 15 YS = 1200, CS = 1350, ε = 23 YS = 1350, CS = 1380, ε = 11 YS = 1750, CS = 2277.79, ε = 15 CS of NbMoTaTi0.5Ni0.5 at 600, 800 and 1000 °C is 1699.75 MPa, 1033.63 MPa and 651.36 MPa |
|
Peng et al. [195] |
Al0.3CoCrFeNi |
FCC + B2 |
YS = 373–476, CS = 473–508, ε = 0.6–2.96, H = 208–221 HV |
|
Kuzminova et al. [196] |
CoCrFeNi |
FCC |
YS = 456–551, UTS = 637–658, H = 209–259 HV |
|
Malatji et al. [197] |
AlCuCrFeNi Heat treated (800–1100 °C) |
FCC + BCC |
H = 310–381 HV |
|
Dong et al. [198] |
AlCoCrFeNi2.1 |
FCC + BCC |
YS = 388, UTS = 719, ε~27, H = 221–228 |
|
Zhou et al. [199] |
CoCrFeNb0.2Ni2.1 Solution treatment (2 h,1250 °C) 96 h aged (650 °C) |
FCC + HCP (Laves C14) + Nb rich carbide |
YS~340, UTS~735 YS~239, UTS~607 YS~896, UTS~1127, ε~17 |
|
Zheng et al. [200] |
CoCrFeNiMn |
FCC |
YS = 330, UTS = 630 |
3. Applications under Extreme Environments
3.1. Nuclear Applications
|
Reactor System |
Core Outlet Temperature (°C) |
Coolant |
|---|---|---|
|
Super critical water-cooled reactor |
350–620 |
Water |
|
Sodium-cooled fast reactor |
~550 |
Na liquid metal |
|
Lead-cooled reactor |
550–800 |
Pb, Pb-Bi liquid Metals |
|
Molten salt reactor |
700–800 |
Fluoride salts |
|
Gas-cooled fast reactor |
~850 |
Helium gas |
|
Very high temperature reactor |
>900 |
Helium gas |
|
Source |
Material (Fabrication) |
Phase |
Irradiation Conditions (Energy, Ion, Fluence, Temperature) |
|---|---|---|---|
|
Jawaharram et al. [237] |
CoCrFeNiMn |
FCC |
2.6 MeV, Ag3+, 1.5 × 10−3 & 1.9 × 10−3 dpa−1 s−1, 23–500 °C |
|
Lu et al. [238] |
NiCoFeCr, CoCrFeNiMn |
FCC |
3 MeV, Ni2+, 5 × 1016 ions·cm−2, 500 °C |
|
Barr et al. [239] |
CoCrFeNiMn |
FCC |
3 MeV, Ni2+, 3 × 1015 ions·cm−2, 500 °C |
|
Lu et al. [240] |
CoCrFeNi, CoCrFeNiMn |
FCC |
1.5 MeV, Ni+, 4 × 1014 & 3 × 1015 ions·cm−2 (peak dose~4 dpa), 500 °C 3 MeV, Ni+, 5 × 1016 ions·cm−2 (peak dose~60 dpa), 500 °C |
|
Tong et al. [241] |
CoCrFeNiMn CoCrFeNi CoCrFeNiPd |
FCC |
16 MeV, Ni5+, 8 MeV Ni3+, 4 MeV Ni1+& 2 MeV Ni1+, 0.1–1 dpa, 420 °C |
|
Jin et al. [242] |
CoCrFeNi, CoCrFeNiMn |
FCC |
3 MeV, Ni2+, 5 × 1016 ions·cm−2 (peak dose~53 dpa), 500 °C |
|
Chen et al. [243] |
CoCrFeMnNi Al0.3CoCrFeNi |
FCC FCC |
1 MeV, Kr ions, 6.3 × 1015 ions·cm−2, 300 °C |
|
Wang et al. [244] |
CoCrFeNiCu |
FCC |
100 keV, He+, 2.5 × 1017, 5 × 1017 & 1 × 1018 ions·cm−2, RT |
|
He et al. [245] |
CoCrFeNi, CoCrFeNiMn, CoCrFeNiPd |
FCC |
electrons, 5 × 1018 e·cm−2·s−1, 400 °C |
|
Yang et al. [246] |
CoCrFeNiMn, CoCrFeNiPd |
FCC |
3MeV, Ni2+, 5 × 1016 ions·cm−2, 420, 500 & 580 °C |
|
Yang et al. [247] |
CoCrFeNiMn |
FCC |
-, He ion, -, RT & 450 °C |
|
Hashimoto et al. [248] |
CoCrFeNiMn, CoCrFeNiAl0.3 |
FCC |
1250 keV, 1.5 dpa, 300–400 °C |
|
Zhang et al. [249] |
CoCrFeNiCu |
FCC |
3 MeV Ni2+, 1014 ions·cm−2, RT |
|
Yang et al. [250] |
CoNi, FeNi, CoCrFeNi |
- FCC |
3 MeV, Ni2+, 1.5 × 1016 (peak dose~17 dpa) & 5.0 × 1016 (peak dose~53 dpa) ions·cm−2, 500 °C |
|
Abhaya et al. [251] |
CrCoFeNi |
FCC |
1.5 MeV, Ni2+, 1 × 1015 (peak dose~2 dpa) & 5 × 1016 (peak dose~96 dpa) ions·cm−2, RT |
|
Sellami et al. [252] |
CoCrFeNi |
1.5 MeV, Ni2+, 1 × 1013–1 × 1014 ions·cm−2 21 MeV, Ni2+, 2 × 1013 & 1 × 1014 ions·cm−2, RT |
|
|
Chen et al. [253] |
CoCrFeNi |
FCC |
275 keV, He+, 5.14 × 1020 ions·m−2, 250, 300, 400 °C |
|
Kombaiah et al. [254] |
CoCrFeNi, Al0.12CoCrFeNi |
FCC |
3 MeV, Ni2+, 1 × 1017 ions·cm−2 (peak dose~100 dpa), 500 °C |
|
Lu et al. [255] |
CoCrFeNiPd |
FCC |
3 MeV, Ni2+, 5 × 1016 ions·cm−2, 580 °C |
|
Tunes et al. [256] |
CrFeNiMn |
FCC |
30 keV, Xe+, 2.6 × 1016 ions·cm−2, 500 °C |
|
Edmondson et al. [257] |
CrFeNiMn |
BCC |
30 keV, Xe+, 9.3×1016 ions·cm−2 6 keV He+, 6.4 × 1016 ions·cm−2, RT |
|
Fan et al. [258] |
CoCrFeNi |
FCC |
3 MeV, Ni ions, 5 × 1016–8 × 1016 ions/cm−2, 580 °C |
|
Chen et al. [5] |
CoCrFeNiTi0.2 |
FCC |
275 keV, He2+, 5.14 × 1020 ions·m−2, 400 °C |
|
Lyu et al. [259] |
CoCrFeNiMo0.2 |
FCC |
27 keV, electrons, -, RT |
|
Xu et al. [260] |
(CoCrFeNi)95Ti1Nb1Al3 |
FCC |
2.5 MeV, Fe ions, 1.5 × 1019 ions·m−2, RT-500 °C |
|
Cao et al. [261] |
(CoCrFeNi)94Ti2Al4 |
FCC |
4 MeV, Au ions, 10–49 dpa, RT |
|
Tolstolutskaya et al. [262] |
Cr0.18Fe0.4Mn0.28Ni0.14 Cr0.18Fe0.28Mn0.27Ni0.28 Cr0.2Fe0.4Mn0.2Ni0.2 |
FCC |
1.4 MeV, Ar ions, 0, 0.3, 1 & 5 dpa, RT |
|
Kumar et al. [263] |
Fe0.27Ni0.28Mn0.27Cr0.18 |
FCC |
3 MeV, Ni2+, 4.2 × 1013, 4.2 × 1014 & 4.2 × 1015 ions·cm−2, RT & 500 °C 3 MeV, Ni2+, 2.43 × 1015 & 2.43 × 1016 ions·cm−2, 400–700 °C |
|
Li et al. [264] |
Cr0.18Fe0.27Ni0.28Mn0.27 |
FCC |
Neutron, 8.9 × 1014 n·cm−2.s, 60 °C |
|
Voyevodin et al. [265] |
Cr0.2Fe0.4Mn0.2Ni0.2+ Y2O3 + ZrO2 |
FCC |
1.4 MeV, Ar ions, 2.2 × 1015 ions·cm−2, RT |
|
Dias et al. [266] |
CuxCrFeTiV (x = 0.21–1.7) |
BCC + FCC |
300 keV, Ar+, 3 × 1020 at·m−2, RT |
|
Yang et al. [234] |
Al0.3CoCrFeNi |
FCC |
3 MeV, Au ions, 6 × 1015 ion·cm−2 (peak dose ~31 dpa), 250–650 °C |
|
Gromov et al. [267] |
AlCoCrFeNi |
- |
18 keV, electrons, -, RT |
|
Zhang et al. [235] |
AlCrMoNbZr, (AlCrMoNbZr)N |
FCC |
400 keV, He+, 8 × 1015 & 8 × 1016 ion·cm−2, RT |
|
Yang et al. [6] |
Al0.1CoCrFeNi, Al0.75CoCrFeNi, Al1.5CoCrFeNi, |
FCC FCC + B2 A2 + B2 |
3 MeV, Au ions, 1 × 1014–1 × 1016 ions·cm−2, RT |
|
Xia et al. [7] |
Al0.1CoCrFeNi, Al0.75CoCrFeNi, Al1.5CoCrFeNi |
FCC FCC + B2 B2 + A2 |
3 MeV, Au ions, 1 × 1014–1 × 1016 ions·cm−2, RT |
|
Yang et al. [268] |
Al0.1CoCrFeNi |
FCC |
3 MeV, Au ions, 6 × 1015 ions·cm−2, 250–650 °C |
|
Zhou et al. [269] |
AlxCoCrFeNi (x = 0–2) |
FCC + BCC |
1 MeV, Kr2+, -, RT |
|
Zhou et al. [270] |
AlxCoCrFeNi, HfNbTaTiZrV |
FCC Amorphous |
MeV Kr & 200 KeV, electrons, 2 dpa, RT & 150 °C |
|
Zhou et al. [271] |
HfNbTaTiZrV |
BCC |
1 MeV Kr2+, -, RT-150 °C |
|
Moschetti et al. [272] |
HfNbTaTiZr |
BCC |
5 MeV, He2+, 1.6 × 1012–4.4 × 1017 ions·cm−2s, 50 °C |
|
Sadeghilaridjani et al. [273] |
HfTaTiZrV |
BCC |
4.4 MeV, Ni2+, 1.08 × 1017 ion·cm−2, RT |
|
Li et al. [274] |
HfNbTiZr |
BCC |
1.5 MeV, He ions, 5 × 1015–1 × 1017 ions·m−2, 700 °C |
|
Kareer et al. [275] |
TaTiVZr, TaTiVCr, TaTiVNb |
BCC BCC BCC |
2 MeV, V+, 2.26 × 1015 ions·cm−2, 500 °C |
|
Wang et al. [276] |
ZrTiHfCuBe, ZrTiHfCuBeNi, ZrTiHfCuNi |
Amorphous |
100 keV, He ions, 5.0 × 1017, 1.0 × 1018 & 2.0 × 1018 ions·cm−2, RT |
|
Lu et al. [4] |
Ti2ZrHfV0.5Mo0.2 |
BCC |
3 MeV, He+, 5 × 1015, 1 × 1016 & 3 × 1016 ions·cm−2, 600 °C |
|
Atwani et al. [277] |
W0.38Ta0.36Cr0.15V0.11 |
BCC |
1 MeV, Kr+2, 0.0006–8 dpa·s−1, 800 °C |
|
Komarov et al. [278] |
(TiHfZrVNb)N |
- |
500 KeV He2+, 5 × 1016–3 × 1017 ions·cm−2, 500 °C |
|
Gandy et al. [279] |
SiFeVCrMo SiFeVCr |
sigma BCC+ sigma |
5 MeV, Au2+, 5 × 1015 ions·cm−2, RT |
|
Patel et al. [280] |
V2.5Cr1.2WMoCo0.04 |
BCC |
5 MeV, Au+, 5 × 1015 ion·cm−2 (peak dose~42 dpa), RT |
|
Zhang et al. [281] |
Mo0.5NbTiVCr0.25, Mo0.5NbTiV0.5Zr0.25 |
BCC |
400 He2+, 1 × 1017–5 × 1017 ions·m−2, 350 °C |
|
Zhang et al. [282] |
Mo0.5NbTiVCr0.25, Mo0.5NbTiV0.5Zr0.25 |
BCC |
400 keV, He2+, peak dose~10.5 dpa, 350 °C |
|
Atwani et al. [283] |
WtaCrV |
BCC |
2 keV, He+, 1.65 × 1017 ions·cm−2, 950 °C |
3.2. Wear Behavior
|
Source |
Composition |
Microstructure |
Method, Medium, Antagonist Material, Temperature, Wear Rate |
|---|---|---|---|
|
Joseph et al. [291] |
CoCrFeNiMn |
FCC |
Pin-on-disc, dry, Al2O3, 600–800 °C, RT, 0.5 × 10−4–3.8 × 10−4 mm3·N−1·m−1 |
|
Wang et al. [292] |
CoCrFeNiMn |
FCC |
Ball-on-disc, MoS2-oil lubrication, GCr15, RT-140 °C |
|
Xiao et al. [293] |
CoCrFeNiMn |
FCC |
Ball-on-flat, dry, WC-Co, RT, 0.5 × 10−4–5.4 × 10−4 mm3·N−1·m−1 |
|
Jones et al. [294] |
CoCrFeNiMn |
FCC |
Rotary tribometer, -, -, ~0.5 × 10−6 mm3·N−1·m−1 |
|
Zhu et al. [295] |
CoCrFeNiMn CoCrFeNiMnV CoCrFeNiMnNb CoCrFeNiMnNbV |
FCC + HCP (Laves) + σ |
Ball-on-disc, dry, Si3N4, RT, 1.85 × 10−5–6.39 × 10−5 mm3·N−1·m−1 |
|
Deng et al. [296] |
CoCrFeNiMox (x = 0–0.3) |
FCC |
Ball-on-disc, dry, GCr15, RT, 0.33 × 10−3–0.53 × 10−3 mm3·N−1·m−1 |
|
Lindner et al. [297] |
CoCrFeNiMn CoCrFeNi |
FCC FCC |
Ball-on-disc, dry, Al2O3, RT |
|
Sha et al. [298] |
(CoCrFeNiMn)N |
FCC + BCC |
Ball-on-disc, dry, ruby, RT, 1 × 10−7–1.4 × 10−6 mm3·N−1·m−1 |
|
Xiao et al. [299] |
CoCrFeNiMnCx (x = 0–1.2) |
FCC |
Ball-on-disk, dry, Si3N4, RT, 0.47 × 10−5–6.5 × 10−5 mm3·N−1·m−1 |
|
Zhu et al. [211] |
CoCrFeNiMn + TiN-Al2O3 |
FCC + TiN |
Ball-on-disc, dry, 440C steel, RT |
|
Cheng et al. [300] |
CoCrFeNiMn Al0.5CoCrFeNiMn AlCoCrFeNiMn |
FCC FCC + BCC FCC + BCC |
Ball-on-disc, dry, Si3N4, RT-800 °C, 0.5 × 10−4–3.8 × 10−4 mm3·N−1·m−1 |
|
Joseph et al. [301] |
CoCrFeNiMn Al0.3CoCrFeNi Al0.6CoCrFeNi AlCoCrFeNi |
FCC FCC FCC + BCC BCC |
Pin-on-disc, dry, Al2O3, 25 & 900 °C |
|
Liu et al. [302] |
CoCrFeNiMn + Y2O3 |
FCC + Y2O3 (particles) |
Ball-on-disc, dry, GCr15, RT |
|
Wang et al. [303] |
(CoCrFeMnNi)85Ti15 |
FCC + BCC |
Ball-on-disc, dry, Si3N4, RT-800 °C, 4 × 10−6–2.23 × 10−5 mm3·N−1·m−1 |
|
Zhang et al. [304] |
CoCrFeNi + (Ag or BaF2/CaF2) |
FCC |
Ball-on-disk, dry, Inconel-718, RT, ~4 × 10−5–40 × 10−5 mm3·N−1·m−1 |
|
Geng et al. [305] |
CoCrFeNi |
FCC |
Pin-on-disc, vacuum (4 Pa) & air, Inconel 718, RT, 0.6 × 10−4–8 × 10−4 mm3·N−1·m−1 |
|
Zhang et al. [306] |
CoCrFeNi + (graphite or MoS2) |
FCC |
Ball-on-disk, dry, Si3N4, RT-800 °C, ~1 × 10−5–23 × 10−5 mm3·N−1·m−1 |
|
Zhou et al. [307] |
CoCrFeNiMo0.85 Al0.5CoCrFeNi |
FCC FCC |
Slurry jet test-rig, HCl+NaCl, -, 40 °C, - |
|
Zhang et al. [308] |
CoCrFeNiMo |
FCC |
Ball-on-disc, dry, -, RT |
|
Huang et al. [309] |
FeCoCrNiSix |
FCC + BCC |
Ball-on-disk, dry, GCr15, RT |
|
Cui et al. [310] |
CoCrFeNiMo Sulfurized at 260 °C for 2 h |
FCC + FeS/MoS2 film |
Pin-on-disk, dry, GCr15, RT |
|
Li et al. [311] |
CoCrFeNiMo0.2 |
FCC |
Ball on disc, dry, GCr15, RT, 3.9 × 10−4–5.4 × 10−4 mm3·N−1·m−1 |
|
Ji et al. [312] |
CoCrFeNiCu + 2% MoS2 CoCrFeNiCu + 5% MoS2 CoCrFeNiCu + 20% WC CoCrFeNiCu + 50% WC CoCrFeNiCu + 80% WC |
FCC + MoS2 (particles) FCC + MoS2 (particles) FCC + WC (particles) FCC + WC (particles) FCC + WC (particles) |
Ball-on-disk, dry, Si3N4, RT |
|
Verma et al. [313] |
CoCrFeNiCux (x = 0–1) |
FCC |
Pin-on-disk, dry, -, RT & 600 °C, ~1.3 × 10−5–2.5 × 10−5 mm3·N−1·m−1 |
|
Liu et al. [314] |
CoCrFeNiBx (x = 0.5–1.5) |
FCC + Borides |
Roller friction wear tester, dry, W18Cr5V, RT |
|
Jiang et al. [315] |
CoCrFeNiNbx (x = 0–1.2) |
FCC + HCP (Laves) HCP (Co2Nb) |
Ball-on-disc, dry, BN, RT |
|
Yu et al. [316] |
CoCrFeNiNbx (x = 0.5–0.8) |
FCC + HCP (Laves) |
Pin-on-disk, dry, Si3N4, RT-800 °C, ~1.8 × 10−4–9 × 10−4 mm3·N−1·m−1 |
|
Liu et al. [317] |
Co10Cr10Fe50Mn30 + graphene nanoplatelets (0.2–0.8 wt%) |
FCC |
Ball-on-plate, dry, GCr15, RT |
|
Wang et al. [318] |
Co10Cr10Fe40Mn40 + WC (10 wt%) |
FCC+ WC + M23C6 |
Ball-on-disc, dry, Si3N4, RT |
|
Derimow et al. [319] |
(CoCrCuTi)100−xMnx (x = 5–10) (CoCrCuTi)100−xMnx (x = 10–20) |
FCC + BCC FCC + HCP (Laves) |
Ball-on-disc, dry, GCr15, RT |
|
Guo et al. [320] |
CoCrFeNiCuSi0.2 (Ti or C)x (x = 0–1.5) |
FCC + TiC |
Brooks sliding friction & wear tester, dry, RT |
|
Zhang et al. [321] |
(CoCrFeNiTi0.5)Cx (x = 3–12 wt%) |
BCC + Cr23C6 + TiC |
ML-100 friction and wear tester, -, -, RT |
|
Erdoğan et al. [322] |
CoCrFeNiTi0.5 CoCrFeNiTi0.5Al0.5 CoCrFeNiTi0.5Al |
FCC BCC BCC |
Ball-on-disc, dry, WC, RT |
|
Liu et al. [323] |
CoCrFeNiMo CoCrFeNiMox (x ≥ 0.3) CoCrFeNiMox (x ≥ 1) |
FCC FCC + σ FCC + σ + µ |
Pin-on-disk, dry, YG6, RT, 1 × 10−5–8.5 × 10−5 mm3·N−1·m−1 |
|
Moazzen et al. [324] |
CoCrFexNi (x = 1–1.6) |
FCC + BCC |
Pin-on-disk, dry, AISI52100 steel, 20–30 °C, - |
|
Yang et al. [325] |
CoCrFeNiMoSix (x = 0.5–1.5) |
FCC |
Pin-on-disk, dry, Si3N4, RT, 0.292 × 10−4–0.892 × 10−4 mm3·N−1·m−1 |
|
Li et al. [326] |
CoCrFeNi2V0.5Tix (x = 0.5–1.25) |
BCC + (Co,Ni)Ti2 |
Ball-on-disc, dry, Si3N4, RT, 4.4 × 10−5- 37.5 × 10−5 mm3·N−1·m−1 |
|
Islak et al. [327] |
CrFeNiMoTi |
FCC |
Ball-on-flat, dry, 100Cr6, RT, 2.7 × 10−3–9.4 × 10−3 mm3·N−1·m−1 |
|
Wen et al. [328] |
CrCoNiTiV |
FCC + BCC + TiO |
HT-1000 tribometer, -, WC, RT & 600 °C |
|
Wang et al. [329] |
CuNiSiTiZr |
BCC |
CJS111A wear tester, dry, -, RT |
|
Cheng et al. [330] |
(Fe25Co25Ni25 (B0.7Si0.3)25)100−xNbx (x = 0–4 wt%) |
BCC + HCP (Laves) + FCC |
Ball-on-disc, dry, GCr15, RT, ~1.5 × 10−6–3.6 × 10−6 mm3·N−1·m−1 |
|
Yadav et al. [331] |
(CuCrFeTiZn)1−xPbx (x = 0.05–0.2) |
FCC + BCC + Pb (particles) |
Ball-on-disk, dry, -, SAE 52100, RT, 1.17 × 10−5–50 × 10−5 mm3·N−1·m−1 |
|
Gou et al. [332] |
CoCrFeNi + WC + Mo2C + NbC |
FCC |
Ball-on-disc, dry, GCr15, 700 °C |
|
Yadav et al. [333] |
(CuCrFeTiZn)100−xPbx (x = 0–10) (CuCrFeTiZn)100−xBix (x = 0–10) |
FCC + BCC BCC |
Ball-on-disk, dry, steel, RT |
|
Cui et al. [334] |
AlxCoCrFeNiMn (x = 0–0.75) |
FCC + BCC |
MDW- 02 abrasive wear tester, RT |
|
Gwalani et al. [335] |
Al0.5CoCrFeNi |
FCC + B2 |
Pin-on-disc, dry, Si3N4, RT, 1.8 × 10−5–11 × 10−5 mm3·N−1·m−1 |
|
Chen et al. [336] |
Al0.6CoCrFeNi |
FCC + BCC |
Ball-on-plate, dry, Si3N4, RT-600 °C, ~0.5 × 10−4–5 × 10−4 mm3·N−1·m−1 |
|
Du et al. [337] |
Al0.25CoCrFeNi |
FCC |
Universal wear testing machine, dry, Si3N4 20–600 °C, ~1.5 × 10−4–3.5 × 10−4 mm3·N−1·m−1 |
|
Chen et al. [338] |
Al0.6CoCrFeNi |
FCC + BCC |
Ball-on-block, deionized water & acid rain (pH = 2), seawater, GCr15, RT, 1.58 × 10−4–6.52 × 10−4 mm3·N−1·m−1 |
|
Ji et al. [339] |
Al3CoCrFeNi |
Jet erosion testing machine, water and 15 wt% SiO2 particles (350–600 mm), RT |
|
|
Haghdadi et al. [340] |
Al0.3CoCrFeNi AlCoCrFeNi |
FCC BCC |
Scratch testing, dry, -, RT |
|
Fang et al. [341] |
Al0.3CoCrFeNi |
FCC |
Pin-on-disc, dry, -, 900 °C |
|
Wu et al. [342] |
Al0.1CoCrFeNi |
FCC |
Ball-on-block, dry and deionized water, Si3N4, RT, ~0.2 × 10−4–1.86 × 10−4 mm3·N−1·m−1 |
|
Nair et al. [343] |
Al0.1CoCrFeNi AlCoCrFeNi Al3CoCrFeNi |
FCC FCC + BCC (B2) BCC (B2) + A2 + σ |
Ball-on-disc, dry, WC, RT |
|
Kumar et al. [344] |
Al0.4CoxCrFeNi (x = 0–1) |
- |
Pin-on-disc, demineralized water & (demineralized water + 3.5 wt% NaCl), EN-31, RT, 0.81 × 10−4–1.86 × 10−4 mm3·N−1·m−1 |
|
Mu et al. [345] |
AlCoCrFeNi |
BCC + FCC |
Ball-on disc, dry, Si3N4, RT |
|
Wu et al. [346] |
AlCoCrFeNi AlCoCrFeNiTi0.5 |
BCC |
Pin-on-disc, dry, Si3N4, RT |
|
Zhao et al. [347] |
Al0.8CoCrFeNi |
FCC + BCC |
Ball-on-disk, dry, deionized water + 0.5 wt% NaCl, RT, ~2 × 10−5–7.5 × 10−5 mm3·N−1·m−1 |
|
Kumar et al. [348] |
Al0.4CoxCrFeNi (x = 0–0.5) Al0.4CoxCrFeNi (x = 1) |
FCC + BCC FCC |
Pin-on-disk, engine oil (SAE Grade:20W-40), EN-31 steel, RT, 2.1 × 10−5–11 × 10−5 mm3·N−1·m−1 |
|
Li et al. [349] |
Al0.8CoCrFeNiCu0.5Six (x = 0–0.5) |
FCC + BCC1 + BCC2 |
-, -, CGr15, RT, 0.9 × 10−6–1.19 × 10−6 mm3·N−1·m−1 |
|
Li et al. [206] |
(AlCoCrFeNi)100-x (NbC)x (x = 0–30 wt%) |
FCC + BCC |
Reciprocating tester, dry, N4Si3, RT |
|
Kafexhiu et al. [350] |
AlCoCrFeNi2.1 |
BCC + FCC |
Ball-on-plate, dry, 100Cr6 steel, RT, 7 × 10−5–11 × 10−5 mm3·N−1·m−1 |
|
Miao et al. [351] |
AlCoCrFeNi2.1 |
FCC (L12) + BCC (B2) |
Ball-on-disk, dry, Al2O3/Si3N4/SiC/GCr15, RT-900 °C, ~1 × 10−4–4.2 × 10−4 mm3·N−1·m−1 |
|
Ye et al. [352] |
AlCoCrFeNi2.1 + TiC (0–15 wt%) |
FCC + B2 + TiC |
MM-200 wear testing machine, dry, -, RT |
|
Wang et al. [353] |
(AlCoCrFeNi)N |
BCC + nitrides (AlN,CrN,Fe4N) |
Ball-on block, dry, deionized water & acid rain (pH = 2), Si3N4, RT, 2.8 × 10−5–7 × 10−5 mm3·N−1·m−1 |
|
Liu et al. [354] |
AlCrCuFeNi2 |
Ball-on-block, dry, simulated rainwater & deionized water, Si3N4, RT, 2.163 × 10−3–0.23 × 10−3 mm3·N−1·m−1 |
|
|
Kong et al. [355] |
Al1.8CrCuFeNi2 |
BCC |
MMS-2A roller friction wear tester, dry, -, RT |
|
Malatji et al. [197] |
AlCrCuFeNi |
FCC + BCC |
Ball-on-disk, dry, SiC, RT |
|
Wang et al. [356] |
Al1.3CoCuFeNi2 |
FCC + BCC |
Ball-on block, dry, deionized water & acid rain (pH = 2), Si3N4, RT, 1 × 10−4–12 × 10−4 mm3·N−1·m−1 |
|
Xiao et al. [357] |
AlxCoCrFeNiSi (x = 0.5–1.5) |
FCC + BCC |
Ball-on-flat, distilled water, WC-12Co, RT, 6.7 × 10−6–5.5 × 10−5 mm3·N−1·m−1 |
|
Liu et al. [358] |
AlCoCrFeNiSix (0–0.5) |
BCC |
Pin-on-disk, dry, ZrO2, RT, 1.3 × 10−4–5.1 × 10−4 mm3·N−1·m−1 |
|
Hsu et al. [359] |
Al0.5CoCrFeNiCuBx (x = 0–1) |
FCC + boride precipitates |
Pin-on-disk, dry, Al2O3, RT |
|
Chen et al. [360] |
Al0.5CoCrFeNiCuTix (x = 0–0.2) Al0.5CoCrFeNiCuTix (x = 0.4–1) Al0.5CoCrFeNiCuTix (x = 1.2–2) |
FCC FCC + BCC FCC + BCC + Ti2N |
Pin-on-disk, dry, Al2O3, RT |
|
Lobel et al. [361] |
AlCoCrFeNiTi |
BCC |
Ball-on-disc, dry, Al2O3, RT |
|
Lobel et al. [362] |
AlCoCrFeNiTi |
BCC |
Ball-on-plate, dry, 100Cr6 Steel, RT |
|
Wu et al. [363] |
AlCoCrFeNiTix (x = 0.5–1) AlCoCrFeNiTix (x = 1.5) AlCoCrFeNiTix (x = 2) |
FCC + BCC FCC + BCC + Ti2Ni FCC + BCC + Ti2Ni + ordered BCC |
Cavitation erosion tests, Distilled water+ 3.5 wt% NaCl, RT |
|
Erdogan et al. [364] |
AlxCoCrFeNiTiy (x = 0–0.5, y = 0–0.5) |
FCC + BCC |
Ball-on-disc, dry, WC, RT, 0.25 × 10−4–1.78 × 10−4 mm3·N−1·m−1, 0.25 × 10−4–1.78 × 10−4 mm3·N−1·m−1 |
|
Xin et al. [365] |
Al0.2Co1.5CrFeNi1.5Ti0.5 + TiC |
FCC |
Ball-on-disc, dry, Si3N4, RT, 0.3 × 10−5–12.6 × 10−5 mm3·N−1·m−1 |
|
Gouvea et al. [366] |
Al0.2Co1.5CrFeNi1.5Ti |
FCC |
Ball-on-plate, dry, AISI 52,100 steel, RT, 1.6 × 10−8–7.5 × 10−5 mm2·N−1 |
|
Chuang et al. [367] |
AlxCo1.5CrFeNi1.5Tiy (x = 0–0.2, y = 0.5–1) |
FCC |
Pin-on-disk, dry, SKH51 steel, RT, ~4 × 10−4–1.8 × 10−4 mm3·N−1·m−1 |
|
Liu et al. [368] |
AlCoCrFeNiTi0.8 |
BCC + B2 |
Ball-on-disc, dry, Si3N4, RT, 1.36 × 10−6–6.96 × 10−6 mm3·N−1·m−1, 0.7 × 10−4–6 × 10−4 mm3·N−1·m−1 |
|
Yu et al. [369] |
AlCoCrFeNiTi0.5 |
BCC1 + BCC2 |
Pin-on-disk, H2O2, SiC & ZrO2, RT |
|
Lobel et al. [370] |
AlCoCrFeNiTi0.5 |
BCC (A2 + B2) |
SRV-Tribometer, dry, Al2O3, 22–900 °C |
|
Chen et al. [371] |
Al0.6CoCrFeNiTi |
BCC |
Pin-on-disc, Dry, Al2O3 RT-500 °C |
|
Yu et al. [372] |
AlCoCrFeNiTi0.5 AlCoCrFeNiCu |
Pin-on-disc, dry, Si3N4 |
|
|
Yu et al. [373] |
AlCoCrFeNiCu AlCoCrFeNiTi0.5 |
FCC + BCC1 BCC1 + BCC2 |
Pin-on-disk, H2O2, 1Cr18Ni9Ti steel & ZrO2/SiC ceramic, RT |
|
Jin et al. [374] |
AlCoFeNiCu |
FCC + BCC |
Ball-on-disk, dry, WC, 200–800 °C |
|
Zhu et al. [375] |
AlCoFeNiCu + TiC (10–30 wt%) |
FCC + BCC |
Ball-on-disk, dry, Si3N420–600 °C, ~0.1 × 10−5–6.5 × 10−5 mm3·N−1·m−1 |
|
Wu et al. [376] |
Al0.5CoCrFeNiCu Al1.0CoCrFeNiCu Al2.0CoCrFeNiCu |
FCC FCC + BCC BCC |
Pin-on-disk, dry, SKH-51 steel, RT |
|
Yan et al. [377] |
AlCoCrFeNiSi + Ti (C, N) |
BCC + FCC |
Ball-on-disc, dry, GCr15, RT, - |
|
Li et al. [378] |
AlCoCrFeNi + Ti (C,N) + TiB2 |
FCC |
Ball-on-disc, dry, WC-6Co, 200–800 °C, 2.69 × 10−5–8.66 × 0−5 mm3·N−1·m−1 |
|
Kumar et al. [379] |
AlCoCrCuFeNiSi0.3 AlCoCrCuFeNiSi0.6 |
FCC + BCC FCC + BCC + σ |
Pin-on-disk, dry, -, RT, - |
|
Xin et al. [380] |
Al0.2Co1.5CrFeNi1.5Ti0.5 |
FCC |
Pin-on-disk, dry, Si3N4, 25–800 °C, 1.21 × 10−5–6.7 × 10−5 mm3·N−1·m−1 |
|
Karakaş et al. [381] |
Al0.07Co1.26Cr1.80Fe1.42Mn1.35Ni1.1 |
FCC |
Ball-on-disc, 3.5%NaCl & 5%H2SO4, -, RT, 16.26 × 10−9–77.84 × 10−8 mm3·N−1·m−1 |
|
Xin et al. [382] |
Al0.2Co1.5CrFeNi1.5Ti (0.5+x) + Cx (x = 0) |
FCC |
Pin-on-disk, dry, Si3N4, 25–800 °C, 3.12 × 10−6–12.59 × 10−5 mm3·N−1·m−1 |
|
Zhao et al. [383] |
AlCrCoFeNiCTax (x = 0–1) |
BCC |
Pin-on-disk, 3.5%NaCl & air, Si3N4, RT, 1.67 × 10−6–2.22 × 10−5 mm3·N−1·m−1 |
|
Ghanbariha et al. [384] |
AlCoCrFeNi + ZrO2 |
FCC + BCC |
Pin-on-disk, dry, WC, RT, 1.11 × 10−3–2.52 × 10−3 mm3·N−1·m−1 |
|
Li et al. [385] |
AlxCrFeCoNiCu (x = 0–0.5) AlxCrFeCoNiCu (x = 0.5–2) |
FCC FCC + BCC |
-, dry, GCr15, RT, 6.64 × 10−7–2.26 × 10−4 mm3·N−1·m−1 |
|
Cai et al. [386] |
AlCrTiV, AlCrTiVSi |
BCC |
Nanoindenter G200, dry, CGr15 &Al2O3, RT, - |
|
Chandrakar et al. [387] |
AlCoCrCuFeNiSix (x = 0–0.9) |
BCC |
Pin-on-disk, dry, -, RT, - |
|
Erdogan et al. [388] |
AlCrFeNiSi AlCrFeNix (x = Cu,Co) |
BCC BCC + FCC |
Ball-on-disc, dry, WC, RT, - |
|
Duan et al. [389] |
AlCoCrFeNiCu |
- |
Pin-on-disc, H2O2, Si3N4, RT |
|
Chen et al. [390] |
Al0.5CoCrFeNiCuVx (x = 0–0.2) Al0.5CoCrFeNiCuVx (x = 0.4–0.8) Al0.5CoCrFeNiCuVx (x = 1–2) |
FCC FCC + BCC BCC |
Pin-on-disk, dry, Al2O3, RT, 1 × 10−4–2.7 × 10−4 mm3·N−1·m−1 |
|
Gu et al. [391] |
AlxMo0.5NbFeTiMn2 (x = 1–2) |
BCC |
Pin-on-disk, dry, Al2O3, RT |
|
Hsu et al. [392] |
AlCoCrFexNiMo0.5 (x = 0.6–2) |
BCC + σ |
Pin-on-disk, dry, SKH51 steel, RT |
|
Liang et al. [393] |
AlCrFe2Ni2W0.2Mo0.75 |
BCC |
Ball-on-disc, deionized water, Al2O3, RT, ~5 × 10−6–22 × 10−6 mm3·N−1·m−1 |
|
Qui et al. [394] |
Al2CoCrFeCuTiNix (x = 0–2) |
FCC + BCC |
Tribometer, -, -, RT |
|
Kanyane et al. [395] |
AlTiSiMoW |
BCC + TiSi2 (ordered FCC) |
Ball-on-disc, dry, stainless steel, RT |
|
Huang et al. [396] |
AlTiSiVCr |
BCC+ (Ti,V)5Si3 precipitates |
Ball-on-disc, dry, GCr15 steel, RT, 2 × 10−5–2.5 × 10−5 mm3·N−1·m−1 |
|
Zhang et al. [397] |
AlTiSiVNi |
B2 (NiAl) + (Ti,V)5Si3 + TiN |
Ball-on-disc, dry, Si3N4, RT & 800 °C |
|
Lin et al. [398] |
AlCoCrNiW AlCoCrNiSi |
W + AlNi + Cr15.58Fe7.42C6 BCC |
Pin-on-disc, dry, AISI 52100, RT |
|
Yadav et al. [399] |
AlCrFeMnV (AlCrFeMnV)90Bi10 (AlCrFeMnV)90Bi10 + 10 wt% TiB2 (AlCrFeMnV)90Bi10 + 15 wt% TiB2 |
BCC BCC + AlV3 + Bi BCC + AlV3 + Bi + TiB2 BCC + AlV3 + Bi + TiB2 |
Ball-on-disk, dry, SAE 52,100 steel, RT, 1.02 × 10−5–7.02 × 10−5 mm3·N−1·m−1 |
|
Bhardwaj et al. [400] |
AlTiZrNbHf |
BCC |
Pin-on-disk, dry, CGr15, RT, - |
|
Zhao et al. [401] |
AlNbTaZrx (x = 0.2–1) |
BCC + HCP |
Ball-on-disc, dry, Si3N4, RT, 1.85 × 10−4–2.41 × 10−4 mm3·N−1·m−1 |
|
Tuten et al. [402] |
TiZrHfNbTa |
Amorphous |
Ball-on-disc, dry, Al2O3, RT |
|
Pole et al. [403] |
TiZrHfTaV, TiZrTaVW |
BCC |
Ball-on-disk, dry, Si3N4, RT-500 °C, ~1 × 10−4–8 × 10−4 mm3·N−1·m−1 |
|
Ye et al. [404] |
TiZrHfNb |
BCC |
Nano-scratch, dry, diamond indenter, RT |
|
Pogrebnjak et al. [405] |
(TiZrHfNbV)N |
FCC |
Ball-on-disc, dry, Al2O3, 20 °C |
|
Gong et al. [406] |
TiZrHfBeCu TiZrHfBeNi Ti20Zr20Hf20Be20Cu10Ni10 Ti13.8Zr41.2Ni10Be22.5Cu12.5 |
Amorphous |
Nano-scratch, dry, diamond indenter, RT |
|
Zhao et al. [407] |
TiZrNiBeCu |
Amorphous |
Nano-scratch, dry, diamond indenter, RT |
|
Jhong et al. [408] |
(TiZrNbCrSi)Cx (x = 36.7–87.8 at.%) |
FCC |
Ball-on-disc, dry, 100Cr6 steel, RT, 0.2 × 10–3.3 × 10−6 mm3·N−1·m−1 |
|
Mathiou et al. [409] |
TiZrNbMoTa |
BCC + HCP |
Ball-on disc, dry, 100Cr6 steel, Al2O3, RT, 0.154 × 10−1–0.199 × 10−1 mm3·N−1·m−1 |
|
Petroglou et al. [410] |
MoTaxNbVTi (x = 0.25–1) |
BCC |
Ball-on-disk, dry, 100Cr6 steel, RT, 0.19 × 10−6–0.38 × 10−6 g·N−1·m−1 |
|
Poulia et al. [411] |
MoTaNbVW |
BCC |
Ball-on-disc, dry, 100Cr6 steel & Al2O3, RT |
|
Poulia et al. [412] |
MoTaNbVW |
BCC |
Ball-on-disc, dry, 100Cr6 steel & Al2O3, RT, 1.05 × 10−4–4.89 × 10−4 mm3·N−1·m−1 |
|
Poulia et al. [413] |
MoTaNbVTi |
BCC + hexagonal C14 Laves + cubic C15 laves |
Ball-on disc, dry, 100Cr6 steel, Al2O3, RT |
|
Alvi et al. [414] |
MoTaWVCu |
BCC |
Ball-on-disc, dry, E52100 steel & Si3N4, RT-600 °C, 2.3 × 10−2–5 × 10−2 mm3·N−1·m−1 |
|
Hua et al. [415] |
TixZrNbTaMo (x = 0.5–2) |
BCC |
HSR-2M tester, dry, Si3N4, RT, 2.22 × 10−7–2.42 × 10−7 mm3·N−1·m−1 |
|
Gu et al. [416] |
Ni1.5CrFeTi2.0.5Mox (x = 0–0.25) Ni1.5CrFeTi2.0.5Mox (x = 0.5–0.25) |
BCC BCC + FCC |
Ball-on-disc, dry, Al2O3, RT, 7.99 × 107–2.7 × 107 µm3 |
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303.
- Yeh, J.W. Recent progress in high-entropy alloys. Ann. Chim. Sci. 2006, 31, 633–648.
- Yeh, J.W. Alloy design strategies and future trends in high-entropy alloys. JOM 2013, 65, 1759–1771.
- Lu, Y.; Huang, H.; Gao, X.; Ren, C.; Gao, J.; Zhang, H.; Zheng, S.; Jin, Q.; Zhao, Y.; Lu, C.; et al. A promising new class of irradiation tolerant materials: Ti2ZrHfV0.5Mo0.2 high-entropy alloy. J. Mater. Sci. Technol. 2019, 35, 369–373.
- Chen, D.; Tong, Y.; Wang, J.; Han, B.; Zhao, Y.L.; He, F.; Kai, J.J. Microstructural response of He+ irradiated FeCoNiCrTi0.2 high-entropy alloy. J. Nucl. Mater. 2018, 510, 187–192.
- Yang, T.; Xia, S.; Liu, S.; Wang, C.; Liu, S.; Fang, Y.; Zhang, Y.; Xue, J.; Yan, S.; Wang, Y. Precipitation behavior of AlxCoCrFeNi high entropy alloys under ion irradiation. Sci. Rep. 2016, 6, 32146.
- Xia, S.; Gao, M.C.; Yang, T.; Liaw, P.K.; Zhang, Y. Phase stability and microstructures of high entropy alloys ion irradiated to high doses. J. Nucl. Mater. 2016, 480, 100–108.
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22.
- Cantor, B. Multicomponent and high entropy alloys. Entropy 2014, 16, 4749–4768.
- Alshataif, Y.A.; Sivasankaran, S.; Al-Mufadi, F.A.; Alaboodi, A.S.; Ammar, H.R. Manufacturing Methods, Microstructural and Mechanical Properties Evolutions of High-Entropy Alloys: A Review. Met. Mater. Int. 2019, 26, 1099–1133.
- He, Q.F.; Tang, P.H.; Chen, H.A.; Lan, S.; Wang, J.G.; Luan, J.H.; Du, M.; Liu, Y.; Liu, C.T.; Pao, C.W.; et al. Understanding chemical short-range ordering/demixing coupled with lattice distortion in solid solution high entropy alloys. Acta Mater. 2021, 216, 117140.
- Stepanov, N.D.; Yurchenko, N.Y.; Zherebtsov, S.V.; Tikhonovsky, M.A.; Salishchev, G.A. Aging behavior of the HfNbTaTiZr high entropy alloy. Mater. Lett. 2018, 211, 87–90.
- Tsai, C.W.; Chen, Y.L.; Tsai, M.H.; Yeh, J.W.; Shun, T.T.; Chen, S.K. Deformation and annealing behaviors of high-entropy alloy Al0.5CoCrCuFeNi. J. Alloys Compd. 2009, 486, 427–435.
- Zhang, K.B.; Fu, Z.Y.; Zhang, J.Y.; Shi, J.; Wang, W.M.; Wang, H.; Wang, Y.C.; Zhang, Q.J. Annealing on the structure and properties evolution of the CoCrFeNiCuAl high-entropy alloy. J. Alloys Compd. 2010, 502, 295–299.
- Bhattacharjee, P.P.; Sathiaraj, G.D.; Zaid, M.; Gatti, J.R.; Lee, C.; Tsai, C.W.; Yeh, J.W. Microstructure and texture evolution during annealing of equiatomic CoCrFeMnNi high-entropy alloy. J. Alloys Compd. 2014, 587, 544–552.
- Stepanov, N.D.; Yurchenko, N.Y.; Tikhonovsky, M.A.; Salishchev, G.A. Effect of carbon content and annealing on structure and hardness of the CoCrFeNiMn-based high entropy alloys. J. Alloys Compd. 2016, 687, 59–71.
- Zhang, K.; Fu, Z. Effects of annealing treatment on phase composition and microstructure of CoCrFeNiTiAlx high-entropy alloys. Intermetallics 2012, 22, 24–32.
- Haase, C.; Barrales-Mora, L.A. Influence of deformation and annealing twinning on the microstructure and texture evolution of face-centered cubic high-entropy alloys. Acta Mater. 2018, 150, 88–103.
- Niu, Z.; Wang, Y.; Geng, C.; Xu, J.; Wang, Y. Microstructural evolution, mechanical and corrosion behaviors of as-annealed CoCrFeNiMox (x = 0, 0.2, 0.5, 0.8, 1) high entropy alloys. J. Alloys Compd. 2020, 820, 153273.
- Abbasi, E.; Dehghani, K. Microstructure and mechanical properties of Co19Cr20Fe20Mn21Ni19 and Co19Cr20Fe20Mn21Ni19Nb0.06C0.8 high-entropy/compositionally-complex alloys after annealing. Mater. Sci. Eng. A 2020, 772, 138812.
- Sathiaraj, G.D.; Pukenas, A.; Skrotzki, W. Texture formation in face-centered cubic high-entropy alloys. J. Alloys Compd. 2020, 826, 154183.
- Munitz, A.; Meshi, L.; Kaufman, M.J. Heat treatments’ effects on the microstructure and mechanical properties of an equiatomic Al-Cr-Fe-Mn-Ni high entropy alloy. Mater. Sci. Eng. A 2017, 689, 384–394.
- Tang, Q.H.; Huang, Y.; Huang, Y.Y.; Liao, X.Z.; Langdon, T.G.; Dai, P.Q. Hardening of an Al0.3CoCrFeNi high entropy alloy via high-pressure torsion and thermal annealing. Mater. Lett. 2015, 151, 126–129.
- Lin, D.; Xu, L.; Jing, H.; Han, Y.; Zhao, L.; Minami, F. Effects of annealing on the structure and mechanical properties of FeCoCrNi high-entropy alloy fabricated via selective laser melting. Addit. Manuf. 2020, 32, 101058.
- Shahmir, H.; He, J.; Lu, Z.; Kawasaki, M.; Langdon, T.G. Effect of annealing on mechanical properties of a nanocrystalline CoCrFeNiMn high-entropy alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2016, 676, 294–303.
- Xu, J.; Zhang, J.Y.; Wang, Y.Q.; Zhang, P.; Kuang, J.; Liu, G.; Zhang, G.J.; Sun, J. Annealing-dependent microstructure, magnetic and mechanical properties of high-entropy FeCoNiAl0.5 alloy. Mater. Sci. Eng. A 2020, 776, 139003.
- Ma, Y.; Peng, G.J.; Wen, D.H.; Zhang, T.H. Nanoindentation creep behavior in a CoCrFeCuNi high-entropy alloy film with two different structure states. Mater. Sci. Eng. A 2015, 621, 111–117.
- Ma, S.G.; Qiao, J.W.; Wang, Z.H.; Yang, H.J.; Zhang, Y. Microstructural features and tensile behaviors of the Al0.5CrCuFeNi2 high-entropy alloys by cold rolling and subsequent annealing. Mater. Des. 2015, 88, 1057–1062.
- Zhuang, Y.X.; Xue, H.D.; Chen, Z.Y.; Hu, Z.Y.; He, J.C. Effect of annealing treatment on microstructures and mechanical properties of FeCoNiCuAl high entropy alloys. Mater. Sci. Eng. A 2013, 572, 30–35.
- Niu, S.; Kou, H.; Guo, T.; Zhang, Y.; Wang, J.; Li, J. Strengthening of nanoprecipitations in an annealed Al0.5CoCrFeNi high entropy alloy. Mater. Sci. Eng. A 2016, 671, 82–86.
- Gwalani, B.; Gorsse, S.; Choudhuri, D.; Styles, M.; Zheng, Y.; Mishra, R.S.; Banerjee, R. Modifying transformation pathways in high entropy alloys or complex concentrated alloys via thermo-mechanical processing. Acta Mater. 2018, 153, 169–185.
- Wani, I.S.; Bhattacharjee, T.; Sheikh, S.; Bhattacharjee, P.P.; Guo, S.; Tsuji, N. Tailoring nanostructures and mechanical properties of AlCoCrFeNi2.1 eutectic high entropy alloy using thermo-mechanical processing. Mater. Sci. Eng. A 2016, 675, 99–109.
- Wani, I.S.; Sathiaraj, G.D.; Ahmed, M.Z.; Reddy, S.R.; Bhattacharjee, P.P. Evolution of microstructure and texture during thermo-mechanical processing of a two phase Al0.5CoCrFeMnNi high entropy alloy. Mater. Charact. 2016, 118, 417–424.
- Sathiaraj, G.D.; Bhattacharjee, P.P. Effect of starting grain size on the evolution of microstructure and texture during thermo-mechanical processing of CoCrFeMnNi high entropy alloy. J. Alloys Compd. 2015, 647, 82–96.
- Munitz, A.; Salhov, S.; Guttmann, G.; Derimow, N.; Nahmany, M. Heat treatment influence on the microstructure and mechanical properties of AlCrFeNiTi0.5 high entropy alloys. Mater. Sci. Eng. A 2019, 742, 1–14.
- Zhang, Y.; Jiang, X.; Sun, H.; Shao, Z. Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing. Nanotechnol. Rev. 2020, 9, 580–595.
- Li, Z.; Fu, L.; Zheng, H.; Yu, R.; Lv, L.; Sun, Y.; Dong, X.; Shan, A. Effect of Annealing Temperature on Microstructure and Mechanical Properties of a Severe Cold-Rolled FeCoCrNiMn High-Entropy Alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2019, 50, 3223–3237.
- Zhang, M.; Zhou, X.; Wang, D.; Zhu, W.; Li, J.; Zhao, Y.F. AlCoCuFeNi high-entropy alloy with tailored microstructure and outstanding compressive properties fabricated via selective laser melting with heat treatment. Mater. Sci. Eng. A 2019, 743, 773–784.
- Lin, C.M.; Tsai, H.L.; Bor, H.Y. Effect of aging treatment on microstructure and properties of high-entropy Cu0.5CoCrFeNi alloy. Intermetallics 2010, 18, 1244–1250.
- Wen, L.H.; Kou, H.C.; Li, J.S.; Chang, H.; Xue, X.Y.; Zhou, L. Effect of aging temperature on microstructure and properties of AlCoCrCuFeNi high-entropy alloy. Intermetallics 2009, 17, 266–269.
- Ren, B.; Liu, Z.X.; Cai, B.; Wang, M.X.; Shi, L. Aging behavior of a CuCr2Fe2NiMn high-entropy alloy. Mater. Des. 2012, 33, 121–126.
- Na, T.W.; Park, K.B.; Lee, S.Y.; Yang, S.M.; Kang, J.W.; Lee, T.W.; Park, J.M.; Park, K.; Park, H.K. Preparation of spherical TaNbHfZrTi high-entropy alloy powders by a hydrogenation–dehydrogenation reaction and thermal plasma treatment. J. Alloys Compd. 2020, 817, 152757.
- Zhang, H.; He, Y.; Pan, Y. Enhanced hardness and fracture toughness of the laser-solidified FeCoNiCrCuTiMoAlSiB0.5 high-entropy alloy by martensite strengthening. Scr. Mater. 2013, 69, 342–345.
- Yang, J.; Jo, Y.H.; Kim, D.W.; Choi, W.M.; Kim, H.S.; Lee, B.J.; Sohn, S.S.; Lee, S. Effects of transformation-induced plasticity (TRIP) on tensile property improvement of Fe45Co30Cr10V10Ni5−xMnx high-entropy alloys. Mater. Sci. Eng. A 2020, 772, 138809.
- Lilensten, L.; Couzinié, J.P.; Perrière, L.; Bourgon, J.; Emery, N.; Guillot, I. New structure in refractory high-entropy alloys. Mater. Lett. 2014, 132, 123–125.
- Aryal, A.; Dubenko, I.; Talapatra, S.; Granovsky, A.; Lähderanta, E.; Stadler, S.; Ali, N. Magnetic field dependence of the martensitic transition and magnetocaloric effects in Ni49BiMn35In15. AIP Adv. 2020, 10, 015138.
- Li, R.X.; Liaw, P.K.; Zhang, Y. Synthesis of AlxCoCrFeNi high-entropy alloys by high-gravity combustion from oxides. Mater. Sci. Eng. A 2017, 707, 668–673.
- Chen, C.; Zhang, H.; Hu, S.; Wei, R.; Wang, T.; Cheng, Y.; Zhang, T.; Shi, N.; Li, F.; Guan, S.; et al. Influences of laser surface melting on microstructure, mechanical properties and corrosion resistance of dual-phase Cr–Fe–Co–Ni–Al high entropy alloys. J. Alloys Compd. 2020, 826, 154100.
- He, J.Y.; Wang, H.; Wu, Y.; Liu, X.J.; Mao, H.H.; Nieh, T.G.; Lu, Z.P. Precipitation behavior and its effects on tensile properties of FeCoNiCr high-entropy alloys. Intermetallics 2016, 79, 41–52.
- Zhang, K.; Wen, H.; Zhao, B.; Dong, X.; Zhang, L. Precipitation behavior and its impact on mechanical properties in an aged carbon-containing Al0.3Cu0.5CrFeNi2 high-entropy alloy. Mater. Charact. 2019, 155, 109792.
- Liu, W.H.; Yang, T.; Liu, C.T. Precipitation hardening in CoCrFeNi-based high entropy alloys. Mater. Chem. Phys. 2018, 210, 2–11.
- He, J.Y.; Wang, H.; Huang, H.L.; Xu, X.D.; Chen, M.W.; Wu, Y.; Liu, X.J.; Nieh, T.G.; An, K.; Lu, Z.P. A precipitation-hardened high-entropy alloy with outstanding tensile properties. Acta Mater. 2016, 102, 187–196.
- Park, J.M.; Kang, J.W.; Lee, W.H.; Lee, S.Y.; Min, S.H.; Ha, T.K.; Park, H.K. Preparation of spherical WTaMoNbV refractory high entropy alloy powder by inductively-coupled thermal plasma. Mater. Lett. 2019, 255, 126513.
- Dong, W.; Zhou, Z.; Zhang, M.; Ma, Y.; Yu, P.; Liaw, P.K.; Li, G. Applications of high-pressure technology for high-entropy alloys: A review. Metals 2019, 9, 876.
- Zhang, F.; Lou, H.; Cheng, B.; Zeng, Z.; Zeng, Q. High-Pressure Induced Phase Transitions in High-Entropy Alloys: A Review. Entropy 2019, 21, 239.
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93.
- Shahmir, H.; He, J.; Lu, Z.; Kawasaki, M.; Langdon, T.G. Evidence for superplasticity in a CoCrFeNiMn high-entropy alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2017, 685, 342–348.
- Haušild, P.; Čížek, J.; Čech, J.; Zýka, J.; Kim, H.S. Indentation size effect in high pressure torsion processed high entropy alloy. Acta Polytech. CTU Proc. 2020, 27, 141–144.
- Zhang, K.; Peng, S.; Li, N.; Liu, X.; Zhang, M.; Wu, Y.D.; Yang, Y.; Greenberg, E.; Prakapenka, V.B.; Hui, X.; et al. Tuning to more compressible phase in TiZrHfNb high entropy alloy by pressure. Appl. Phys. Lett. 2020, 116, 031901.
- Sonkusare, R.; Biswas, K.; Al-Hamdany, N.; Brokmeier, H.G.; Kalsar, R.; Schell, N.; Gurao, N.P. A critical evaluation of microstructure-texture-mechanical behavior heterogeneity in high pressure torsion processed CoCuFeMnNi high entropy alloy. Mater. Sci. Eng. A 2020, 782, 139187.
- Podolskiy, A.V.; Shapovalov, Y.O.; Tabachnikova, E.D.; Tortika, A.S.; Tikhonovsky, M.A.; Joni, B.; Ódor, E.; Ungar, T.; Maier, S.; Rentenberger, C.; et al. Anomalous Evolution of Strength and Microstructure of High-Entropy Alloy CoCrFeNiMn after High-Pressure Torsion at 300 and 77 K. Adv. Eng. Mater. 2020, 22, 1900752.
- Skrotzki, W.; Pukenas, A.; Odor, E.; Joni, B.; Ungar, T.; Völker, B.; Hohenwarter, A.; Pippan, R.; George, E.P. Microstructure, texture, and strength development during high-pressure torsion of crmnfeconi high-entropy alloy. Crystals 2020, 10, 336.
- Asghari-Rad, P.; Sathiyamoorthi, P.; Thi-Cam Nguyen, N.; Bae, J.W.; Shahmir, H.; Kim, H.S. Fine-tuning of mechanical properties in V10Cr15Mn5Fe35Co10Ni25 high-entropy alloy through high-pressure torsion and annealing. Mater. Sci. Eng. A 2020, 771, 138604.
- Ahmad, A.S.; Su, Y.; Liu, S.Y.; Ståhl, K.; Wu, Y.D.; Hui, X.D.; Ruett, U.; Gutowski, O.; Glazyrin, K.; Liermann, H.P.; et al. Structural stability of high entropy alloys under pressure and temperature. J. Appl. Phys. 2017, 121, 235901.
- LuŽnik, J.; KoŽelj, P.; Vrtnik, S.; Jelen, A.; Jagličić, Z.; Meden, A.; Feuerbacher, M.; Dolinšek, J. Complex magnetism of Ho-Dy-Y-Gd-Tb hexagonal high-entropy alloy. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 92, 224201.
- Feuerbacher, M.; Heidelmann, M.; Thomas, C. Hexagonal High-entropy Alloys. Mater. Res. Lett. 2014, 3, 1–6.
- Heczel, A.; Kawasaki, M.; Lábár, J.L.; Jang, J.I.; Langdon, T.G.; Gubicza, J. Defect structure and hardness in nanocrystalline CoCrFeMnNi High-Entropy Alloy processed by High-Pressure Torsion. J. Alloys Compd. 2017, 711, 143–154.
- Kilmametov, A.; Kulagin, R.; Mazilkin, A.; Seils, S.; Boll, T.; Heilmaier, M.; Hahn, H. High-pressure torsion driven mechanical alloying of CoCrFeMnNi high entropy alloy. Scr. Mater. 2019, 158, 29–33.
- Asghari-Rad, P.; Sathiyamoorthi, P.; Bae, J.W.; Moon, J.; Park, J.M.; Zargaran, A.; Kim, H.S. Effect of grain size on the tensile behavior of V10Cr15Mn5Fe35Co10Ni25 high entropy alloy. Mater. Sci. Eng. A 2019, 744, 610–617.
- Edalati, P.; Floriano, R.; Tang, Y.; Mohammadi, A.; Pereira, K.D.; Luchessi, A.D.; Edalati, K. Ultrahigh hardness and biocompatibility of high-entropy alloy TiAlFeCoNi processed by high-pressure torsion. Mater. Sci. Eng. C 2020, 112, 110908.
- Yu, P.F.; Zhang, L.J.; Cheng, H.; Zhang, H.; Ma, M.Z.; Li, Y.C.; Li, G.; Liaw, P.K.; Liu, R.P. The high-entropy alloys with high hardness and soft magnetic property prepared by mechanical alloying and high-pressure sintering. Intermetallics 2016, 70, 82–87.
- Tracy, C.L.; Park, S.; Rittman, D.R.; Zinkle, S.J.; Bei, H.; Lang, M.; Ewing, R.C.; Mao, W.L. High pressure synthesis of a hexagonal close-packed phase of the high-entropy alloy CrMnFeCoNi. Nat. Commun. 2017, 8, 15634.
- Liu, W.H.; Tong, Y.; Chen, S.W.; Xu, W.W.; Wu, H.H.; Zhao, Y.L.; Yang, T.; Wang, X.L.; Liu, X.; Kai, J.J.; et al. Unveiling the Electronic Origin for Pressure-Induced Phase Transitions in High-Entropy Alloys. Matter 2020, 2, 751–763.
- Yu, P.F.; Zhang, L.J.; Ning, J.L.; Ma, M.Z.; Zhang, X.Y.; Li, Y.C.; Liaw, P.K.; Li, G.; Liu, R.P. Pressure-induced phase transitions in HoDyYGdTb high-entropy alloy. Mater. Lett. 2017, 196, 137–140.
- Zhang, F.; Lou, H.; Chen, S.; Chen, X.; Zeng, Z.; Yan, J.; Zhao, W.; Wu, Y.; Lu, Z.; Zeng, Q. Effects of non-hydrostaticity and grain size on the pressure-induced phase transition of the CoCrFeMnNi high-entropy alloy. J. Appl. Phys. 2018, 124, 115901.
- Zhang, F.X.; Zhao, S.; Jin, K.; Bei, H.; Popov, D.; Park, C.; Neuefeind, J.C.; Weber, W.J.; Zhang, Y. Pressure-induced fcc to hcp phase transition in Ni-based high entropy solid solution alloys. Appl. Phys. Lett. 2017, 110, 011902.
- Cheng, B.; Zhang, F.; Lou, H.; Chen, X.; Liaw, P.K.; Yan, J.; Zeng, Z.; Ding, Y.; Zeng, Q. Pressure-induced phase transition in the AlCoCrFeNi high-entropy alloy. Scr. Mater. 2019, 161, 88–92.
- Zhang, C.; Bhandari, U.; Zeng, C.; Ding, H.; Guo, S.; Yan, J.; Yang, S. Carbide formation in refractory Mo15Nb20Re15Ta30W20 alloy under a combined high-pressure and high-temperature condition. Entropy 2020, 22, 718.
- Guo, J.; Tang, C.; Rothwell, G.; Li, L.; Wang, Y.C.; Yang, Q.; Ren, X. Welding of high entropy alloys—A review. Entropy 2019, 21, 431.
- Lopes, J.G.; Oliveira, J.P. A short review on welding and joining of high entropy alloys. Metals 2020, 10, 212.
- Filho, F.C.G.; Monteiro, S.N. Welding joints in high entropy alloys: A short-review on recent trends. Materials 2020, 13, 1411.
- Scutelnicu, E.; Simion, G.; Rusu, C.C.; Corneliu Gheonea, M.; Voiculescu, I.; Geanta, V. High Entropy Alloys Behaviour During Welding. Rev. Chim. 2020, 71, 219–233.
- Tillmann, W.; Ulitzka, T.; Wojarski, L.; Manka, M.; Ulitzka, H.; Wagstyl, D. Development of high entropy alloys for brazing applications. Weld. World 2020, 64, 201–208.
- Liu, R.; Wang, Z.; Sparks, T.; Liou, F.; Newkirk, J. Aerospace applications of laser additive manufacturing. In Laser Additive Manufacturing: Materials, Design, Technologies, and Applications; Brandt, M., Ed.; Matthew Deans; Woodhead Publishing: Sawston, England, 2017; pp. 351–371. ISBN 9780081004340.
- Zhu, Z.G.; Nguyen, Q.B.; Ng, F.L.; An, X.H.; Liao, X.Z.; Liaw, P.K.; Nai, S.M.L.; Wei, J. Hierarchical microstructure and strengthening mechanisms of a CoCrFeNiMn high entropy alloy additively manufactured by selective laser melting. Scr. Mater. 2018, 154, 20–24.
- Park, J.M.; Choe, J.; Kim, J.G.; Bae, J.W.; Moon, J.; Yang, S.; Kim, K.T.; Yu, J.H.; Kim, H.S. Superior tensile properties of 1%C-CoCrFeMnNi high-entropy alloy additively manufactured by selective laser melting. Mater. Res. Lett. 2020, 8, 1–7.
- Zhou, R.; Liu, Y.; Zhou, C.; Li, S.; Wu, W.; Song, M.; Liu, B.; Liang, X.; Liaw, P.K. Microstructures and mechanical properties of C-containing FeCoCrNi high-entropy alloy fabricated by selective laser melting. Intermetallics 2018, 94, 165–171.
- Brif, Y.; Thomas, M.; Todd, I. The use of high-entropy alloys in additive manufacturing. Scr. Mater. 2015, 99, 93–96.
- Peyrouzet, F.; Hachet, D.; Soulas, R.; Navone, C.; Godet, S.; Gorsse, S. Selective Laser Melting of Al0.3CoCrFeNi High-Entropy Alloy: Printability, Microstructure, and Mechanical Properties. JOM 2019, 71, 3443–3451.
- Sun, K.; Peng, W.; Yang, L.; Fang, L. Effect of SLM processing parameters on microstructures and mechanical properties of Al0. 5CoCrFeNi high entropy alloys. Metals 2020, 10, 292.
- Wang, J.; Niu, S.; Guo, T.; Kou, H.; Li, J. The FCC to BCC phase transformation kinetics in an Al0.5CoCrFeNi high entropy alloy. J. Alloys Compd. 2017, 710, 144–150.
- Zhou, P.F.; Xiao, D.H.; Wu, Z.; Ou, X.Q. Al0.5FeCoCrNi high entropy alloy prepared by selective laser melting with gas-atomized pre-alloy powders. Mater. Sci. Eng. A 2019, 739, 86–89.
- Li, X. Additive Manufacturing of Advanced Multi-Component Alloys: Bulk Metallic Glasses and High Entropy Alloys. Adv. Eng. Mater. 2018, 20, 1490.
- Li, X.P.; Wang, X.J.; Saunders, M.; Suvorova, A.; Zhang, L.C.; Liu, Y.J.; Fang, M.H.; Huang, Z.H.; Sercombe, T.B. A selective laser melting and solution heat treatment refined Al-12Si alloy with a controllable ultrafine eutectic microstructure and 25% tensile ductility. Acta Mater. 2015, 95, 74–82.
- Li, X.P.; Ji, G.; Chen, Z.; Addad, A.; Wu, Y.; Wang, H.W.; Vleugels, J.; Van Humbeeck, J.; Kruth, J.P. Selective laser melting of nano-TiB2 decorated AlSi10Mg alloy with high fracture strength and ductility. Acta Mater. 2017, 129, 183–193.
- Attar, H.; Bönisch, M.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Selective laser melting of in situ titanium-titanium boride composites: Processing, microstructure and mechanical properties. Acta Mater. 2014, 76, 13–22.
- Vrancken, B.; Thijs, L.; Kruth, J.P.; Van Humbeeck, J. Microstructure and mechanical properties of a novel β titanium metallic composite by selective laser melting. Acta Mater. 2014, 68, 150–158.
- Li, X.P.; Van Humbeeck, J.; Kruth, J.P. Selective laser melting of weak-textured commercially pure titanium with high strength and ductility: A study from laser power perspective. Mater. Des. 2017, 116, 352–358.
- Wang, Q.; Ren, L.; Li, X.; Zhang, S.; Sercombe, T.B.; Yang, K. Antimicrobial Cu-bearing stainless steel scaffolds. Mater. Sci. Eng. C 2016, 68, 519–522.
- Sercombe, T.B.; Li, X. Selective laser melting of aluminium and aluminium metal matrix composites: Review. Mater. Technol. 2016, 31, 77–85.
- Niu, P.; Li, R.; Zhu, S.; Wang, M.; Chen, C.; Yuan, T. Hot cracking, crystal orientation and compressive strength of an equimolar CoCrFeMnNi high-entropy alloy printed by selective laser melting. Opt. Laser Technol. 2020, 127, 106147.
- Karlsson, D.; Marshal, A.; Johansson, F.; Schuisky, M.; Sahlberg, M.; Schneider, J.M.; Jansson, U. Elemental segregation in an AlCoCrFeNi high-entropy alloy—A comparison between selective laser melting and induction melting. J. Alloys Compd. 2019, 784, 195–203.
- Chen, S.; Tong, Y.; Liaw, P.K. Additive manufacturing of high-entropy alloys: A review. Entropy 2018, 20, 937.
- Cui, W.; Zhang, X.; Liou, F. Additive Manufacturing of High-Entropy Alloys—A Review. In Proceedings of the 2017 28th Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Rolla, MO, USA, 7–9 August 2017; pp. 712–724.
- Ostovari Moghaddam, A.; Shaburova, N.A.; Samodurova, M.N.; Abdollahzadeh, A.; Trofimov, E.A. Additive manufacturing of high entropy alloys: A practical review. J. Mater. Sci. Technol. 2021, 77, 131–162.
- Murty, B.; Yeh, J.; Ranganathan, S.; Bhattacharjee, P. High-Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2019.
- Yeh, M.C.G.J.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9783319270111.
- Riva, S.; Brown, S.G.R.; Lavery, N.P.; Tudball, A.; Yusenko, K.V. Spark Plasma Sintering of Materials; Cavaliere, P., Ed.; Springer: Lecce, Italy, 2019; ISBN 978-3-030-05326-0.
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686.
- Chen, P.; Li, S.; Zhou, Y.; Yan, M.; Attallah, M.M. Fabricating CoCrFeMnNi high entropy alloy via selective laser melting in-situ alloying. J. Mater. Sci. Technol. 2020, 43, 40–43.
- Li, B.; Zhang, L.; Xu, Y.; Liu, Z.; Qian, B.; Xuan, F. Selective laser melting of CoCrFeNiMn high entropy alloy powder modified with nano-TiN particles for additive manufacturing and strength enhancement: Process, particle behavior and effects. Powder Technol. 2020, 360, 509–521.
- Li, N.; Wu, S.; Ouyang, D.; Zhang, J.; Liu, L. Fe-based metallic glass reinforced FeCoCrNiMn high entropy alloy through selective laser melting. J. Alloys Compd. 2020, 822, 153695.
- Li, B.; Zhang, L.; Yang, B. Grain refinement and localized amorphization of additively manufactured high-entropy alloy matrix composites reinforced by nano ceramic particles via selective-laser-melting/remelting. Compos. Commun. 2020, 19, 56–60.
- Kim, J.G.; Park, J.M.; Seol, J.B.; Choe, J.; Yu, J.H.; Yang, S.; Kim, H.S. Nano-scale solute heterogeneities in the ultrastrong selectively laser melted carbon-doped CoCrFeMnNi alloy. Mater. Sci. Eng. A 2020, 773, 138726.
- Li, B.; Qian, B.; Xu, Y.; Liu, Z.; Xuan, F. Fine-structured CoCrFeNiMn high-entropy alloy matrix composite with 12 wt% TiN particle reinforcements via selective laser melting assisted additive manufacturing. Mater. Lett. 2019, 252, 88–91.
- Piglione, A.; Dovgyy, B.; Liu, C.; Gourlay, C.M.; Hooper, P.A.; Pham, M.S. Printability and microstructure of the CoCrFeMnNi high-entropy alloy fabricated by laser powder bed fusion. Mater. Lett. 2018, 224, 22–25.
- Xu, Z.; Zhang, H.; Li, W.; Mao, A.; Wang, L.; Song, G.; He, Y. Microstructure and nanoindentation creep behavior of CoCrFeMnNi high-entropy alloy fabricated by selective laser melting. Addit. Manuf. 2019, 28, 766–771.
- Ren, J.; Mahajan, C.; Liu, L.; Follette, D.; Chen, W.; Mukherjee, S. Corrosion behavior of selectively laser melted CoCrFeMnNi high entropy alloy. Metals 2019, 9, 1029.
- Dovgyy, B.; Pham, M.S. Epitaxial growth in 316L steel and CoCrFeMnNi high entropy alloy made by powder-bed laser melting. AIP Conf. Proc. 2018, 1960, 140008.
- Wu, W.; Zhou, R.; Wei, B.; Ni, S.; Liu, Y.; Song, M. Nanosized precipitates and dislocation networks reinforced C-containing CoCrFeNi high-entropy alloy fabricated by selective laser melting. Mater. Charact. 2018, 144, 605–610.
- Sun, Z.; Tan, X.P.; Descoins, M.; Mangelinck, D.; Tor, S.B.; Lim, C.S. Revealing hot tearing mechanism for an additively manufactured high-entropy alloy via selective laser melting. Scr. Mater. 2019, 168, 129–133.
- Song, M.; Zhou, R.; Gu, J.; Wang, Z.; Ni, S.; Liu, Y. Nitrogen induced heterogeneous structures overcome strength-ductility trade-off in an additively manufactured high-entropy alloy. Appl. Mater. Today 2020, 18, 100498.
- Zhou, R.; Chen, G.; Liu, B.; Wang, J.; Han, L.; Liu, Y. Microstructures and wear behaviour of (FeCoCrNi)1−x(WC)x high entropy alloy composites. Int. J. Refract. Met. Hard Mater. 2018, 75, 56–62.
- Niu, P.D.; Li, R.D.; Yuan, T.C.; Zhu, S.Y.; Chen, C.; Wang, M.B.; Huang, L. Microstructures and properties of an equimolar AlCoCrFeNi high entropy alloy printed by selective laser melting. Intermetallics 2019, 104, 24–32.
- Luo, S.; Gao, P.; Yu, H.; Yang, J.; Wang, Z.; Zeng, X. Selective laser melting of an equiatomic AlCrCuFeNi high-entropy alloy: Processability, non-equilibrium microstructure and mechanical behavior. J. Alloys Compd. 2019, 771, 387–397.
- Luo, S.; Zhao, C.; Su, Y.; Liu, Q.; Wang, Z. Selective laser melting of dual phase AlCrCuFeNix high entropy alloys: Formability, heterogeneous microstructures and deformation mechanisms. Addit. Manuf. 2020, 31, 100925.
- Yao, H.; Tan, Z.; He, D.; Zhou, Z.; Zhou, Z.; Xue, Y.; Cui, L.; Chen, L.; Wang, G.; Yang, Y. High strength and ductility AlCrFeNiV high entropy alloy with hierarchically heterogeneous microstructure prepared by selective laser melting. J. Alloys Compd. 2020, 813, 152196.
- Wang, Y.; Li, R.; Niu, P.; Zhang, Z.; Yuan, T.; Yuan, J.; Li, K. Microstructures and properties of equimolar AlCoCrCuFeNi high-entropy alloy additively manufactured by selective laser melting. Intermetallics 2020, 120, 106746.
- Wang, M.; Li, R.; Yuan, T.; Chen, C.; Zhou, L.; Chen, H.; Zhang, M.; Xie, S. Microstructures and mechanical property of AlMgScZrMn—A comparison between selective laser melting, spark plasma sintering and cast. Mater. Sci. Eng. A 2019, 756, 354–364.
- Sarswat, P.K.; Sarkar, S.; Murali, A.; Huang, W.; Tan, W.; Free, M.L. Additive manufactured new hybrid high entropy alloys derived from the AlCoFeNiSmTiVZr system. Appl. Surf. Sci. 2019, 476, 242–258.
- Agrawal, P.; Thapliyal, S.; Nene, S.S.; Mishra, R.S.; McWilliams, B.A.; Cho, K.C. Excellent strength-ductility synergy in metastable high entropy alloy by laser powder bed additive manufacturing. Addit. Manuf. 2020, 32, 101098.
- Zhang, H.; Zhao, Y.; Huang, S.; Zhu, S.; Wang, F.; Li, D. Manufacturing and analysis of high-performance refractory high-entropy alloy via selective laser melting (SLM). Materials 2019, 12, 720.
- Zhang, H.; Xu, W.; Xu, Y.; Lu, Z.; Li, D. The thermal-mechanical behavior of WTaMoNb high-entropy alloy via selective laser melting (SLM): Experiment and simulation. Int. J. Adv. Manuf. Technol. 2018, 96, 461–474.
- Yang, X.; Zhou, Y.; Xi, S.; Chen, Z.; Wei, P.; He, C.; Li, T.; Gao, Y.; Wu, H. Additively manufactured fine grained Ni6Cr4WFe9Ti high entropy alloys with high strength and ductility. Mater. Sci. Eng. A 2019, 767, 138394.
- Yang, X.; Zhou, Y.; Xi, S.; Chen, Z.; Wei, P.; He, C.; Li, T.; Gao, Y.; Wu, H. Grain-anisotropied high-strength Ni6Cr4WFe9Ti high entropy alloys with outstanding tensile ductility. Mater. Sci. Eng. A 2019, 767, 138382.
- Chen, P.; Yang, C.; Li, S.; Attallah, M.M.; Yan, M. In-situ alloyed, oxide-dispersion-strengthened CoCrFeMnNi high entropy alloy fabricated via laser powder bed fusion. Mater. Des. 2020, 194, 108966.
- Litwa, P.; Hernandez-Nava, E.; Guan, D.; Goodall, R.; Wika, K.K. The additive manufacture processing and machinability of CrMnFeCoNi high entropy alloy. Mater. Des. 2021, 198, 109380.
- Zhang, C.; Feng, K.; Kokawa, H.; Han, B.; Li, Z. Cracking mechanism and mechanical properties of selective laser melted CoCrFeMnNi high entropy alloy using different scanning strategies. Mater. Sci. Eng. A 2020, 789, 139672.
- Kim, Y.K.; Suh, J.Y.; Lee, K.A. Effect of gaseous hydrogen embrittlement on the mechanical properties of additively manufactured CrMnFeCoNi high-entropy alloy strengthened by in-situ formed oxide. Mater. Sci. Eng. A 2020, 796, 140039.
- Choi, N.; Kulitckii, V.; Kottke, J.; Tas, B.; Choe, J.; Yu, J.H.; Yang, S.; Park, J.H.; Lee, J.S.; Wilde, G.; et al. Analyzing the ‘non-equilibrium state’ of grain boundaries in additively manufactured high-entropy CoCrFeMnNi alloy using tracer diffusion measurements. J. Alloys Compd. 2020, 844, 155757.
- Su, Y.; Luo, S.; Wang, Z. Microstructure evolution and cracking behaviors of additively manufactured AlxCrCuFeNi2 high entropy alloys via selective laser melting. J. Alloys Compd. 2020, 842, 155823.
- Peng, Y.; Kong, Y.; Zhang, W.; Zhang, M.; Wang, H. Effect of diffusion barrier and interfacial strengthening on the interface behavior between high entropy alloy and diamond. J. Alloys Compd. 2021, 852, 157023.
- Wang, H.; Zhu, Z.G.; Chen, H.; Wang, A.G.; Liu, J.Q.; Liu, H.W.; Zheng, R.K.; Nai, S.M.L.; Primig, S.; Babu, S.S.; et al. Effect of cyclic rapid thermal loadings on the microstructural evolution of a CrMnFeCoNi high-entropy alloy manufactured by selective laser melting. Acta Mater. 2020, 196, 609–625.
- Sun, Z.; Tan, X.; Wang, C.; Descoins, M.; Mangelinck, D.; Tor, S.B.; Jägle, E.A.; Zaefferer, S.; Raabe, D. Reducing hot tearing by grain boundary segregation engineering in additive manufacturing: Example of an AlxCoCrFeNi high-entropy alloy. Acta Mater. 2021, 204, 116505.
- Ishimoto, T.; Ozasa, R.; Nakano, K.; Weinmann, M.; Schnitter, C.; Stenzel, M.; Matsugaki, A.; Nagase, T.; Matsuzaka, T.; Todai, M.; et al. Development of TiNbTaZrMo bio-high entropy alloy (BioHEA) super-solid solution by selective laser melting, and its improved mechanical property and biocompatibility. Scr. Mater. 2021, 194, 113658.
- Park, J.M.; Choe, J.; Park, H.K.; Son, S.; Jung, J.; Kim, T.S.; Yu, J.H.; Kim, J.G.; Kim, H.S. Synergetic strengthening of additively manufactured (CoCrFeMnNi)99C1 high-entropy alloy by heterogeneous anisotropic microstructure. Addit. Manuf. 2020, 35, 101333.
- Lin, D.; Xu, L.; Li, X.; Jing, H.; Qin, G.; Pang, H.; Minami, F. A Si-containing FeCoCrNi high-entropy alloy with high strength and ductility synthesized in situ via selective laser melting. Addit. Manuf. 2020, 35, 101340.
- Kim, Y.K.; Yang, S.; Lee, K.A. Compressive creep behavior of selective laser melted CoCrFeMnNi high-entropy alloy strengthened by in-situ formation of nano-oxides. Addit. Manuf. 2020, 36, 101543.
- Jin, M.; Piglione, A.; Dovgyy, B.; Hosseini, E.; Hooper, P.A.; Holdsworth, S.R.; Pham, M.S. Cyclic plasticity and fatigue damage of CrMnFeCoNi high entropy alloy fabricated by laser powder-bed fusion. Addit. Manuf. 2020, 36, 101584.
- Lin, W.C.; Chang, Y.J.; Hsu, T.H.; Gorsse, S.; Sun, F.; Furuhara, T.; Yeh, A.C. Microstructure and tensile property of a precipitation strengthened high entropy alloy processed by selective laser melting and post heat treatment. Addit. Manuf. 2020, 36, 101601.
- Peng, H.; Lin, Z.; Li, R.; Niu, P.; Zhang, Z. Corrosion Behavior of an Equiatomic CoCrFeMnNi High-Entropy Alloy—A Comparison Between Selective Laser Melting and Cast. Front. Mater. 2020, 7, 244.
- Vogiatzief, D.; Evirgen, A.; Gein, S.; Molina, V.R.; Weisheit, A.; Pedersen, M. Laser Powder Bed Fusion and Heat Treatment of an AlCrFe2Ni2 High Entropy Alloy. Front. Mater. 2020, 7, 248.
- Liao, Y.; Zhu, P.; Li, S. Synthesis of AlFeCrNiV high entropy alloy by gas atomization and selective laser melting. Synthesis (Stuttg) 2020, 7, 11591–11594.
- Guo, L.; Gu, J.; Gan, B.; Ni, S.; Bi, Z.; Wang, Z.; Song, M. Effects of elemental segregation and scanning strategy on the mechanical properties and hot cracking of a selective laser melted FeCoCrNiMn-(N,Si) high entropy alloy. J. Alloys Compd. 2021, 865, 158892.
- Kim, Y.K.; Yu, J.H.; Kim, H.S.; Lee, K.A. In-situ carbide-reinforced CoCrFeMnNi high-entropy alloy matrix nanocomposites manufactured by selective laser melting: Carbon content effects on microstructure, mechanical properties, and deformation mechanism. Compos. Part B Eng. 2021, 210, 108638.
- Zhao, W.; Han, J.-K.; Kuzminova, Y.O.; Evlashin, S.A.; Zhilyaev, A.P.; Pesin, A.M.; Jang, J.; Liss, K.-D.; Kawasaki, M. Significance of grain refinement on micro-mechanical properties and structures of additively-manufactured CoCrFeNi high-entropy alloy. Mater. Sci. Eng. A 2021, 807, 140898.
- Gu, Z.; Su, X.; Peng, W.; Guo, W.; Xi, S.; Zhang, X.; Tu, H.; Gao, Y.; Wu, H. An important improvement of strength and ductility on a new type of CoCr2.5FeNi2TiW0.5 high entropy alloys under two different protective gases by selective laser melting. J. Alloys Compd. 2021, 868, 159088.
- Peng, S.; Mooraj, S.; Feng, R.; Liu, L.; Ren, J.; Liu, Y.; Kong, F.; Xiao, Z.; Zhu, C.; Liaw, P.K.; et al. Additive manufacturing of three-dimensional (3D)-architected CoCrFeNiMn high- entropy alloy with great energy absorption. Scr. Mater. 2021, 190, 46–51.
- Wang, P.; Huang, P.; Ng, F.L.; Sin, W.J.; Lu, S.; Nai, M.L.S.; Dong, Z.L.; Wei, J. Additively manufactured CoCrFeNiMn high-entropy alloy via pre-alloyed powder. Mater. Des. 2019, 168, 107576.
- Kuwabara, K.; Shiratori, H.; Fujieda, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A. Mechanical and corrosion properties of AlCoCrFeNi high-entropy alloy fabricated with selective electron beam melting. Addit. Manuf. 2018, 23, 264–271.
- Yang, S.; Liu, Z.; Pi, J. Microstructure and wear behavior of the AlCrFeCoNi high-entropy alloy fabricated by additive manufacturing. Mater. Lett. 2020, 261, 127004.
- Fujieda, T.; Chen, M.; Shiratori, H.; Kuwabara, K.; Yamanaka, K.; Koizumi, Y.; Chiba, A.; Watanabe, S. Mechanical and corrosion properties of CoCrFeNiTi-based high-entropy alloy additive manufactured using selective laser melting. Addit. Manuf. 2019, 25, 412–420.
- Popov, V.V.; Katz-Demyanetz, A.; Koptyug, A.; Bamberger, M. Selective electron beam melting of Al0.5CrMoNbTa0.5 high entropy alloys using elemental powder blend. Heliyon 2019, 5, e01188.
- Guan, S.; Wan, D.; Solberg, K.; Berto, F.; Welo, T.; Yue, T.M.; Chan, K.C. Additive manufacturing of fine-grained and dislocation-populated CrMnFeCoNi high entropy alloy by laser engineered net shaping. Mater. Sci. Eng. A 2019, 761, 138056.
- Melia, M.A.; Carroll, J.D.; Whetten, S.R.; Esmaeely, S.N.; Locke, J.; White, E.; Anderson, I.; Chandross, M.; Michael, J.R.; Argibay, N.; et al. Mechanical and Corrosion Properties of Additively Manufactured CoCrFeMnNi High Entropy Alloy. Addit. Manuf. 2019, 29, 100833.
- Li, H.G.; Lee, T.L.; Zheng, W.; Lu, Y.Z.; Yin, H.B.C.; Yang, J.X.; Huang, Y.J.; Sun, J.F. Characterization of residual stress in laser melting deposited CoCrFeMnNi high entropy alloy by neutron diffraction. Mater. Lett. 2020, 263, 127247.
- Gao, X.; Lu, Y. Laser 3D printing of CoCrFeMnNi high-entropy alloy. Mater. Lett. 2019, 236, 77–80.
- Xiang, S.; Li, J.; Luan, H.; Amar, A.; Lu, S.; Li, K.; Zhang, L.; Liu, X.; Le, G.; Wang, X.; et al. Effects of process parameters on microstructures and tensile properties of laser melting deposited CrMnFeCoNi high entropy alloys. Mater. Sci. Eng. A 2019, 743, 412–417.
- Xiang, S.; Luan, H.; Wu, J.; Yao, K.F.; Li, J.; Liu, X.; Tian, Y.; Mao, W.; Bai, H.; Le, G.; et al. Microstructures and mechanical properties of CrMnFeCoNi high entropy alloys fabricated using laser metal deposition technique. J. Alloys Compd. 2019, 773, 387–392.
- Chew, Y.; Bi, G.J.; Zhu, Z.G.; Ng, F.L.; Weng, F.; Liu, S.B.; Nai, S.M.L.; Lee, B.Y. Microstructure and enhanced strength of laser aided additive manufactured CoCrFeNiMn high entropy alloy. Mater. Sci. Eng. A 2019, 744, 137–144.
- Qiu, Z.; Yao, C.; Feng, K.; Li, Z.; Chu, P.K. Cryogenic deformation mechanism of CrMnFeCoNi high-entropy alloy fabricated by laser additive manufacturing process. Int. J. Light. Mater. Manuf. 2018, 1, 33–39.
- Li, J.; Xiang, S.; Luan, H.; Amar, A.; Liu, X.; Lu, S.; Zeng, Y.; Le, G.; Wang, X.; Qu, F.; et al. Additive manufacturing of high-strength CrMnFeCoNi high-entropy alloys-based composites with WC addition. J. Mater. Sci. Technol. 2019, 35, 2430–2434.
- Amar, A.; Li, J.; Xiang, S.; Liu, X.; Zhou, Y.; Le, G.; Wang, X.; Qu, F.; Ma, S.; Dong, W.; et al. Additive manufacturing of high-strength CrMnFeCoNi-based High Entropy Alloys with TiC addition. Intermetallics 2019, 109, 162–166.
- Guan, S.; Wan, D.; Solberg, K.; Berto, F.; Welo, T.; Yue, T.M.; Chan, K.C. Additively manufactured CrMnFeCoNi/AlCoCrFeNiTi0.5 laminated high-entropy alloy with enhanced strength-plasticity synergy. Scr. Mater. 2020, 183, 133–138.
- Wang, Q.; Amar, A.; Jiang, C.; Luan, H.; Zhao, S.; Zhang, H.; Le, G.; Liu, X.; Wang, X.; Yang, X.; et al. CoCrFeNiMo0.2 high entropy alloy by laser melting deposition: Prospective material for low temperature and corrosion resistant applications. Intermetallics 2020, 119, 106727.
- Zhou, K.; Li, J.; Wang, L.; Yang, H.; Wang, Z.; Wang, J. Direct laser deposited bulk CoCrFeNiNbx high entropy alloys. Intermetallics 2019, 114, 106592.
- Gwalani, B.; Gangireddy, S.; Shukla, S.; Yannetta, C.J.; Valentin, S.G.; Mishra, R.S.; Banerjee, R. Compositionally graded high entropy alloy with a strong front and ductile back. Mater. Today Commun. 2019, 20, 100602.
- Nartu, M.S.K.K.Y.; Alam, T.; Dasari, S.; Mantri, S.A.; Gorsse, S.; Siller, H.; Dahotre, N.; Banerjee, R. Enhanced tensile yield strength in laser additively manufactured Al0.3CoCrFeNi high entropy alloy. Materialia 2020, 9, 100522.
- Mohanty, A.; Sampreeth, J.K.; Bembalge, O.; Hascoet, J.Y.; Marya, S.; Immanuel, R.J.; Panigrahi, S.K. High temperature oxidation study of direct laser deposited AlXCoCrFeNi (X = 0.3, 0.7) high entropy alloys. Surf. Coat. Technol. 2019, 380, 125028.
- Vikram, R.J.; Murty, B.S.; Fabijanic, D.; Suwas, S. Insights into micro-mechanical response and texture of the additively manufactured eutectic high entropy alloy AlCoCrFeNi2.1. J. Alloys Compd. 2020, 827, 154034.
- Gwalani, B.; Soni, V.; Waseem, O.A.; Mantri, S.A.; Banerjee, R. Laser additive manufacturing of compositionally graded AlCrFeMoVx (x = 0 to 1) high-entropy alloy system. Opt. Laser Technol. 2019, 113, 330–337.
- Guan, S.; Solberg, K.; Wan, D.; Berto, F.; Welo, T.; Yue, T.M.; Chan, K.C. Formation of fully equiaxed grain microstructure in additively manufactured AlCoCrFeNiTi0.5 high entropy alloy. Mater. Des. 2019, 184, 108202.
- Malatji, N.; Popoola, A.P.I.; Lengopeng, T.; Pityana, S. Tribological and corrosion properties of laser additive manufactured AlCrFeNiCu high entropy alloy. Mater. Today Proc. 2020, 28, 944–948.
- Dada, M.; Patricia, P.; Mathe, N.; Pityana, S.; Adeosun, S.; Lengopeng, T. Fabrication and Hardness Behaviour of High Entropy Alloys. In Proceedings of the TMS 2020 149th Annual Meeting & Exhibition Supplemental Proceedings, San Diego, CA, USA, 23–27 February 2020; pp. 1581–1591.
- Dada, M.; Popoola, P.; Mathe, N.; Pityana, S.; Adeosun, S. Effect of laser parameters on the properties of high entropy alloys: A preliminary study. Mater. Today Proc. 2020, 38, 756–761.
- Moorehead, M.; Bertsch, K.; Niezgoda, M.; Parkin, C.; Elbakhshwan, M.; Sridharan, K.; Zhang, C.; Thoma, D.; Couet, A. High-throughput synthesis of Mo-Nb-Ta-W high-entropy alloys via additive manufacturing. Mater. Des. 2020, 187, 108358.
- Kunce, I.; Polanski, M.; Bystrzycki, J. Microstructure and hydrogen storage properties of a TiZrNbMoV high entropy alloy synthesized using Laser Engineered Net Shaping (LENS). Int. J. Hydrogen Energy 2014, 39, 9904–9910.
- Dobbelstein, H.; Gurevich, E.L.; George, E.P.; Ostendorf, A.; Laplanche, G. Laser metal deposition of a refractory TiZrNbHfTa high-entropy alloy. Addit. Manuf. 2018, 24, 386–390.
- Pegues, J.W.; Melia, M.A.; Puckett, R.; Whetten, S.R.; Argibay, N.; Kustas, A.B. Exploring additive manufacturing as a high-throughput screening tool for multiphase high entropy alloys. Addit. Manuf. 2021, 37, 101598.
- Li, H.; Huang, Y.; Jiang, S.; Lu, Y.; Gao, X.; Lu, X.; Ning, Z.; Sun, J. Columnar to equiaxed transition in additively manufactured CoCrFeMnNi high entropy alloy. Mater. Des. 2021, 197, 109262.
- Tong, Z.; Liu, H.; Jiao, J.; Zhou, W.; Yang, Y.; Ren, X. Improving the strength and ductility of laser directed energy deposited CrMnFeCoNi high-entropy alloy by laser shock peening. Addit. Manuf. 2020, 35, 101417.
- Shen, Q.; Kong, X.; Chen, X.; Yao, X.; Deev, V.B.; Prusov, E.S. Powder plasma arc additive manufactured CoCrFeNi(SiC)x high-entropy alloys: Microstructure and mechanical properties. Mater. Lett. 2021, 282, 128736.
- Cai, Y.; Zhu, L.; Cui, Y.; Han, J. Manufacturing of FeCoCrNi + FeCoCrNiAl laminated high-entropy alloy by laser melting deposition (LMD). Mater. Lett. 2021, 289, 129445.
- Zhang, H.; Zhao, Y.; Cai, J.; Ji, S.; Geng, J.; Sun, X.; Li, D. High-strength NbMoTaX refractory high-entropy alloy with low stacking fault energy eutectic phase via laser additive manufacturing. Mater. Des. 2021, 201, 109462.
- Peng, H.; Xie, S.; Niu, P.; Zhang, Z.; Yuan, T.; Ren, Z.; Wang, X.; Zhao, Y.; Li, R. Additive manufacturing of Al0.3CoCrFeNi high-entropy alloy by powder feeding laser melting deposition. J. Alloys Compd. 2021, 863, 158286.
- Kuzminova, Y.O.; Firsov, D.G.; Dagesyan, S.A.; Konev, S.D.; Sergeev, S.N.; Zhilyaev, A.P.; Kawasaki, M.; Akhatov, I.S.; Evlashin, S.A. Fatigue behavior of additive manufactured CrFeCoNi medium-entropy alloy. J. Alloys Compd. 2021, 863, 158609.
- Malatji, N.; Lengopeng, T.; Pityana, S.; Popoola, A.P.I. Effect of heat treatment on the microstructure, microhardness, and wear characteristics of AlCrFeCuNi high-entropy alloy. Int. J. Adv. Manuf. Technol. 2020, 111, 2021–2029.
- Dong, B.; Wang, Z.; Pan, Z.; Muránsky, O.; Shen, C.; Reid, M.; Wu, B.; Chen, X.; Li, H. On the development of pseudo-eutectic AlCoCrFeNi2.1 high entropy alloy using Powder-bed Arc Additive Manufacturing (PAAM) process. Mater. Sci. Eng. A 2021, 802, 140639.
- Zhou, K.; Wang, Z.; He, F.; Liu, S.; Li, J.; Kai, J.J.; Wang, J. A precipitation-strengthened high-entropy alloy for additive manufacturing. Addit. Manuf. 2020, 35, 101410.
- Zheng, M.; Li, C.; Zhang, X.; Ye, Z.; Yang, X.; Gu, J. The influence of columnar to equiaxed transition on deformation behavior of FeCoCrNiMn high entropy alloy fabricated by laser-based directed energy deposition. Addit. Manuf. 2020, 37, 101660.
- Luo, S.; Wang, Z.; Zeng, X. Study on the formability, microstructures and mechanical properties of AlCrCuFeNi high-entropy alloys prepared by selective laser melting. In Proceedings of the Solid Freeform Fabrication 2019: Proceedings of the 30th Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Wuhan, China, 12–14 August 2019; pp. 625–635.
- Jiang, F.; Zhao, C.; Liang, D.; Zhu, W.; Zhang, Y.; Pan, S.; Ren, F. In-situ formed heterogeneous grain structure in spark-plasma-sintered CoCrFeMnNi high-entropy alloy overcomes the strength-ductility trade-off. Mater. Sci. Eng. A 2020, 771, 138625.
- Rogal, Ł.; Kalita, D.; Tarasek, A.; Bobrowski, P.; Czerwinski, F. Effect of SiC nano-particles on microstructure and mechanical properties of the CoCrFeMnNi high entropy alloy. J. Alloys Compd. 2017, 708, 344–352.
- Yim, D.; Sathiyamoorthi, P.; Hong, S.J.; Kim, H.S. Fabrication and mechanical properties of TiC reinforced CoCrFeMnNi high-entropy alloy composite by water atomization and spark plasma sintering. J. Alloys Compd. 2019, 781, 389–396.
- Rogal, Ł.; Kalita, D.; Litynska-Dobrzynska, L. CoCrFeMnNi high entropy alloy matrix nanocomposite with addition of Al2O3. Intermetallics 2017, 86, 104–109.
- Li, X.; Feng, Y.; Liu, B.; Yi, D.; Yang, X.; Zhang, W.; Chen, G.; Liu, Y.; Bai, P. Influence of NbC particles on microstructure and mechanical properties of AlCoCrFeNi high-entropy alloy coatings prepared by laser cladding. J. Alloys Compd. 2019, 788, 485–494.
- Chen, S.; Chen, X.; Wang, L.; Liang, J.; Liu, C. Laser cladding FeCrCoNiTiAl high entropy alloy coatings reinforced with self-generated TiC particles. J. Laser Appl. 2017, 29, 012004.
- Fan, Q.C.; Li, B.S.; Zhang, Y. The microstructure and properties of (FeCrNiCo)AlxCuy high-entropy alloys and their TiC-reinforced composites. Mater. Sci. Eng. A 2014, 598, 244–250.
- Jiang, L.; Jiang, H.; Lu, Y.; Wang, T.; Cao, Z.; Li, T. Mechanical properties improvement of AlCrFeNi2Ti0.5 high entropy alloy through annealing design and its relationship with its particle-reinforced microstructures. J. Mater. Sci. Technol. 2015, 31, 397–402.
- Guo, L.; Ou, X.; Ni, S.; Liu, Y.; Song, M. Effects of carbon on the microstructures and mechanical properties of FeCoCrNiMn high entropy alloys. Mater. Sci. Eng. A 2019, 746, 356–362.
- Zhu, S.; Yu, Y.; Zhang, B.; Zhang, Z.; Yan, X.; Wang, Z. Microstructure and wear behaviour of in-situ TiN-Al2O3 reinforced CoCrFeNiMn high-entropy alloys composite coatings fabricated by plasma cladding. Mater. Lett. 2020, 272, 127870.
- Lim, K.R.; Kwon, H.J.; Kang, J.H.; Won, J.W.; Na, Y.S. A novel ultra-high-strength duplex Al–Co–Cr–Fe–Ni high-entropy alloy reinforced with body-centered-cubic ordered-phase particles. Mater. Sci. Eng. A 2020, 771, 138638.
- Fu, A.; Guo, W.; Liu, B.; Cao, Y.; Xu, L.; Fang, Q.; Yang, H.; Liu, Y. A particle reinforced NbTaTiV refractory high entropy alloy based composite with attractive mechanical properties. J. Alloys Compd. 2020, 815, 152466.
- Wang, L.; Wang, L.; Tang, Y.C.; Luo, L.; Luo, L.S.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Microstructure and mechanical properties of CoCrFeNiWx high entropy alloys reinforced by μ phase particles. J. Alloys Compd. 2020, 843, 155997.
- Jinhong, P.; Ye, P.; Hui, Z.; Lu, Z. Microstructure and properties of AlCrFeCuNi x (0.6 ≤ x ≤ 1.4) high-entropy alloys. Mater. Sci. Eng. A 2012, 543, 228–233.
- He, J.Y.; Liu, W.H.; Wang, H.; Wu, Y.; Liu, X.J.; Nieh, T.G.; Lu, Z.P. Effects of Al addition on structural evolution and tensile properties of the FeCoNiCrMn high-entropy alloy system. Acta Mater. 2014, 62, 105–113.
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765.
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706.
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303.
- Yvon, P.; Carré, F. Structural materials challenges for advanced reactor systems. J. Nucl. Mater. 2009, 385, 217–222.
- Allen, T.; Busby, J.; Meyer, M.; Petti, D. Materials challenges for nuclear systems. Mater. Today 2010, 13, 14–23.
- Murty, K.L.; Charit, I. Structural materials for Gen-IV nuclear reactors: Challenges and opportunities. J. Nucl. Mater. 2008, 383, 189–195.
- Zinkle, S.J.; Boutard, J.L.; Hoelzer, D.T.; Kimura, A.; Lindau, R.; Odette, G.R.; Rieth, M.; Tan, L.; Tanigawa, H. Development of next generation tempered and ODS reduced activation ferritic/martensitic steels for fusion energy applications. Nucl. Fusion 2017, 57, 092005.
- Zinkle, S.J.; Busby, J.T. Structural materials for fission & fusion energy. Mater. Today 2009, 12, 12–19.
- Li, C. Characterization of Radiation Effects and Ab Initio Modeling of Defects in a High Entropy Alloy for Nuclear Power Application; The University of Tennessee: Knoxville, TN, USA, 2018.
- Hoffman, A.K. Development and characterization of nanostructured steels and high entropy alloys for nuclear applications. J. Mater. Res. 2019, 33, 3077–3091.
- King, D.J.M. Investigation of High-Entropy Alloys for Use in Advanced Nuclear Applications; University of Technology Sydney: Sydney, Australia, 2016.
- Yeh, J.W.; Lin, S.J. Breakthrough applications of high-entropy materials. J. Mater. Res. 2018, 33, 3129–3137.
- Yang, T.; Li, C.; Zinkle, S.J.; Zhao, S.; Bei, H.; Zhang, Y. Irradiation responses and defect behavior of single-phase concentrated solid solution alloys. J. Mater. Res. 2018, 33, 3077–3091.
- Jin, K.; Bei, H. Single-phase concentrated solid-solution alloys: Bridging intrinsic transport properties and irradiation resistance. Front. Mater. 2018, 5, 26.
- Xia, S.Q.; Wang, Z.; Yang, T.F.; Zhang, Y. Irradiation Behavior in High Entropy Alloys. J. Iron Steel Res. Int. 2015, 22, 879–884.
- Barron, P.J.; Carruthers, A.W.; Fellowes, J.W.; Jones, N.G.; Dawson, H.; Pickering, E.J. Towards V-based high-entropy alloys for nuclear fusion applications. Scr. Mater. 2020, 176, 12–16.
- Xiang, C.; Fu, H.M.; Zhang, Z.M.; Han, E.H.; Zhang, H.F.; Wang, J.Q.; Hu, G.D. Effect of Cr content on microstructure and properties of Mo0.5VNbTiCrx high-entropy alloys. J. Alloys Compd. 2020, 818, 153352.
- Yang, T.; Guo, W.; Poplawsky, J.D.; Li, D.; Wang, L.; Li, Y.; Hu, W.; Crespillo, M.L.; Yan, Z.; Zhang, Y.; et al. Structural damage and phase stability of Al0.3CoCrFeNi high entropy alloy under high temperature ion irradiation. Acta Mater. 2020, 188, 1–15.
- Zhang, W.; Wang, M.; Wang, L.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Interface stability, mechanical and corrosion properties of AlCrMoNbZr/(AlCrMoNbZr)N high-entropy alloy multilayer coatings under helium ion irradiation. Appl. Surf. Sci. 2019, 485, 108–118.
- Xiang, C.; Han, E.H.; Zhang, Z.M.; Fu, H.M.; Wang, J.Q.; Zhang, H.F.; Hu, G.D. Design of single-phase high-entropy alloys composed of low thermal neutron absorption cross-section elements for nuclear power plant application. Intermetallics 2019, 104, 143–153.
- Jawaharram, G.S.; Barr, C.M.; Monterrosa, A.M.; Hattar, K.; Averback, R.S.; Dillon, S.J. Irradiation induced creep in nanocrystalline high entropy alloys. Acta Mater. 2020, 182, 68–76.
- Lu, C.; Yang, T.; Jin, K.; Gao, N.; Xiu, P.; Zhang, Y.; Gao, F.; Bei, H.; Weber, W.J.; Sun, K.; et al. Radiation-induced segregation on defect clusters in single-phase concentrated solid-solution alloys. Acta Mater. 2017, 127, 98–107.
- Barr, C.M.; Nathaniel, J.E.; Unocic, K.A.; Liu, J.; Zhang, Y.; Wang, Y.; Taheri, M.L. Exploring radiation induced segregation mechanisms at grain boundaries in equiatomic CoCrFeNiMn high entropy alloy under heavy ion irradiation. Scr. Mater. 2018, 156, 80–84.
- Lu, C.; Niu, L.; Chen, N.; Jin, K.; Yang, T.; Xiu, P.; Zhang, Y.; Gao, F.; Bei, H.; Shi, S.; et al. Enhancing radiation tolerance by controlling defect mobility and migration pathways in multicomponent single-phase alloys. Nat. Commun. 2016, 7, 13564.
- Tong, Y.; Velisa, G.; Zhao, S.; Guo, W.; Yang, T.; Jin, K.; Lu, C.; Bei, H.; Ko, J.Y.P.; Pagan, D.C.; et al. Evolution of local lattice distortion under irradiation in medium- and high-entropy alloys. Materialia 2018, 2, 73–81.
- Jin, K.; Lu, C.; Wang, L.M.; Qu, J.; Weber, W.J.; Zhang, Y.; Bei, H. Effects of compositional complexity on the ion-irradiation induced swelling and hardening in Ni-containing equiatomic alloys. Scr. Mater. 2016, 119, 65–70.
- Chen, W.Y.; Liu, X.; Chen, Y.; Yeh, J.W.; Tseng, K.K.; Natesan, K. Irradiation effects in high entropy alloys and 316H stainless steel at 300 °C. J. Nucl. Mater. 2018, 510, 421–430.
- Wang, Y.; Zhang, K.; Feng, Y.; Li, Y.; Tang, W.; Wei, B. Evaluation of radiation response in CoCrFeCuNi high-entropy alloys. Entropy 2018, 20, 835.
- He, M.R.; Wang, S.; Shi, S.; Jin, K.; Bei, H.; Yasuda, K.; Matsumura, S.; Higashida, K.; Robertson, I.M. Mechanisms of radiation-induced segregation in CrFeCoNi-based single-phase concentrated solid solution alloys. Acta Mater. 2017, 126, 182–193.
- Yang, T.N.; Lu, C.; Velisa, G.; Jin, K.; Xiu, P.; Zhang, Y.; Bei, H.; Wang, L. Influence of irradiation temperature on void swelling in NiCoFeCrMn and NiCoFeCrPd. Scr. Mater. 2019, 158, 57–61.
- Yang, L.; Ge, H.; Zhang, J.; Xiong, T.; Jin, Q.; Zhou, Y.; Shao, X.; Zhang, B.; Zhu, Z.; Zheng, S.; et al. High He-ion irradiation resistance of CrMnFeCoNi high-entropy alloy revealed by comparison study with Ni and 304SS. J. Mater. Sci. Technol. 2019, 35, 300–305.
- Hashimoto, N.; Ono, Y. Mobility of point defects in CoCrFeNi-base high entropy alloys. Intermetallics 2021, 133, 107182.
- Zhang, Y.; Tunes, M.A.; Crespillo, M.L.; Zhang, F.; Boldman, W.L.; Rack, P.D.; Jiang, L.; Xu, C.; Greaves, G.; Donnelly, S.E.; et al. Thermal stability and irradiation response of nanocrystalline CoCrCuFeNi high-entropy alloy. Nanotechnology 2019, 30, 294004.
- Yang, T.N.; Lu, C.; Jin, K.; Crespillo, M.L.; Zhang, Y.; Bei, H.; Wang, L. The effect of injected interstitials on void formation in self-ion irradiated nickel containing concentrated solid solution alloys. J. Nucl. Mater. 2017, 488, 328–337.
- Abhaya, S.; Rajaraman, R.; Kalavathi, S.; David, C.; Panigrahi, B.K.; Amarendra, G. Effect of dose and post irradiation annealing in Ni implanted high entropy alloy FeCrCoNi using slow positron beam. J. Alloys Compd. 2016, 669, 117–122.
- Sellami, N.; Debelle, A.; Ullah, M.W.; Christen, H.M.; Keum, J.K.; Bei, H.; Xue, H.; Weber, W.J.; Zhang, Y. Effect of electronic energy dissipation on strain relaxation in irradiated concentrated solid solution alloys. Curr. Opin. Solid State Mater. Sci. 2019, 23, 107–115.
- Chen, D.; Tong, Y.; Li, H.; Wang, J.; Zhao, Y.L.; Hu, A.; Kai, J.J. Helium accumulation and bubble formation in FeCoNiCr alloy under high fluence He+ implantation. J. Nucl. Mater. 2018, 501, 208–216.
- Kombaiah, B.; Jin, K.; Bei, H.; Edmondson, P.D.; Zhang, Y. Phase stability of single phase Al0.12CrNiFeCo high entropy alloy upon irradiation. Mater. Des. 2018, 160, 1208–1216.
- Lu, C.; Yang, T.; Jin, K.; Velisa, G.; Xiu, P.; Song, M.; Peng, Q.; Gao, F.; Zhang, Y.; Bei, H.; et al. Enhanced void swelling in NiCoFeCrPd high-entropy alloy by indentation-induced dislocations. Mater. Res. Lett. 2018, 6, 584–591.
- Tunes, M.A.; Edmondson, P.D.; Vishnyakov, V.M.; Donnelly, S.E. Displacement damage and self-healing in high-entropy alloys: A TEM with in situ ion irradiation study. In Fusion Materials Research at Oak Ridge National Laboratory in Fiscal Year 2017; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 2017; Volume 1, pp. 62–64.
- AlTabbaa, O.; Ankrah, S. Social capital to facilitate ‘engineered’ university–industry collaboration for technology transfer: A dynamic perspective. Technol. Forecast. Soc. Chang. 2016, 104, 1–15.
- Fan, Z.; Zhong, W.; Jin, K.; Bei, H.; Osetsky, Y.N.; Zhang, Y. Diffusion-mediated chemical concentration variation and void evolution in ion-irradiated NiCoFeCr high-entropy alloy. J. Mater. Res. 2021, 36, 298–310.
- Lyu, P.; Peng, T.; Miao, Y.; Liu, Z.; Gao, Q.; Zhang, C.; Jin, Y.; Qingfeng Guan, J.C. Microstructure and properties of CoCrFeNiMo0.2 high-entropy alloy enhanced by high-current pulsed electron beam. Surf. Coat. Technol. 2021, 410, 126911.
- Xu, Q.; Zhu, T.; Zhong, Z.H.; Cao, X.Z.; Tsuchida, H. Investigation of irradiation resistance characteristics of precipitation strengthened high-entropy alloy (CoCrFeNi)95Ti1Nb1Al3 using slow positron beam. J. Alloys Compd. 2021, 888, 161518.
- Cao, P.P.; Wang, H.; He, J.Y.; Xuc, C.; Jiang, S.H.; Du, J.L.; Cao, X.Z.; Fu, E.G.; Lu, Z.P. Effects of nanosized precipitates on irradiation behavior of CoCrFeNi high entropy alloys. J. Alloys Compd. 2021, 859, 158291.
- Tolstolutskaya, G.D.; Rostova, G.Y.; Voyevodin, V.N.; Velikodnyi, A.N.; Tikhonovsky, M.A.; Tolmachova, G.N.; Kalchenko, A.S.; Vasilenko, R.L.; Kopanets, I.E. Section 2 thermal and fast reactor materials hardening of Cr-Fe-Ni-Mn high-entropy alloys caused by the irradiation with argon ions. Probl. At. Sci. Technol. 2017, 5, 40–47. Available online: http://dspace.nbuv.gov.ua/handle/123456789/136159 (accessed on 30 November 2021).
- Kumar, N.A.P.K.; Li, C.; Leonard, K.J.; Bei, H.; Zinkle, S.J. Microstructural stability and mechanical behavior of FeNiMnCr high entropy alloy under ion irradiation. Acta Mater. 2016, 113, 230–244.
- Li, C.; Hu, X.; Yang, T.; Kumar, N.K.; Wirth, B.D.; Zinkle, S.J. Neutron irradiation response of a Co-free high entropy alloy. J. Nucl. Mater. 2019, 527, 151838.
- Voyevodin, V.N.; Karpov, S.A.; Tolstolutskaya, G.D.; Tikhonovsky, M.A.; Velikodnyi, A.N.; Kopanets, I.E.; Tolmachova, G.N.; Kalchenko, A.S.; Vasilenko, R.L.; Kolodiy, I.V. Effect of irradiation on microstructure and hardening of Cr–Fe–Ni–Mn high-entropy alloy and its strengthened version. Philos. Mag. 2020, 100, 822–836.
- Dias, M.; Antão, F.; Catarino, N.; Galatanu, A.; Galatanu, M.; Ferreira, P.; Correia, J.B.; da Silva, R.C.; Gonçalves, A.P.; Alves, E. Sintering and irradiation of copper-based high entropy alloys for nuclear fusion. Fusion Eng. Des. 2020, 146, 1824–1828.
- Gromov, V.; Ivanov, Y.; Konovalov, S.; Osintsev, K.; Semin, A.; Rubannikova, Y. Modification of high-entropy alloy AlCoCrFeNi by electron beam treatment. J. Mater. Sci. Technol. 2021, 13, 787–797.
- Yang, T.; Xia, S.; Guo, W.; Hu, R.; Poplawsky, J.D.; Sha, G.; Fang, Y.; Yan, Z.; Wang, C.; Li, C.; et al. Effects of temperature on the irradiation responses of Al0.1CoCrFeNi high entropy alloy. Scr. Mater. 2018, 144, 31–35.
- Zhou, J.; Islam, M.I.; Guo, S.; Zhang, Y.; Lu, F. Radiation-induced grain growth of nanocrystalline alxcocrfeni high-entropy alloys. J. Phys. Chem. C 2021, 125, 3509–3516.
- Zhou, J. Radiation Effects in Apatite and High Entropy Alloy under Energetic Ions and Electrons; Louisiana State University and Agricultural and Mechanical College: Baton Rouge, LA, USA, 2020.
- Zhou, J.; Kirk, M.; Baldo, P.; Guo, S.; Lu, F. Phase stability of novel HfNbTaTiVZr refractory high entropy alloy under ion irradiation. Mater. Lett. 2021, 305, 130789.
- Moschetti, M.; Xu, A.; Schuh, B.; Hohenwarter, A.; Couzinié, J.P.; Kruzic, J.J.; Bhattacharyya, D.; Gludovatz, B. On the Room-Temperature Mechanical Properties of an Ion-Irradiated TiZrNbHfTa Refractory High Entropy Alloy. JOM 2020, 72, 130–138.
- Sadeghilaridjani, M.; Ayyagari, A.; Muskeri, S.; Hasannaeimi, V.; Salloom, R.; Chen, W.Y.; Mukherjee, S. Ion irradiation response and mechanical behavior of reduced activity high entropy alloy. J. Nucl. Mater. 2020, 529, 151955.
- Li, D.; Jia, N.; Huang, H.; Chen, S.; Dou, Y.; He, X.; Yang, W.; Xue, Y.; Hua, Z.; Zhang, F.; et al. Helium ion irradiation enhanced precipitation and the impact on cavity formation in a HfNbZrTi refractory high entropy alloy. J. Nucl. Mater. 2021, 552, 153023.
- Kareer, A.; Waite, J.C.; Li, B.; Couet, A.; Armstrong, D.E.J.; Wilkinson, A.J. Short communication: ‘Low activation, refractory, high entropy alloys for nuclear applications’. J. Nucl. Mater. 2019, 526, 151744.
- Wang, Y.; Zhang, K.; Feng, Y.; Li, Y.; Tang, W.; Zhang, Y.; Wei, B.; Hu, Z. Excellent irradiation tolerance and mechanical behaviors in high-entropy metallic glasses. J. Nucl. Mater. 2019, 527, 151785.
- El-Atwani, O.; Li, N.; Li, M.; Devaraj, A.; Baldwin, J.K.S.; Schneider, M.M.; Sobieraj, D.; Wróbel, J.S.; Nguyen-Manh, D.; Maloy, S.A.; et al. Outstanding radiation resistance of tungsten-based high-entropy alloys. Sci. Adv. 2019, 5, eaav2002.
- Komarov, F.F.; Konstantinov, S.V.; Pogrebnyak, A.D. Effect of high-fluence ion irradiation on the structure and mechanical properties of coatings based on nanostructured nitrides of high-entropy alloys (Ti, Hf, Zr, V, Nb). Dokl. Natsional’noj Akad. Nauk Belarusi 2015, 48, 24–30.
- Gandy, A.S.; Jim, B.; Coe, G.; Patel, D.; Hardwick, L.; Akhmadaliev, S.; Reeves-McLaren, N.; Goodall, R. High temperature and ion implantation-induced phase transformations in novel reduced activation si-fe-v-cr (-mo) high entropy alloys. Front. Mater. 2019, 6, 146.
- Patel, D.; Richardson, M.D.; Jim, B.; Akhmadaliev, S.; Goodall, R.; Gandy, A.S. Radiation damage tolerance of a novel metastable refractory high entropy alloy V2.5Cr1.2WMoCo0.04. J. Nucl. Mater. 2020, 531, 152005.
- Zhang, Z.; Han, E.H.; Xiang, C. Irradiation behaviors of two novel single-phase bcc-structure high-entropy alloys for accident-tolerant fuel cladding. J. Mater. Sci. Technol. 2021, 84, 230–238.
- Zhang, Z.; Han, E.H.; Xiang, C. Effect of helium ion irradiation on short-time corrosion behavior of two novel high-entropy alloys in simulated PWR primary water. Corros. Sci. 2021, 191, 109742.
- El-Atwani, O.; Alvarado, A.; Unal, K.; Fensin, S.; Hinks, J.A.; Greaves, G.; Baldwin, J.K.S.; Maloy, S.A.; Martinez, E. Helium implantation damage resistance in nanocrystalline W-Ta-V-Cr high entropy alloys. Mater. Today Energy 2021, 19, 100599.
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123.
- Kasar, A.K.; Scalaro, K.; Menezes, P.L. Tribological properties of high-entropy alloys under dry conditions for a wide temperature range—a review. Materials 2021, 14, 5814.
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128.
- Sharma, A.S.; Yadav, S.; Biswas, K.; Basu, B. High-entropy alloys and metallic nanocomposites: Processing challenges, microstructure development and property enhancement. Mater. Sci. Eng. R Rep. 2018, 131, 1–42.
- Li, Z.; Zhao, S.; Ritchie, R.O.; Meyers, M.A. Mechanical properties of high-entropy alloys with emphasis on face-centered cubic alloys. Prog. Mater. Sci. 2019, 102, 296–345.
- Menghani, J.; Vyas, A.; Patel, P.; Natu, H.; More, S. Wear, erosion and corrosion behavior of laser cladded high entropy alloy coatings—A review. Mater. Today Proc. 2020, 38, 2824–2829.
- Ayyagari, A.; Hasannaeimi, V.; Grewal, H.S.; Arora, H.; Mukherjee, S. Corrosion, erosion andwear behavior of complex concentrated alloys: A review. Metals 2018, 8, 603.
- Joseph, J.; Haghdadi, N.; Annasamy, M.; Kada, S.; Hodgson, P.D.; Barnett, M.R.; Fabijanic, D.M. On the enhanced wear resistance of CoCrFeMnNi high entropy alloy at intermediate temperature. Scr. Mater. 2020, 186, 230–235.
- Wang, H.; Ren, K.; Xie, J.; Zhang, C.; Tang, W. Friction and wear behavior of single-phase high-entropy alloyFeCoNiCrMn under MoS2-oil lubrication. Ind. Lubr. Tribol. 2019, 2019, 2–9.
- Xiao, J.K.; Tan, H.; Wu, Y.Q.; Chen, J.; Zhang, C. Microstructure and wear behavior of FeCoNiCrMn high entropy alloy coating deposited by plasma spraying. Surf. Coat. Technol. 2020, 385, 125430.
- Jones, M.R.; Nation, B.L.; Wellington-Johnson, J.A.; Curry, J.F.; Kustas, A.B.; Lu, P.; Chandross, M.; Argibay, N. Evidence of Inverse Hall-Petch Behavior and Low Friction and Wear in High Entropy Alloys. Sci. Rep. 2020, 10, 14336.
- Zhu, S.; Zhang, B.; Tao, X.; Yu, Y.; Zhang, Z.; Wang, Z.; Lu, B. Microstructure and tribology performance of plasma clad intermetallics reinforced CoCrFeMnNi-based high-entropy alloy composite coatings. Tribol. Trans. 2020, 64, 264–274.
- Deng, G.; Tieu, A.K.; Su, L.; Wang, P.; Wang, L.; Lan, X.; Cui, S.; Zhu, H. Investigation into reciprocating dry sliding friction and wear properties of bulk CoCrFeNiMo high entropy alloys fabricated by spark plasma sintering and subsequent cold rolling processes: Role of Mo element concentration. Wear 2020, 460–461, 203440.
- Lindner, T.; Löbel, M.; Saborowski, E.; Rymer, L.M.; Lampke, T. Wear and corrosion behaviour of supersaturated surface layers in the high-entropy alloy systems CrMnFeCoNi and CrFeCoNi. Crystals 2020, 10, 110.
- Sha, C.; Zhou, Z.; Xie, Z.; Munroe, P. FeMnNiCoCr-based high entropy alloy coatings: Effect of nitrogen additions on microstructural development, mechanical properties and tribological performance. Appl. Surf. Sci. 2020, 507, 145101.
- Xiao, J.K.; Tan, H.; Chen, J.; Martini, A.; Zhang, C. Effect of carbon content on microstructure, hardness and wear resistance of CoCrFeMnNiCx high-entropy alloys. J. Alloys Compd. 2020, 847, 156533.
- Cheng, H.; Fang, Y.; Xu, J.; Zhu, C.; Dai, P.; Xue, S. Tribological properties of nano/ultrafine-grained FeCoCrNiMnAlx high-entropy alloys over a wide range of temperatures. J. Alloys Compd. 2020, 817, 153305.
- Joseph, J.; Haghdadi, N.; Shamlaye, K.; Hodgson, P.; Barnett, M.; Fabijanic, D. The sliding wear behaviour of CoCrFeMnNi and AlxCoCrFeNi high entropy alloys at elevated temperatures. Wear 2019, 428–429, 32–44.
- Liu, X.; Yin, H.; Xu, Y. Microstructure, mechanical and tribological properties of Oxide Dispersion Strengthened high-entropy alloys. Materials 2017, 10, 1312.
- Wang, J.; Zhang, B.; Yu, Y.; Zhang, Z.; Zhu, S.; Lou, X.; Wang, Z. Study of high temperature friction and wear performance of (CoCrFeMnNi)85Ti15 high-entropy alloy coating prepared by plasma cladding. Surf. Coat. Technol. 2020, 384, 125337.
- Zhang, A.; Han, J.; Su, B.; Meng, J. A novel CoCrFeNi high entropy alloy matrix self-lubricating composite. J. Alloys Compd. 2017, 725, 700–710.
- Geng, Y.; Chen, J.; Tan, H.; Cheng, J.; Yang, J.; Liu, W. Vacuum tribological behaviors of CoCrFeNi high entropy alloy at elevated temperatures. Wear 2020, 456, 203368.
- Zhang, A.; Han, J.; Su, B.; Li, P.; Meng, J. Microstructure, mechanical properties and tribological performance of CoCrFeNi high entropy alloy matrix self-lubricating composite. Mater. Des. 2017, 114, 253–263.
- Brownlie, F.; Hodgkiess, T.; Fanicchia, F. Erosion-corrosion behaviour of CoCrFeNiMo0.85 and Al0.5CoCrFeNi complex concentrated alloys produced by laser metal deposition. Surf. Coatings Technol. 2021, 423, 127634.
- Zhang, M.; Zhang, W.; Liu, Y.; Liu, B.; Wang, J. FeCoCrNiMo high-entropy alloys prepared by powder metallurgy processing for diamond tool applications. Powder Metall. 2018, 61, 123–130.
- Huang, L.; Wang, X.; Jia, F.; Zhao, X.; Huang, B.; Ma, J.; Wang, C. Effect of Si element on phase transformation and mechanical properties for FeCoCrNiSix high entropy alloys. Mater. Lett. 2021, 282, 128809.
- Cui, G.; Han, B.; Yang, Y.; Wang, Y.; Chunyang, H. Microstructure and tribological property of CoCrFeMoNi High entropy alloy treated by ion sulfurization. J. Mater. Res. Technol. 2020, 9, 2598–2609.
- Li, T.; Liu, Y.; Liu, B.; Guo, W.; Xu, L. Microstructure and wear behavior of FeCoCrNiMo0.2 high entropy coatings prepared by air plasma spray and the high velocity oxy-fuel spray processes. Coatings 2017, 7, 151.
- Ji, X.; Zhao, J.; Wang, H.; Luo, C. Sliding wear of spark plasma sintered CrFeCoNiCu high entropy alloy coatings with MoS2 and WC additions. Int. J. Adv. Manuf. Technol. 2018, 96, 1685–1691.
- Verma, A.; Tarate, P.; Abhyankar, A.C.; Mohape, M.R.; Gowtam, D.S.; Deshmukh, V.P.; Shanmugasundaram, T. High temperature wear in CoCrFeNiCux high entropy alloys: The role of Cu. Scr. Mater. 2019, 161, 28–31.
- Liu, D.; Zhao, J.; Li, Y.; Zhu, W.; Lin, L. Effects of boron content on microstructure and wear properties of FeCoCrNiBx high-entropy alloy coating by laser cladding. Appl. Sci. 2020, 10, 49.
- Jiang, H.; Jiang, L.; Qiao, D.; Lu, Y.; Wang, T.; Cao, Z.; Li, T. Effect of Niobium on Microstructure and Properties of the CoCrFeNbxNi High Entropy Alloys. J. Mater. Sci. Technol. 2017, 33, 712–717.
- Yu, Y.; He, F.; Qiao, Z.; Wang, Z.; Liu, W.; Yang, J. Effects of temperature and microstructure on the triblogical properties of CoCrFeNiNbx eutectic high entropy alloys. J. Alloys Compd. 2019, 775, 1376–1385.
- Liu, X.; Zhou, S.; Xu, Y. Microstructure and tribological performance of Fe50Mn30Co10Cr10 high-entropy alloy based self-lubricating composites. Mater. Lett. 2018, 233, 142–145.
- Wang, J.; Yang, H.; Liu, Z.; Li, R.; Ruan, J.; Ji, S. Synergistic effects of WC nanoparticles and MC nanoprecipitates on the mechanical and tribological properties of Fe40Mn40Cr10Co10 medium-entropy alloy. J. Mater. Res. Technol. 2019, 8, 3550–3564.
- Derimow, N.; MacDonald, B.E.; Lavernia, E.J.; Abbaschian, R. Duplex phase hexagonal-cubic multi-principal element alloys with high hardness. Mater. Today Commun. 2019, 21, 100658.
- Guo, Y.; Li, C.; Zeng, M.; Wang, J.; Deng, P.; Wang, Y. In-situ TiC reinforced CoCrCuFeNiSi0.2 high-entropy alloy coatings designed for enhanced wear performance by laser cladding. Mater. Chem. Phys. 2020, 242, 122522.
- Zhang, Y.; Han, T.; Xiao, M.; Shen, Y. Tribological behavior of diamond reinforced FeNiCoCrTi0.5 carbonized high-entropy alloy coating. Surf. Coat. Technol. 2020, 401, 126233.
- Erdoğan, A.; Gök, M.S.; Zeytin, S. Analysis of the high-temperature dry sliding behavior of CoCrFeNiTi0.5Alx high-entropy alloys. Friction 2020, 8, 198–207.
- Liu, Y.; Xie, Y.; Cui, S.; Yi, Y.; Xing, X.; Wang, X.; Li, W. Effect of mo element on the mechanical properties and tribological responses of cocrfenimox high-entropy alloys. Metals 2021, 11, 486.
- Moazzen, P.; Toroghinejad, M.R.; Cavaliere, P. Effect of Iron content on the microstructure evolution, mechanical properties and wear resistance of FeXCoCrNi high-entropy alloy system produced via MA-SPS. J. Alloys Compd. 2021, 870, 159410.
- Yang, Y.; Ren, Y.; Tian, Y.; Li, K.; Zhang, W.; Shan, Q.; Tian, Y.; Huang, Q.; Wu, H. Microstructure and properties of FeCoCrNiMoSix high-entropy alloys fabricated by spark plasma sintering. J. Alloys Compd. 2021, 884, 161070.
- Li, Y.; Liang, H.; Nie, Q.; Qi, Z.; Deng, D.; Jiang, H.; Cao, Z. Microstructures and Wear Resistance of CoCrFeNi2V0.5Tix High-Entropy Alloy Coatings Prepared by Laser Cladding. Crystals 2020, 10, 352.
- Islak, S.; Eski, Ö.; Koç, V.; Özorak, C. Wear properties and synthesis of crfenimoti high entropy alloy coatings produced by TIG process. Indian J. Eng. Mater. Sci. 2020, 27, 659–664.
- Wen, X.; Cai, Z.; Yin, B.; Cui, X.; Zhang, X.; Jin, G. Tribological and Corrosion Properties of Ni-Cr-Co-Ti-V Multi-Principal Element Alloy Prepared by Vacuum Hot-Pressing Sintering. Adv. Eng. Mater. 2019, 21, 1801239.
- Wang, X.R.; Wang, Z.Q.; He, P.; Lin, T.S.; Shi, Y. Microstructure and wear properties of CuNiSiTiZr high-entropy alloy coatings on TC11 titanium alloy produced by electrospark—computer numerical control deposition process. Surf. Coat. Technol. 2015, 283, 156–161.
- Cheng, J.; Sun, B.; Ge, Y.; Hu, X.; Zhang, L.; Liang, X.; Zhang, X. Nb doping in laser-cladded Fe25Co25Ni25(B0.7Si0.3)25 high entropy alloy coatings: Microstructure evolution and wear behavior. Surf. Coat. Technol. 2020, 402, 126321.
- Yadav, S.; Sarkar, S.; Aggarwal, A.; Kumar, A.; Biswas, K. Wear and mechanical properties of novel (CuCrFeTiZn)100−xPbx high entropy alloy composite via mechanical alloying and spark plasma sintering. Wear 2018, 410–411, 93–109.
- Gou, Q.; Xiong, J.; Guo, Z.; Liu, J.; Yang, L.; Li, X. Influence of NbC additions on microstructure and wear resistance of Ti(C,N)-based cermets bonded by CoCrFeNi high-entropy alloy. Int. J. Refract. Met. Hard Mater. 2020, 94, 105375.
- Yadav, S.; Kumar, A.; Biswas, K. Wear behavior of high entropy alloys containing soft dispersoids (Pb, Bi). Mater. Chem. Phys. 2018, 210, 222–232.
- Cui, Y.; Shen, J.; Manladan, S.M.; Geng, K.; Hu, S. Wear resistance of FeCoCrNiMnAlx high-entropy alloy coatings at high temperature. Appl. Surf. Sci. 2020, 512, 145736.
- Gwalani, B.; Torgerson, T.; Dasari, S.; Jagetia, A.; Nartu, M.S.K.K.Y.; Gangireddy, S.; Pole, M.; Wang, T.; Scharf, T.W.; Banerjee, R. Influence of fine-scale B2 precipitation on dynamic compression and wear properties in hypo-eutectic Al0.5CoCrFeNi high-entropy alloy. J. Alloys Compd. 2021, 853, 157126.
- Chen, M.; Lan, L.; Shi, X.; Yang, H.; Zhang, M.; Qiao, J. The tribological properties of Al0.6CoCrFeNi high-entropy alloy with the σ phase precipitation at elevated temperature. J. Alloys Compd. 2019, 777, 180–189.
- Du, L.M.; Lan, L.W.; Zhu, S.; Yang, H.J.; Shi, X.H.; Liaw, P.K.; Qiao, J.W. Effects of temperature on the tribological behavior of Al0.25CoCrFeNi high-entropy alloy. J. Mater. Sci. Technol. 2019, 35, 917–925.
- Chen, M.; Shi, X.H.; Yang, H.J.; Liaw, P.K.; Gao, M.C.; Hawk, J.A. Wear behavior of Al0.6CoCrFeNi high-entropy alloy: Effect of environments. J. Mater. Res. 2018, 33, 3310–3320.
- Ji, X.; Duan, H.; Zhang, H.; Ma, J. Slurry Erosion Resistance of Laser Clad NiCoCrFeAl3 High-Entropy Alloy Coatings. Tribol. Trans. 2015, 58, 1119–1123.
- Haghdadi, N.; Guo, T.; Ghaderi, A.; Hodgson, P.D.; Barnett, M.R.; Fabijanic, D.M. The scratch behaviour of AlXCoCrFeNi (x = 0.3 and 1.0) high entropy alloys. Wear 2019, 428–429, 293–301.
- Fang, Y.; Chen, N.; Du, G.; Zhang, M.; Zhao, X.; Cheng, H.; Wu, J. High-temperature oxidation resistance, mechanical and wear resistance properties of Ti(C,N)-based cermets with Al0.3CoCrFeNi high-entropy alloy as a metal binder. J. Alloys Compd. 2020, 815, 152486.
- Wu, Y.H.; Yang, H.J.; Guo, R.P.; Wang, X.J.; Shi, X.H.; Liaw, P.K.; Qiao, J.W. Tribological behavior of boronized Al0.1CoCrFeNi high-entropy alloys under dry and lubricated conditions. Wear 2020, 460–461, 203452.
- Nair, R.B.; Arora, H.S.; Boyana, A.V.; Saiteja, P.; Grewal, H.S. Tribological behavior of microwave synthesized high entropy alloy claddings. Wear 2019, 436–437, 203028.
- Kumar, S.; Rani, P.; Patnaik, A.; Pradhan, A.K.; Kumar, V. Effect of cobalt content on wear behaviour of Al0.4FeCrNiCox (x = 0, 0.25, 0.5, 1.0 mol) high entropy alloys tested under demineralised water with and without 3.5% NaCl solution. Mater. Res. Express 2019, 6, 0865b3.
- Mu, Y.; Zhang, L.; Xu, L.; Prashanth, K.; Zhang, N.; Ma, X.; Jia, Y.; Xu, Y.; Jia, Y.; Wang, G. Frictional wear and corrosion behavior of AlCoCrFeNi high-entropy alloy coatings synthesized by atmospheric plasma spraying. Entropy 2020, 22, 740.
- Wu, M.; Chen, K.; Xu, Z.; Li, D.Y. Effect of Ti addition on the sliding wear behavior of AlCrFeCoNi high-entropy alloy. Wear 2020, 462–463, 203493.
- Zhao, D.; Yamaguchi, T.; Wang, W. Fabrication and wear performance of Al0.8FeCrCoNi high entropy alloy coating on magnesium alloy by resistance seam welding. Mater. Lett. 2020, 265, 127250.
- Kumar, S.; Patnaik, A.; Pradhan, A.K.; Kumar, V. Room temperature wear study of Al0.4FeCrNiCox (x = 0, 0.25, 0.5, 1.0 mol) high-entropy alloys under oil lubricating conditions. J. Mater. Res. 2019, 34, 841–853.
- Li, Y.; Shi, Y. Phase assemblage and wear resistance of laser-cladding Al0.8FeCoNiCrCu0.5Six high-entropy alloys on aluminum. Mater. Res. Express 2020, 7, 086504.
- Kafexhiu, F.; Podgornik, B.; Feizpour, D. Tribological behavior of as-cast and aged AlCoCrFeNi2.1 CCA. Metals 2020, 10, 208.
- Miao, J.; Liang, H.; Zhang, A.; He, J.; Meng, J.; Lu, Y. Tribological behavior of an AlCoCrFeNi2.1 eutectic high entropy alloy sliding against different counterfaces. Tribol. Int. 2021, 153, 106599.
- Ye, F.; Yang, Y.; Lou, Z.; Feng, L.; Guo, L.; Yu, J. Microstructure and wear resistance of TiC reinforced AlCoCrFeNi2.1 eutectic high entropy alloy layer fabricated by micro-plasma cladding. Mater. Lett. 2021, 284, 128859.
- Wang, Y.; Yang, Y.; Yang, H.; Zhang, M.; Ma, S.; Qiao, J. Microstructure and wear properties of nitrided AlCoCrFeNi high-entropy alloy. Mater. Chem. Phys. 2018, 210, 233–239.
- Liu, Y.; Ma, S.; Gao, M.C.; Zhang, C.; Zhang, T.; Yang, H.; Wang, Z.; Qiao, J. Tribological Properties of AlCrCuFeNi2 High-Entropy Alloy in Different Conditions. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2016, 47, 3312–3321.
- Kong, D.; Guo, J.; Cui, X.; Zhang, X. Effect of superheating on microstructure and wear resistance of high-entropy Al1.8CrCuFeNi2 alloy. Mater. Lett. 2020, 274, 128021.
- Wang, Y.; Yang, Y.; Yang, H.; Zhang, M.; Qiao, J. Effect of nitriding on the tribological properties of Al1.3CoCuFeNi2 high-entropy alloy. J. Alloys Compd. 2017, 725, 365–372.
- Xiao, J.K.; Wu, Y.Q.; Chen, J.; Zhang, C. Microstructure and tribological properties of plasma sprayed FeCoNiCrSiAlx high entropy alloy coatings. Wear 2020, 448–449, 203209.
- Liu, H.; Sun, S.; Zhang, T.; Zhang, G.; Yang, H.; Hao, J. Effect of Si addition on microstructure and wear behavior of AlCoCrFeNi high-entropy alloy coatings prepared by laser cladding. Surf. Coat. Technol. 2020, 405, 126522.
- Hsu, C.Y.; Yeh, J.W.; Chen, S.K.; Shun, T.T. Wear resistance and high-temperature compression strength of Fcc CuCoNiCrAl0.5Fe alloy with boron addition. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2004, 35A, 1465–1469.
- Chen, M.R.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Tu, C.P. Microstructure and properties of Al0.5CoCrCuFeNiTix (x = 0–2.0) high-entropy alloys. Mater. Trans. 2006, 47, 1395–1401.
- Löbel, M.; Lindner, T.; Mehner, T.; Lampke, T. Microstructure and wear resistance of AlCoCrFeNiTi high-entropy alloy coatings produced by HVOF. Coatings 2017, 7, 144.
- Kane, S.N.; Mishra, A.; Dutta, A.K. Preface: International Conference on Recent Trends in Physics (ICRTP 2016). J. Phys. Conf. Ser. 2016, 755, 011001.
- Wu, C.L.; Zhang, S.; Zhang, C.H.; Zhang, H.; Dong, S.Y. Phase evolution and cavitation erosion-corrosion behavior of FeCoCrAlNiTix high entropy alloy coatings on 304 stainless steel by laser surface alloying. J. Alloys Compd. 2017, 698, 761–770.
- Erdogan, A.; Döleker, K.M.; Zeytin, S. Effect of laser re-melting on electric current assistive sintered CoCrFeNiAlxTiy high entropy alloys: Formation, micro-hardness and wear behaviors. Surf. Coat. Technol. 2020, 399, 126179.
- Xin, B.; Zhang, A.; Han, J.; Su, B.; Meng, J. Tuning composition and microstructure by doping Ti and C for enhancing mechanical property and wear resistance of Al0.2Co1.5CrFeNi1.5Ti0.5 high entropy alloy matrix composites. J. Alloys Compd. 2020, 836, 155273.
- Moravcikova-Gouvea, L.; Moravcik, I.; Omasta, M.; Veselý, J.; Cizek, J.; Minárik, P.; Cupera, J.; Záděra, A.; Jan, V.; Dlouhy, I. High-strength Al0.2Co1.5CrFeNi1.5Ti high-entropy alloy produced by powder metallurgy and casting: A comparison of microstructures, mechanical and tribological properties. Mater. Charact. 2020, 159, 110046.
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo 1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317.
- Liu, H.; Liu, J.; Li, X.; Chen, P.; Yang, H.; Hao, J. Effect of heat treatment on phase stability and wear behavior of laser clad AlCoCrFeNiTi0.8 high-entropy alloy coatings. Surf. Coat. Technol. 2020, 392, 125758.
- Yu, Y.; Liu, W.M.; Zhang, T.B.; Li, J.S.; Wang, J.; Kou, H.C.; Li, J. Microstructure and tribological properties of AlCoCrFeNiTi0.5 high-entropy alloy in hydrogen peroxide solution. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 201–207.
- Löbel, M.; Lindner, T.; Lampke, T. High-temperature wear behaviour of AlCoCrFeNiTi0.5 coatings produced by HVOF. Surf. Coatings Technol. 2020, 403, 126379.
- Chen, L.; Bobzin, K.; Zhou, Z.; Zhao, L.; Öte, M.; Königstein, T.; Tan, Z.; He, D. Wear behavior of HVOF-sprayed Al0.6TiCrFeCoNi high entropy alloy coatings at different temperatures. Surf. Coat. Technol. 2019, 358, 215–222.
- Yu, Y.; Wang, J.; Yang, J.; Qiao, Z.; Duan, H.; Li, J.; Li, J.; Liu, W. Corrosive and tribological behaviors of AlCoCrFeNi-M high entropy alloys under 90 wt. % H2O2 solution. Tribol. Int. 2019, 131, 24–32.
- Yu, Y.; Wang, J.; Li, J.; Kou, H.; Duan, H.; Li, J.; Liu, W. Tribological behavior of AlCoCrCuFeNi and AlCoCrFeNiTi0.5 high entropy alloys under hydrogen peroxide solution against different counterparts. Tribol. Int. 2015, 92, 203–210.
- Jin, G.; Cai, Z.; Guan, Y.; Cui, X.; Liu, Z.; Li, Y.; Dong, M.; Zhang, D. High temperature wear performance of laser-cladded FeNiCoAlCu high-entropy alloy coating. Appl. Surf. Sci. 2018, 445, 113–122.
- Zhu, T.; Wu, H.; Zhou, R.; Zhang, N.; Yin, Y.; Liang, L.; Liu, Y.; Li, J.; Shan, Q.; Li, Q.; et al. Microstructures and Tribological Properties of TiC Reinforced FeCoNiCuAl High-Entropy Alloy at Normal and Elevated Temperature. Metals 2020, 10, 387.
- Wu, J.M.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Chen, H.C. Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519.
- Yan, G.; Zheng, M.; Ye, Z.; Gu, J.; Li, C.; Wu, C.; Wang, B. In-situ Ti(C, N) reinforced AlCoCrFeNiSi-based high entropy alloy coating with functional gradient double-layer structure fabricated by laser cladding. J. Alloys Compd. 2021, 886, 161252.
- Li, Z.; Fu, P.; Hong, C.; Chang, F.; Dai, P. Tribological behavior of Ti(C, N)-TiB2 composite cermets using FeCoCrNiAl high entropy alloys as binder over a wide range of temperatures. Mater. Today Commun. 2021, 26, 102095.
- Kumar, A.; Chandrakar, R.; Chandraker, S.; Rao, K.R.; Chopkar, M. Microstructural and mechanical properties of AlCoCrCuFeNiSix (x = 0.3 and 0.6) high entropy alloys synthesized by spark plasma sintering. J. Alloys Compd. 2021, 184, 158193.
- Xin, B.; Zhang, A.; Han, J.; Meng, J. Improving mechanical properties and tribological performance of Al0.2Co1.5CrFeNi1.5Ti0.5 high entropy alloys via doping Si. J. Alloys Compd. 2021, 869, 159122.
- Karakaş, M.S.; Günen, A.; Çarboğa, C.; Karaca, Y.; Demir, M.; Altınay, Y.; Erdoğan, A. Microstructure, some mechanical properties and tribocorrosion wear behavior of boronized Al0.07Co1.26Cr1.80Fe1.42Mn1.35Ni1.10 high entropy alloy. J. Alloys Compd. 2021, 886, 161222.
- Xin, B.; Zhang, A.; Han, J.; Meng, J. The tribological properties of carbon doped Al0.2Co1.5CrFeNi1.5Ti0.5 high entropy alloys. Wear 2021, 484, 204045.
- Zhao, P.; Li, J.; Lei, R.; Yuan, B.; Xia, M.; Li, X.; Zhang, Y. Investigation into microstructure, wear resistance in air and nacl solution of alcrconifectax high-entropy alloy coatings fabricated by laser cladding. Coatings 2021, 11, 358.
- Ghanbariha, M.; Farvizi, M.; Ebadzadeh, T.; Alizadeh Samiyan, A. Effect of ZrO2 particles on the nanomechanical properties and wear behavior of AlCoCrFeNi–ZrO2 high entropy alloy composites. Wear 2021, 484–485, 204032.
- Li, Y.; Shi, Y. Microhardness, wear resistance, and corrosion resistance of AlxCrFeCoNiCu high-entropy alloy coatings on aluminum by laser cladding. Opt. Laser Technol. 2021, 134, 106632.
- Cai, Z.; Wang, Z.; Hong, Y.; Lu, B.; Liu, J.; Li, Y.; Pu, J. Improved tribological behavior of plasma-nitrided AlCrTiV and AlCrTiVSi high-entropy alloy films. Tribol. Int. 2021, 163, 107195.
- Chandrakar, R.; Kumar, A.; Chandraker, S.; Rao, K.R.; Chopkar, M. Microstructural and mechanical properties of AlCoCrCuFeNiSix (x = 0 and 0.9) high entropy alloys. Vacuum 2021, 184, 109943.
- Erdogan, A.; Sunbul, S.E.; Icin, K.; Doleker, K.M. Microstructure, wear and oxidation behavior of AlCrFeNiX (X = Cu, Si, Co) high entropy alloys produced by powder metallurgy. Vacuum 2021, 187, 110143.
- Duan, H.; Wu, Y.; Hua, M.; Yuan, C.; Wang, D.; Tu, J.; Kou, H.; Li, J. Tribological properties of AlCoCrFeNiCu high-entropy alloy in hydrogen peroxide solution and in oil lubricant. Wear 2013, 297, 1045–1051.
- Chen, M.R.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Chuang, M.H. Effect of vanadium addition on the microstructure, hardness, and wear resistance of Al0.5CoCrCuFeNi high-entropy alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2006, 37, 1363–1369.
- Gu, Z.; Xi, S.; Mao, P.; Wang, C. Microstructure and wear behavior of mechanically alloyed powder AlxMo0.5NbFeTiMn2 high entropy alloy coating formed by laser cladding. Surf. Coat. Technol. 2020, 401, 126244.
- Hsu, C.Y.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. Effect of iron content on wear behavior of AlCoCrFexMo0.5Ni high-entropy alloys. Wear 2010, 268, 653–659.
- Liang, H.; Miao, J.; Gao, B.; Deng, D.; Wang, T.; Lu, Y.; Cao, Z.; Jiang, H.; Li, T.; Kang, H. Microstructure and tribological properties of AlCrFe2Ni2W0.2Mo0.75 high-entropy alloy coating prepared by laser cladding in seawater, NaCl solution and deionized water. Surf. Coat. Technol. 2020, 400, 126214.
- Qiu, X.W.; Liu, C.G. Microstructure and properties of Al2CrFeCoCuTiNix high-entropy alloys prepared by laser cladding. J. Alloys Compd. 2013, 553, 216–220.
- Kanyane, L.R.; Popoola, A.P.; Malatji, N. Influence of Sintering Temperature on Microhardness and Tribological Properties of Equi-Atomic Ti-Al-Mo-Si-W Multicomponent Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2019, 538, 012009.
- Huang, C.; Zhang, Y.; Vilar, R.; Shen, J. Dry sliding wear behavior of laser clad TiVCrAlSi high entropy alloy coatings on Ti-6Al-4V substrate. Mater. Des. 2012, 41, 338–343.
- Zhang, H.X.; Dai, J.J.; Sun, C.X.; Li, S.Y. Microstructure and wear resistance of TiAlNiSiV high-entropy laser cladding coating on Ti-6Al-4V. J. Mater. Process. Technol. 2020, 282, 116671.
- Lin, Y.C.; Cho, Y.H. Elucidating the microstructure and wear behavior for multicomponent alloy clad layers by in situ synthesis. Surf. Coat. Technol. 2008, 202, 4666–4672.
- Yadav, S.; Aggrawal, A.; Kumar, A.; Biswas, K. Effect of TiB2 addition on wear behavior of (AlCrFeMnV)90Bi10 high entropy alloy composite. Tribol. Int. 2019, 132, 62–74.
- Bhardwaj, V.; Zhou, Q.; Zhang, F.; Han, W.; Du, Y.; Hua, K.; Wang, H. Effect of Al addition on the microstructure, mechanical and wear properties of TiZrNbHf refractory high entropy alloys. Tribol. Int. 2021, 160, 107031.
- Zhao, P.; Li, J.; Zhang, Y.; Li, X.; Xia, M.M.; Yuan, B.G. Wear and high-temperature oxidation resistances of AlNbTaZrx high-entropy alloys coatings fabricated on Ti6Al4V by laser cladding. J. Alloys Compd. 2021, 862, 158405.
- Tüten, N.; Canadinc, D.; Motallebzadeh, A.; Bal, B. Microstructure and tribological properties of TiTaHfNbZr high entropy alloy coatings deposited on Ti–6Al–4V substrates. Intermetallics 2019, 105, 99–106.
- Pole, M.; Sadeghilaridjani, M.; Shittu, J.; Ayyagari, A.; Mukherjee, S. High temperature wear behavior of refractory high entropy alloys based on 4-5-6 elemental palette. J. Alloys Compd. 2020, 843, 156004.
- Ye, Y.X.; Liu, C.Z.; Wang, H.; Nieh, T.G. Friction and wear behavior of a single-phase equiatomic TiZrHfNb high-entropy alloy studied using a nanoscratch technique. Acta Mater. 2018, 147, 78–89.
- Pogrebnjak, A.D.; Yakushchenko, I.V.; Abadias, G.; Chartier, P.; Bondar, O.V.; Beresnev, V.M.; Takeda, Y.; Sobol’, O.V.; Oyoshi, K.; Andreyev, A.A.; et al. The effect of the deposition parameters of nitrides of high-entropy alloys (TiZrHfVNb)N on their structure, composition, mechanical and tribological properties. J. Superhard Mater. 2013, 35, 356–368.
- Gong, P.; Li, F.; Deng, L.; Wang, X.; Jin, J. Research on nano-scratching behavior of TiZrHfBeCu(Ni) high entropy bulk metallic glasses. J. Alloys Compd. 2020, 817, 153240.
- Zhao, Y.Y.; Ye, Y.X.; Liu, C.Z.; Feng, R.; Yao, K.F.; Nieh, T.G. Tribological behavior of an amorphous Zr20Ti20Cu20Ni20Be20 high-entropy alloy studied using a nanoscratch technique. Intermetallics 2019, 113, 1065601.
- Jhong, Y.S.; Huang, C.W.; Lin, S.J. Effects of CH4 flow ratio on the structure and properties of reactively sputtered (CrNbSiTiZr)Cx coatings. Mater. Chem. Phys. 2018, 210, 348–352.
- Mathiou, C.; Poulia, A.; Georgatis, E.; Karantzalis, A.E. Microstructural features and dry—Sliding wear response of MoTaNbZrTi high entropy alloy. Mater. Chem. Phys. 2018, 210, 126–135.
- Petroglou, D.; Poulia, A.; Mathiou, C.; Georgatis, E.; Karantzalis, A.E. A further examination of MoTaxNbVTi (x = 0.25, 0.50, 0.75 and 1.00 at.%) high-entropy alloy system: Microstructure, mechanical behavior and surface degradation phenomena. Appl. Phys. A Mater. Sci. Process. 2020, 126, 364.
- Poulia, A.; Georgatis, E.; Lekatou, A.; Karantzalis, A.E. Microstructure and wear behavior of a refractory high entropy alloy. Int. J. Refract. Met. Hard Mater. 2016, 57, 50–63.
- Poulia, A.; Georgatis, E.; Lekatou, A.; Karantzalis, A. Dry-Sliding Wear Response of MoTaWNbV High Entropy Alloy. Adv. Eng. Mater. 2017, 19, 1600535.
- Poulia, A.; Georgatis, E.; Karantzalis, A. Evaluation of the Microstructural Aspects, Mechanical Properties and Dry Sliding Wear Response of MoTaNbVTi Refractory High Entropy Alloy. Met. Mater. Int. 2019, 25, 1529–1540.
- Alvi, S.; Akhtar, F. High temperature tribology of CuMoTaWV high entropy alloy. Wear 2019, 426–427, 412–419.
- Hua, N.; Wang, W.; Wang, Q.; Ye, Y.; Lin, S.; Zhang, L.; Guo, Q.; Brechtl, J.; Liaw, P.K. Mechanical, corrosion, and wear properties of biomedical Ti–Zr–Nb–Ta–Mo high entropy alloys. J. Alloys Compd. 2021, 861, 157997.
- Gu, Z.; Peng, W.; Guo, W.; Zhang, Y.; Hou, J.; He, Q.; Zhao, K.; Xi, S. Design and characterization on microstructure evolution and properties of laser-cladding Ni1.5CrFeTi2B0.5Mox high-entropy alloy coatings. Surf. Coat. Technol. 2021, 408, 126793.




