Alloying has been very common practice in materials engineering to fabricate metals of desirable properties for specific applications. Traditionally, a small amount of the desired material is added to the principal metal. However, a new alloying technique emerged in 2004 with the concept of adding several principal elements in or near equi-atomic concentrations. These are popularly known as high entropy alloys (HEAs) which can have a wide composition range.
- high entropy alloys (HEAs)
- additive manufacturing (AM)
- wear
- nuclear applications
- irradiation
1. Introduction
1.1. The Definitions of High Entropy Alloys
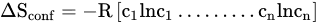
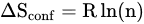
2. Manufacturing of HEAs
2.1. Background and Conventional Methods
2.2. Additive Manufacturing of HEAs
|
Source |
Alloy Composition |
Microstructure (Grain Size) |
Result UTS (MPa), YS (MPa), BS (MPa), δb (mm), ε (%), H, CS (MPa), C (%) |
|---|---|---|---|
|
Chen et al. [176] |
CoCrFeMnNi |
FCC (53.1 µm) |
UTS = 281 ± 18, YS = 12.5 ± 0.5, H = 261 ± 7 HV |
|
Niu et al. [169] |
CoCrFeMnNi |
FCC (<5 µm) |
CS = 2447.7 |
|
Li et al. [177] |
CoCrFeMnNi + TiNp nanoparticles |
FCC |
UTS = 601–1036, ε = 12–30 |
|
Li et al. [178] |
CoCrFeMnNi + Fe based metallic glass |
FCC |
UTS = 916–1517 |
|
Li et al. [179] |
CoCrFeMnNi + TiN nanoparticles |
FCC |
- |
|
Kim et al. [180] |
(CoCrFeMnNi)C |
FCC (180–330 nm) |
YS = 800–900, ε = 25–30 |
|
Li et al. [181] |
CoCrFeMnNi + 12 wt% nano-TiNp |
FCC (<2 µm) |
UTS = 1100 |
|
Piglione et al. [182] |
CoCrFeMnNi |
FCC (0.52–0.64 µm) |
H = 212 HV |
|
Zhu et al. [153] |
CoCrFeMnNi |
FCC |
- |
|
Xu et al. [183] |
CoCrFeMnNi |
FCC (1–2 µm) |
H = 2.84 ± 0.13 GPa |
|
Park et al. [154] |
CoCrFeMnNi +1 at%C |
FCC (20–35 µm) |
UTS = 829–989, YS = 741, ε = 24.3 |
|
Ren et al. [184] |
CoCrFeMnNi |
- |
- |
|
Dovgyy et al. [185] |
CoCrFeMnNi |
FCC & cubic (0.2–0.8 µm) |
- |
|
Zhou et al. [155] |
CoCrFeNi + 0.5 at%C |
FCC (40–50 µm) |
UTS = 776–797, YS = 630–656, ε = 7.7–13.5 |
|
Wu et al. [186] |
CoCrFeNi + 0.5 at%C |
FCC (40–50 µm) |
UTS = 795, YS = 638 |
|
Lin et al. [98] |
CoCrFeNi |
FCC |
|
|
Sun et al. [187] |
CoCrFeNi |
-, ~3 mm in length and ~200 μm in width |
UTS = 676.7–691, YS = 556.7–572, ε = 12.4–17.9 |
|
Song et al. [188] |
CoCrFeNi + N (1.8%) |
FCC |
UTS = 600–853, YS = 520–650, ε = 27 |
|
Zhou et al. [189] |
(CoCrFeNi)1−x (WC)x |
FCC |
H = 603–768 HV |
|
Brif et al. [156] |
CoCrFeNi |
FCC |
UTS = 480–745, YS = 402–600, ε = 8–32, H = 205–238 |
|
Niu et al. [190] |
AlCoCrFeNi |
Disordered (A2) + Ordered (B2) BCC |
H = 632.8 HV |
|
Karlsson et al. [170] |
AlCoCrFeNi |
FCC & BCC (<20 µm) |
- |
|
Peyrouzet et al. [157] |
Al0.3CoCrFeNi |
FCC (width~13 and length~70–120 µm) |
UTS = 896, YS = 730, ε = 29 |
|
Sun et al. [158] |
Al0.5CoCrFeNi |
FCC & BCC (1 µm) |
UTS = 878, YS = 609, H = 270HV |
|
Zhou et al. [160] |
Al0.5CoCrFeNi |
FCC |
UTS = 721, YS = 579, ε = 22 |
|
Luo et al. [191] |
AlCrCuFeNi |
BCC (avg. width~4 µm) |
CS = 1655.2–2052.8, C = 6.5–6.8 |
|
Luo et al. [192] |
AlCrCuFeNix (2 ≤ x ≤ 3) |
FCC (thickness~490 nm) & BCC (~140 nm) Avg. thickness of both ~ 650 nm |
UTS = 957, ε = 14.3 |
|
Li et al. [112] |
AlCoCuFeNi |
BCC |
YS = 744, ε = 13.1, CS = 1600 |
|
Yao et al. [193] |
AlCrFeNiV |
FCC (width~15 µm, length~75–200 µm) |
UTS = 1057.47, ε = 30.3 |
|
Wang et al. [194] |
AlCoCrCuFeNi |
FCC & BCC |
H = 710.4 HV |
|
Wang et al. [195] |
AlMgScZrMn |
Al3 (Sc, Zr) (1–10 nm + 7 µm) |
UTS = 394, ε = 10.5 |
|
Sarawat et al. [196] |
AlCoFeNiV0.9Sm0.1 AlCoFeNiSm0.1TiV0.9 AlCoFeNiSm0.05TiV0.95Zr, AlCoFeNiTiVZr |
FCC |
H~42.8–86.7 HV |
|
Agrawal et al. [197] |
Fe40Mn20Co20Cr15Si5 |
HCP |
UTS = 1100, YS = 530, ε = 30 |
|
NbMoTaW |
BCC (13.4 μm) |
H = 826 HV |
|
|
Ni6Cr4WFe9Ti |
FCC (300–1000 nm) + unknown phase |
UTS = 972, YS = 742, ε = 12.2 |
|
|
Chen et al. [202] |
CoCrFeNiMn |
FCC + Mn2O3 particles |
YS = 620, UTS = 730, ε~12 |
|
Litwa et al. [203] |
CoCrFeNiMn |
FCC |
H~320 HV |
|
Zhang et al. [204] |
CoCrFeNiMn |
FCC |
YS~729.6 |
|
Kim et al. [205] |
CoCrFeNiMn |
FCC |
YS = 752.6 |
|
Choi et al. [206] |
CoCrFeNiMn |
FCC |
|
|
Su et al. [207] |
CrCuFeNi2 Al0.5CrCuFeNi2 Al0.75CrCuFeNi2 AlCrCuFeNi2 |
FCC FCC FCC + BCC/B2 FCC + BCC/B2 |
|
|
Peng et al. [208] |
CoCrFeNi + Ti coated diamond CoCrFeNi + diamond |
FCC + diamond particles FCC + Cr7C3 + diamond particles |
H = 622 HV, BS = 530, δb = 0.64 H = 615 HV, BS = 925, δb = 0.48 |
|
Wang et al. [209] |
CoCrFeNiMn |
FCC |
H = 164–370 HV |
|
Sun et al. [210] |
Al0.1CrCuFeNi Al0.5CrCuFeNi AlCrCuFeNi |
FCC FCC FCC + BCC/B2 (NiAl) |
|
|
Ishimoto et al. [211] |
Ti1.4Nb0.6Ta0.6Zr1.4Mo0.6 |
BCC |
YS = 1690, |
|
Park et al. [212] |
(CoCrFeMnNi)99C1 |
FCC |
YS~741, UTS~874 |
|
Lin et al. [213] |
CoCrFeNi |
FCC |
YS = 701 ± 14, UTS = 907 ± 25 |
|
Kim et al. [214] |
CoCrFeNiMn |
FCC |
- |
|
Jin et al. [215] |
CoCrFeNiMn |
FCC |
YS = 520 ± 10, UTS = 770 ± 10, ε~25 |
|
Lin et al. [216] |
Al0.2Co1.5CrFeNi1.5Ti0.3 |
FCC + σ + L12 |
YS = 1235, UTS = 1550 |
|
Peng et al. [217] |
CoCrFeNiMn |
FCC |
- |
|
Vogiatzief et al. [218] |
AlCrFe2Ni2 Heat treatment (750–950 °C, 3 h & 6 h) |
FCC + BCC |
H = 276–483 HV |
|
Liao et al. [219] |
Al0.5FeCrNi2.5V0.2 |
FCC |
H = 220–240 HV |
|
Guo et al. [220] |
CoCrFeNiMn |
FCC |
YS = 622, UTS = 763, ε~16 |
|
Kim et al. [221] |
(CoCrFeNiMn)100−xCx |
FCC (15–22 µm) |
YS = 653–753, UTS = 766–911 |
|
Zhao et al. [222] |
CoCrFeNi |
FCC |
H = 238–525 HV |
|
Gu et al. [223] |
CoCr2.5FeNi2TiW0.5 |
FCC |
YS = 449–581, CS = 823–893, ε = 4.4–9.9, H = 436.7–499.2 HV |
|
Source |
Alloy Composition |
Microstructure (Grain Size) |
Result UTS (MPa), YS (MPa), ε (%), H, CS (MPa), C (%) |
|---|---|---|---|
|
Peng et al. [224] |
CoCrFeNiMn |
FCC |
YS = 196 |
|
Wang et al. [225] |
CoCrFeMnNi |
FCC (65) |
UTS = 497, 205, H = 157.1HV |
|
Kuwabara et al. [226] |
AlCoCrFeNi |
BCC & FCC |
UTS = 1073, YS = 769, ε = 0–1.2 YS = 944–1015, CS = 1447–1668, C = 14.5–26.4 |
|
Wang et al. [227] |
AlCoCrFeNi |
BCC |
- |
|
Fujieda et al. [228] |
CoCrFeNiTi |
FCC + Cubic |
UTS = 1178, YS = 773, ε = 25.8 |
|
Popov et al. [229] |
Al0.5CrMoNbTa0.5 |
BCC |
|
Scheme |
Alloy Composition |
Microstructure (Grain Size) |
Result UTS (MPa), YS (MPa), ε (%), H, CS (MPa), C (%) |
|---|---|---|---|
|
Guan et al. [230] |
CoCrFeMnNi |
FCC (13 μm) |
YS = 517, ε = 26 |
|
Melia et al. [231] |
CoCrFeMnNi |
FCC (~4 μm) |
UTS = 647–651, YS = 232–424 |
|
Li et al. [232] |
CoCrFeMnNi |
FCC |
|
|
Gao et al. [233] |
CoCrFeMnNi |
FCC (30–150 μm) + BCC |
UTS = 620, YS = 448 |
|
CoCrFeNiMn |
FCC |
UTS = 400–600 |
|
|
Chew et al. [236] |
CoCrFeNiMn |
FCC (3.68 ± 0.85 μm) |
UTS = 660, YS = 518 |
|
Qiu et al. [237] |
CoCrFeMnNi |
FCC |
UTS = 891, YS = 564 |
|
Li et al. [238] |
CoCrFeMnNi + WC (0–10 wt%) |
FCC |
UTS = 550–845, YS = 300–675, ε = 9 |
|
Amar et al. [239] |
CoCrFeMnNi + TiC (0–5 wt%) |
FCC |
UTS = 550–723, YS = 300–385, ε = 32 |
|
Guan et al. [240] |
CoCrFeMnNi AlCoCrFeNiTi0.5 |
FCC (24 µm) BCC (7 µm) + FCC |
YS = 888–1100, H = 197–657 HV |
|
Wang et al. [241] |
CoCrFeNiMo0.2 |
FCC |
UTS = 532–928, ε = 37 |
|
Zhou et al. [242] |
CoCrFeNiNbx (x = 0–0.2) |
FCC |
UTS = 400–820, YS = 220–750 |
|
Gwalani et al. [243] |
AlxCoCrFeNi (x = 0.3–0.7) |
FCC |
|
|
Nartu et al. [244] |
Al0.3CoCrFeNi |
FCC |
YS = 410–630, ε = 18–28 |
|
Mohanty et al. [245] |
AlxCoCrFeNi (x = 0.3–0.7) |
FCC + BCC |
H = 170–380 HV |
|
Vikram et al. [246] |
AlCoCrFeNi2.1 |
FCC & BCC |
YS = 309–711, H = 278 ± 11–316 ± 14 HV |
|
Gwalani et al. [247] |
AlCrFeMoVx (x = 0–1) |
BCC (68–165 μm) |
H = 485–581 HV |
|
Guan et al. [248] |
AlCoCrFeNiTi0.5 |
BCC (12 μm) |
- |
|
Malatji et al. [249] |
AlCrCuFeNi |
BCC & FCC |
H = 350 HV, |
|
AlCoCrFeNiCu AlTiCrFeCoNi |
H = 600 HV, H = 850 HV |
||
|
Moorehead et al. [252] |
NbMoTaW |
BCC |
- |
|
Kunce et al. [253] |
TiZrNbMoV |
BCC |
- |
|
Dobbelstein et al. [254] |
TiZrNbHfTa |
BCC |
H = 509 HV0.2 |
|
Pegues et al. [255] |
CoCrFeNiMn |
FCC |
- |
|
Li et al. [256] |
CoCrFeNiMn |
FCC |
- |
|
Tong et al. [257] |
CoCrFeNiMn Vacuum arc melting 1 impact Laser shock peening 3 impact Laser shock peening 5 impact Laser shock peening |
FCC |
YS = 320.7, UTS = 531.7 YS = 427.4, UTS = 570.7 YS~435, UTS~600 YS = 489.9, UTS = 639.9 |
|
Shen et al. [258] |
CoCrFeNi (SiC)x |
FCC + Cr7C3 (1 µm) |
UTS = 2155–2499, YS = 142–713, H = 139–310 |
|
Cai et al. [259] |
CoCrFeNi AlCoCrFeNi |
BCC (102.27 µm) BCC (18.75 µm) |
YS = 318, UTS = 440, ε = 8.56 YS = 383, UTS = 533, ε = 10.6 |
|
Zhang et al. [260] |
NbMoTa NbMoTaTi NbMoTaNi NbMoTaTi0.5Ni0.5 |
BCC BCC + α-Ti BCC BCC + Ni3Ta + β-Ti |
YS = 1252, CS = 1282, ε = 15 YS = 1200, CS = 1350, ε = 23 YS = 1350, CS = 1380, ε = 11 YS = 1750, CS = 2277.79, ε = 15 CS of NbMoTaTi0.5Ni0.5 at 600, 800 and 1000 °C is 1699.75 MPa, 1033.63 MPa and 651.36 MPa |
|
Peng et al. [261] |
Al0.3CoCrFeNi |
FCC + B2 |
YS = 373–476, CS = 473–508, ε = 0.6–2.96, H = 208–221 HV |
|
Kuzminova et al. [262] |
CoCrFeNi |
FCC |
YS = 456–551, UTS = 637–658, H = 209–259 HV |
|
Malatji et al. [263] |
AlCuCrFeNi Heat treated (800–1100 °C) |
FCC + BCC |
H = 310–381 HV |
|
Dong et al. [264] |
AlCoCrFeNi2.1 |
FCC + BCC |
YS = 388, UTS = 719, ε~27, H = 221–228 |
|
Zhou et al. [265] |
CoCrFeNb0.2Ni2.1 Solution treatment (2 h,1250 °C) 96 h aged (650 °C) |
FCC + HCP (Laves C14) + Nb rich carbide |
YS~340, UTS~735 YS~239, UTS~607 YS~896, UTS~1127, ε~17 |
|
Zheng et al. [266] |
CoCrFeNiMn |
FCC |
YS = 330, UTS = 630 |
3. Applications under Extreme Environments
3.1. Nuclear Applications
|
Reactor System |
Core Outlet Temperature (°C) |
Coolant |
|---|---|---|
|
Super critical water-cooled reactor |
350–620 |
Water |
|
Sodium-cooled fast reactor |
~550 |
Na liquid metal |
|
Lead-cooled reactor |
550–800 |
Pb, Pb-Bi liquid Metals |
|
Molten salt reactor |
700–800 |
Fluoride salts |
|
Gas-cooled fast reactor |
~850 |
Helium gas |
|
Very high temperature reactor |
>900 |
Helium gas |
|
Source |
Material (Fabrication) |
Phase |
Irradiation Conditions (Energy, Ion, Fluence, Temperature) |
|---|---|---|---|
|
Jawaharram et al. [301] |
CoCrFeNiMn |
FCC |
2.6 MeV, Ag3+, 1.5 × 10−3 & 1.9 × 10−3 dpa−1 s−1, 23–500 °C |
|
Lu et al. [302] |
NiCoFeCr, CoCrFeNiMn |
FCC |
3 MeV, Ni2+, 5 × 1016 ions·cm−2, 500 °C |
|
Barr et al. [303] |
CoCrFeNiMn |
FCC |
3 MeV, Ni2+, 3 × 1015 ions·cm−2, 500 °C |
|
Lu et al. [304] |
CoCrFeNi, CoCrFeNiMn |
FCC |
1.5 MeV, Ni+, 4 × 1014 & 3 × 1015 ions·cm−2 (peak dose~4 dpa), 500 °C 3 MeV, Ni+, 5 × 1016 ions·cm−2 (peak dose~60 dpa), 500 °C |
|
Tong et al. [305] |
CoCrFeNiMn CoCrFeNi CoCrFeNiPd |
FCC |
16 MeV, Ni5+, 8 MeV Ni3+, 4 MeV Ni1+& 2 MeV Ni1+, 0.1–1 dpa, 420 °C |
|
Jin et al. [306] |
CoCrFeNi, CoCrFeNiMn |
FCC |
3 MeV, Ni2+, 5 × 1016 ions·cm−2 (peak dose~53 dpa), 500 °C |
|
Chen et al. [307] |
CoCrFeMnNi Al0.3CoCrFeNi |
FCC FCC |
1 MeV, Kr ions, 6.3 × 1015 ions·cm−2, 300 °C |
|
Wang et al. [308] |
CoCrFeNiCu |
FCC |
100 keV, He+, 2.5 × 1017, 5 × 1017 & 1 × 1018 ions·cm−2, RT |
|
He et al. [309] |
CoCrFeNi, CoCrFeNiMn, CoCrFeNiPd |
FCC |
electrons, 5 × 1018 e·cm−2·s−1, 400 °C |
|
Yang et al. [310] |
CoCrFeNiMn, CoCrFeNiPd |
FCC |
3MeV, Ni2+, 5 × 1016 ions·cm−2, 420, 500 & 580 °C |
|
Yang et al. [311] |
CoCrFeNiMn |
FCC |
-, He ion, -, RT & 450 °C |
|
Hashimoto et al. [312] |
CoCrFeNiMn, CoCrFeNiAl0.3 |
FCC |
1250 keV, 1.5 dpa, 300–400 °C |
|
Zhang et al. [313] |
CoCrFeNiCu |
FCC |
3 MeV Ni2+, 1014 ions·cm−2, RT |
|
Yang et al. [314] |
CoNi, FeNi, CoCrFeNi |
- FCC |
3 MeV, Ni2+, 1.5 × 1016 (peak dose~17 dpa) & 5.0 × 1016 (peak dose~53 dpa) ions·cm−2, 500 °C |
|
Abhaya et al. [315] |
CrCoFeNi |
FCC |
1.5 MeV, Ni2+, 1 × 1015 (peak dose~2 dpa) & 5 × 1016 (peak dose~96 dpa) ions·cm−2, RT |
|
Sellami et al. [316] |
CoCrFeNi |
1.5 MeV, Ni2+, 1 × 1013–1 × 1014 ions·cm−2 21 MeV, Ni2+, 2 × 1013 & 1 × 1014 ions·cm−2, RT |
|
|
Chen et al. [317] |
CoCrFeNi |
FCC |
275 keV, He+, 5.14 × 1020 ions·m−2, 250, 300, 400 °C |
|
Kombaiah et al. [318] |
CoCrFeNi, Al0.12CoCrFeNi |
FCC |
3 MeV, Ni2+, 1 × 1017 ions·cm−2 (peak dose~100 dpa), 500 °C |
|
Lu et al. [319] |
CoCrFeNiPd |
FCC |
3 MeV, Ni2+, 5 × 1016 ions·cm−2, 580 °C |
|
Tunes et al. [320] |
CrFeNiMn |
FCC |
30 keV, Xe+, 2.6 × 1016 ions·cm−2, 500 °C |
|
Edmondson et al. [321] |
CrFeNiMn |
BCC |
30 keV, Xe+, 9.3×1016 ions·cm−2 6 keV He+, 6.4 × 1016 ions·cm−2, RT |
|
Fan et al. [322] |
CoCrFeNi |
FCC |
3 MeV, Ni ions, 5 × 1016–8 × 1016 ions/cm−2, 580 °C |
|
Chen et al. [81] |
CoCrFeNiTi0.2 |
FCC |
275 keV, He2+, 5.14 × 1020 ions·m−2, 400 °C |
|
Lyu et al. [323] |
CoCrFeNiMo0.2 |
FCC |
27 keV, electrons, -, RT |
|
Xu et al. [324] |
(CoCrFeNi)95Ti1Nb1Al3 |
FCC |
2.5 MeV, Fe ions, 1.5 × 1019 ions·m−2, RT-500 °C |
|
Cao et al. [325] |
(CoCrFeNi)94Ti2Al4 |
FCC |
4 MeV, Au ions, 10–49 dpa, RT |
|
Tolstolutskaya et al. [326] |
Cr0.18Fe0.4Mn0.28Ni0.14 Cr0.18Fe0.28Mn0.27Ni0.28 Cr0.2Fe0.4Mn0.2Ni0.2 |
FCC |
1.4 MeV, Ar ions, 0, 0.3, 1 & 5 dpa, RT |
|
Kumar et al. [327] |
Fe0.27Ni0.28Mn0.27Cr0.18 |
FCC |
3 MeV, Ni2+, 4.2 × 1013, 4.2 × 1014 & 4.2 × 1015 ions·cm−2, RT & 500 °C 3 MeV, Ni2+, 2.43 × 1015 & 2.43 × 1016 ions·cm−2, 400–700 °C |
|
Li et al. [328] |
Cr0.18Fe0.27Ni0.28Mn0.27 |
FCC |
Neutron, 8.9 × 1014 n·cm−2.s, 60 °C |
|
Voyevodin et al. [329] |
Cr0.2Fe0.4Mn0.2Ni0.2+ Y2O3 + ZrO2 |
FCC |
1.4 MeV, Ar ions, 2.2 × 1015 ions·cm−2, RT |
|
Dias et al. [330] |
CuxCrFeTiV (x = 0.21–1.7) |
BCC + FCC |
300 keV, Ar+, 3 × 1020 at·m−2, RT |
|
Yang et al. [298] |
Al0.3CoCrFeNi |
FCC |
3 MeV, Au ions, 6 × 1015 ion·cm−2 (peak dose ~31 dpa), 250–650 °C |
|
Gromov et al. [331] |
AlCoCrFeNi |
- |
18 keV, electrons, -, RT |
|
Zhang et al. [299] |
AlCrMoNbZr, (AlCrMoNbZr)N |
FCC |
400 keV, He+, 8 × 1015 & 8 × 1016 ion·cm−2, RT |
|
Yang et al. [82] |
Al0.1CoCrFeNi, Al0.75CoCrFeNi, Al1.5CoCrFeNi, |
FCC FCC + B2 A2 + B2 |
3 MeV, Au ions, 1 × 1014–1 × 1016 ions·cm−2, RT |
|
Xia et al. [83] |
Al0.1CoCrFeNi, Al0.75CoCrFeNi, Al1.5CoCrFeNi |
FCC FCC + B2 B2 + A2 |
3 MeV, Au ions, 1 × 1014–1 × 1016 ions·cm−2, RT |
|
Yang et al. [332] |
Al0.1CoCrFeNi |
FCC |
3 MeV, Au ions, 6 × 1015 ions·cm−2, 250–650 °C |
|
Zhou et al. [333] |
AlxCoCrFeNi (x = 0–2) |
FCC + BCC |
1 MeV, Kr2+, -, RT |
|
Zhou et al. [334] |
AlxCoCrFeNi, HfNbTaTiZrV |
FCC Amorphous |
MeV Kr & 200 KeV, electrons, 2 dpa, RT & 150 °C |
|
Zhou et al. [335] |
HfNbTaTiZrV |
BCC |
1 MeV Kr2+, -, RT-150 °C |
|
Moschetti et al. [336] |
HfNbTaTiZr |
BCC |
5 MeV, He2+, 1.6 × 1012–4.4 × 1017 ions·cm−2s, 50 °C |
|
Sadeghilaridjani et al. [337] |
HfTaTiZrV |
BCC |
4.4 MeV, Ni2+, 1.08 × 1017 ion·cm−2, RT |
|
Li et al. [338] |
HfNbTiZr |
BCC |
1.5 MeV, He ions, 5 × 1015–1 × 1017 ions·m−2, 700 °C |
|
Kareer et al. [339] |
TaTiVZr, TaTiVCr, TaTiVNb |
BCC BCC BCC |
2 MeV, V+, 2.26 × 1015 ions·cm−2, 500 °C |
|
Wang et al. [340] |
ZrTiHfCuBe, ZrTiHfCuBeNi, ZrTiHfCuNi |
Amorphous |
100 keV, He ions, 5.0 × 1017, 1.0 × 1018 & 2.0 × 1018 ions·cm−2, RT |
|
Lu et al. [80] |
Ti2ZrHfV0.5Mo0.2 |
BCC |
3 MeV, He+, 5 × 1015, 1 × 1016 & 3 × 1016 ions·cm−2, 600 °C |
|
Atwani et al. [341] |
W0.38Ta0.36Cr0.15V0.11 |
BCC |
1 MeV, Kr+2, 0.0006–8 dpa·s−1, 800 °C |
|
Komarov et al. [342] |
(TiHfZrVNb)N |
- |
500 KeV He2+, 5 × 1016–3 × 1017 ions·cm−2, 500 °C |
|
Gandy et al. [343] |
SiFeVCrMo SiFeVCr |
sigma BCC+ sigma |
5 MeV, Au2+, 5 × 1015 ions·cm−2, RT |
|
Patel et al. [344] |
V2.5Cr1.2WMoCo0.04 |
BCC |
5 MeV, Au+, 5 × 1015 ion·cm−2 (peak dose~42 dpa), RT |
|
Zhang et al. [345] |
Mo0.5NbTiVCr0.25, Mo0.5NbTiV0.5Zr0.25 |
BCC |
400 He2+, 1 × 1017–5 × 1017 ions·m−2, 350 °C |
|
Zhang et al. [346] |
Mo0.5NbTiVCr0.25, Mo0.5NbTiV0.5Zr0.25 |
BCC |
400 keV, He2+, peak dose~10.5 dpa, 350 °C |
|
Atwani et al. [347] |
WtaCrV |
BCC |
2 keV, He+, 1.65 × 1017 ions·cm−2, 950 °C |
3.2. Wear Behavior
|
Source |
Composition |
Microstructure |
Method, Medium, Antagonist Material, Temperature, Wear Rate |
|---|---|---|---|
|
Joseph et al. [355] |
CoCrFeNiMn |
FCC |
Pin-on-disc, dry, Al2O3, 600–800 °C, RT, 0.5 × 10−4–3.8 × 10−4 mm3·N−1·m−1 |
|
Wang et al. [356] |
CoCrFeNiMn |
FCC |
Ball-on-disc, MoS2-oil lubrication, GCr15, RT-140 °C |
|
Xiao et al. [357] |
CoCrFeNiMn |
FCC |
Ball-on-flat, dry, WC-Co, RT, 0.5 × 10−4–5.4 × 10−4 mm3·N−1·m−1 |
|
Jones et al. [358] |
CoCrFeNiMn |
FCC |
Rotary tribometer, -, -, ~0.5 × 10−6 mm3·N−1·m−1 |
|
Zhu et al. [359] |
CoCrFeNiMn CoCrFeNiMnV CoCrFeNiMnNb CoCrFeNiMnNbV |
FCC + HCP (Laves) + σ |
Ball-on-disc, dry, Si3N4, RT, 1.85 × 10−5–6.39 × 10−5 mm3·N−1·m−1 |
|
Deng et al. [360] |
CoCrFeNiMox (x = 0–0.3) |
FCC |
Ball-on-disc, dry, GCr15, RT, 0.33 × 10−3–0.53 × 10−3 mm3·N−1·m−1 |
|
Lindner et al. [361] |
CoCrFeNiMn CoCrFeNi |
FCC FCC |
Ball-on-disc, dry, Al2O3, RT |
|
Sha et al. [362] |
(CoCrFeNiMn)N |
FCC + BCC |
Ball-on-disc, dry, ruby, RT, 1 × 10−7–1.4 × 10−6 mm3·N−1·m−1 |
|
Xiao et al. [363] |
CoCrFeNiMnCx (x = 0–1.2) |
FCC |
Ball-on-disk, dry, Si3N4, RT, 0.47 × 10−5–6.5 × 10−5 mm3·N−1·m−1 |
|
Zhu et al. [277] |
CoCrFeNiMn + TiN-Al2O3 |
FCC + TiN |
Ball-on-disc, dry, 440C steel, RT |
|
Cheng et al. [364] |
CoCrFeNiMn Al0.5CoCrFeNiMn AlCoCrFeNiMn |
FCC FCC + BCC FCC + BCC |
Ball-on-disc, dry, Si3N4, RT-800 °C, 0.5 × 10−4–3.8 × 10−4 mm3·N−1·m−1 |
|
Joseph et al. [365] |
CoCrFeNiMn Al0.3CoCrFeNi Al0.6CoCrFeNi AlCoCrFeNi |
FCC FCC FCC + BCC BCC |
Pin-on-disc, dry, Al2O3, 25 & 900 °C |
|
Liu et al. [366] |
CoCrFeNiMn + Y2O3 |
FCC + Y2O3 (particles) |
Ball-on-disc, dry, GCr15, RT |
|
Wang et al. [367] |
(CoCrFeMnNi)85Ti15 |
FCC + BCC |
Ball-on-disc, dry, Si3N4, RT-800 °C, 4 × 10−6–2.23 × 10−5 mm3·N−1·m−1 |
|
Zhang et al. [368] |
CoCrFeNi + (Ag or BaF2/CaF2) |
FCC |
Ball-on-disk, dry, Inconel-718, RT, ~4 × 10−5–40 × 10−5 mm3·N−1·m−1 |
|
Geng et al. [369] |
CoCrFeNi |
FCC |
Pin-on-disc, vacuum (4 Pa) & air, Inconel 718, RT, 0.6 × 10−4–8 × 10−4 mm3·N−1·m−1 |
|
Zhang et al. [370] |
CoCrFeNi + (graphite or MoS2) |
FCC |
Ball-on-disk, dry, Si3N4, RT-800 °C, ~1 × 10−5–23 × 10−5 mm3·N−1·m−1 |
|
Zhou et al. [371] |
CoCrFeNiMo0.85 Al0.5CoCrFeNi |
FCC FCC |
Slurry jet test-rig, HCl+NaCl, -, 40 °C, - |
|
Zhang et al. [372] |
CoCrFeNiMo |
FCC |
Ball-on-disc, dry, -, RT |
|
Huang et al. [373] |
FeCoCrNiSix |
FCC + BCC |
Ball-on-disk, dry, GCr15, RT |
|
Cui et al. [374] |
CoCrFeNiMo Sulfurized at 260 °C for 2 h |
FCC + FeS/MoS2 film |
Pin-on-disk, dry, GCr15, RT |
|
Li et al. [375] |
CoCrFeNiMo0.2 |
FCC |
Ball on disc, dry, GCr15, RT, 3.9 × 10−4–5.4 × 10−4 mm3·N−1·m−1 |
|
Ji et al. [376] |
CoCrFeNiCu + 2% MoS2 CoCrFeNiCu + 5% MoS2 CoCrFeNiCu + 20% WC CoCrFeNiCu + 50% WC CoCrFeNiCu + 80% WC |
FCC + MoS2 (particles) FCC + MoS2 (particles) FCC + WC (particles) FCC + WC (particles) FCC + WC (particles) |
Ball-on-disk, dry, Si3N4, RT |
|
Verma et al. [377] |
CoCrFeNiCux (x = 0–1) |
FCC |
Pin-on-disk, dry, -, RT & 600 °C, ~1.3 × 10−5–2.5 × 10−5 mm3·N−1·m−1 |
|
Liu et al. [378] |
CoCrFeNiBx (x = 0.5–1.5) |
FCC + Borides |
Roller friction wear tester, dry, W18Cr5V, RT |
|
Jiang et al. [379] |
CoCrFeNiNbx (x = 0–1.2) |
FCC + HCP (Laves) HCP (Co2Nb) |
Ball-on-disc, dry, BN, RT |
|
Yu et al. [380] |
CoCrFeNiNbx (x = 0.5–0.8) |
FCC + HCP (Laves) |
Pin-on-disk, dry, Si3N4, RT-800 °C, ~1.8 × 10−4–9 × 10−4 mm3·N−1·m−1 |
|
Liu et al. [381] |
Co10Cr10Fe50Mn30 + graphene nanoplatelets (0.2–0.8 wt%) |
FCC |
Ball-on-plate, dry, GCr15, RT |
|
Wang et al. [382] |
Co10Cr10Fe40Mn40 + WC (10 wt%) |
FCC+ WC + M23C6 |
Ball-on-disc, dry, Si3N4, RT |
|
Derimow et al. [383] |
(CoCrCuTi)100−xMnx (x = 5–10) (CoCrCuTi)100−xMnx (x = 10–20) |
FCC + BCC FCC + HCP (Laves) |
Ball-on-disc, dry, GCr15, RT |
|
Guo et al. [384] |
CoCrFeNiCuSi0.2 (Ti or C)x (x = 0–1.5) |
FCC + TiC |
Brooks sliding friction & wear tester, dry, RT |
|
Zhang et al. [385] |
(CoCrFeNiTi0.5)Cx (x = 3–12 wt%) |
BCC + Cr23C6 + TiC |
ML-100 friction and wear tester, -, -, RT |
|
Erdoğan et al. [386] |
CoCrFeNiTi0.5 CoCrFeNiTi0.5Al0.5 CoCrFeNiTi0.5Al |
FCC BCC BCC |
Ball-on-disc, dry, WC, RT |
|
Liu et al. [387] |
CoCrFeNiMo CoCrFeNiMox (x ≥ 0.3) CoCrFeNiMox (x ≥ 1) |
FCC FCC + σ FCC + σ + µ |
Pin-on-disk, dry, YG6, RT, 1 × 10−5–8.5 × 10−5 mm3·N−1·m−1 |
|
Moazzen et al. [388] |
CoCrFexNi (x = 1–1.6) |
FCC + BCC |
Pin-on-disk, dry, AISI52100 steel, 20–30 °C, - |
|
Yang et al. [389] |
CoCrFeNiMoSix (x = 0.5–1.5) |
FCC |
Pin-on-disk, dry, Si3N4, RT, 0.292 × 10−4–0.892 × 10−4 mm3·N−1·m−1 |
|
Li et al. [390] |
CoCrFeNi2V0.5Tix (x = 0.5–1.25) |
BCC + (Co,Ni)Ti2 |
Ball-on-disc, dry, Si3N4, RT, 4.4 × 10−5- 37.5 × 10−5 mm3·N−1·m−1 |
|
Islak et al. [391] |
CrFeNiMoTi |
FCC |
Ball-on-flat, dry, 100Cr6, RT, 2.7 × 10−3–9.4 × 10−3 mm3·N−1·m−1 |
|
Wen et al. [392] |
CrCoNiTiV |
FCC + BCC + TiO |
HT-1000 tribometer, -, WC, RT & 600 °C |
|
Wang et al. [393] |
CuNiSiTiZr |
BCC |
CJS111A wear tester, dry, -, RT |
|
Cheng et al. [394] |
(Fe25Co25Ni25 (B0.7Si0.3)25)100−xNbx (x = 0–4 wt%) |
BCC + HCP (Laves) + FCC |
Ball-on-disc, dry, GCr15, RT, ~1.5 × 10−6–3.6 × 10−6 mm3·N−1·m−1 |
|
Yadav et al. [395] |
(CuCrFeTiZn)1−xPbx (x = 0.05–0.2) |
FCC + BCC + Pb (particles) |
Ball-on-disk, dry, -, SAE 52100, RT, 1.17 × 10−5–50 × 10−5 mm3·N−1·m−1 |
|
Gou et al. [396] |
CoCrFeNi + WC + Mo2C + NbC |
FCC |
Ball-on-disc, dry, GCr15, 700 °C |
|
Yadav et al. [397] |
(CuCrFeTiZn)100−xPbx (x = 0–10) (CuCrFeTiZn)100−xBix (x = 0–10) |
FCC + BCC BCC |
Ball-on-disk, dry, steel, RT |
|
Cui et al. [398] |
AlxCoCrFeNiMn (x = 0–0.75) |
FCC + BCC |
MDW- 02 abrasive wear tester, RT |
|
Gwalani et al. [399] |
Al0.5CoCrFeNi |
FCC + B2 |
Pin-on-disc, dry, Si3N4, RT, 1.8 × 10−5–11 × 10−5 mm3·N−1·m−1 |
|
Chen et al. [400] |
Al0.6CoCrFeNi |
FCC + BCC |
Ball-on-plate, dry, Si3N4, RT-600 °C, ~0.5 × 10−4–5 × 10−4 mm3·N−1·m−1 |
|
Du et al. [401] |
Al0.25CoCrFeNi |
FCC |
Universal wear testing machine, dry, Si3N4 20–600 °C, ~1.5 × 10−4–3.5 × 10−4 mm3·N−1·m−1 |
|
Chen et al. [402] |
Al0.6CoCrFeNi |
FCC + BCC |
Ball-on-block, deionized water & acid rain (pH = 2), seawater, GCr15, RT, 1.58 × 10−4–6.52 × 10−4 mm3·N−1·m−1 |
|
Ji et al. [403] |
Al3CoCrFeNi |
Jet erosion testing machine, water and 15 wt% SiO2 particles (350–600 mm), RT |
|
|
Haghdadi et al. [404] |
Al0.3CoCrFeNi AlCoCrFeNi |
FCC BCC |
Scratch testing, dry, -, RT |
|
Fang et al. [405] |
Al0.3CoCrFeNi |
FCC |
Pin-on-disc, dry, -, 900 °C |
|
Wu et al. [406] |
Al0.1CoCrFeNi |
FCC |
Ball-on-block, dry and deionized water, Si3N4, RT, ~0.2 × 10−4–1.86 × 10−4 mm3·N−1·m−1 |
|
Nair et al. [407] |
Al0.1CoCrFeNi AlCoCrFeNi Al3CoCrFeNi |
FCC FCC + BCC (B2) BCC (B2) + A2 + σ |
Ball-on-disc, dry, WC, RT |
|
Kumar et al. [408] |
Al0.4CoxCrFeNi (x = 0–1) |
- |
Pin-on-disc, demineralized water & (demineralized water + 3.5 wt% NaCl), EN-31, RT, 0.81 × 10−4–1.86 × 10−4 mm3·N−1·m−1 |
|
Mu et al. [409] |
AlCoCrFeNi |
BCC + FCC |
Ball-on disc, dry, Si3N4, RT |
|
Wu et al. [410] |
AlCoCrFeNi AlCoCrFeNiTi0.5 |
BCC |
Pin-on-disc, dry, Si3N4, RT |
|
Zhao et al. [411] |
Al0.8CoCrFeNi |
FCC + BCC |
Ball-on-disk, dry, deionized water + 0.5 wt% NaCl, RT, ~2 × 10−5–7.5 × 10−5 mm3·N−1·m−1 |
|
Kumar et al. [412] |
Al0.4CoxCrFeNi (x = 0–0.5) Al0.4CoxCrFeNi (x = 1) |
FCC + BCC FCC |
Pin-on-disk, engine oil (SAE Grade:20W-40), EN-31 steel, RT, 2.1 × 10−5–11 × 10−5 mm3·N−1·m−1 |
|
Li et al. [413] |
Al0.8CoCrFeNiCu0.5Six (x = 0–0.5) |
FCC + BCC1 + BCC2 |
-, -, CGr15, RT, 0.9 × 10−6–1.19 × 10−6 mm3·N−1·m−1 |
|
Li et al. [272] |
(AlCoCrFeNi)100-x (NbC)x (x = 0–30 wt%) |
FCC + BCC |
Reciprocating tester, dry, N4Si3, RT |
|
Kafexhiu et al. [414] |
AlCoCrFeNi2.1 |
BCC + FCC |
Ball-on-plate, dry, 100Cr6 steel, RT, 7 × 10−5–11 × 10−5 mm3·N−1·m−1 |
|
Miao et al. [415] |
AlCoCrFeNi2.1 |
FCC (L12) + BCC (B2) |
Ball-on-disk, dry, Al2O3/Si3N4/SiC/GCr15, RT-900 °C, ~1 × 10−4–4.2 × 10−4 mm3·N−1·m−1 |
|
Ye et al. [416] |
AlCoCrFeNi2.1 + TiC (0–15 wt%) |
FCC + B2 + TiC |
MM-200 wear testing machine, dry, -, RT |
|
Wang et al. [417] |
(AlCoCrFeNi)N |
BCC + nitrides (AlN,CrN,Fe4N) |
Ball-on block, dry, deionized water & acid rain (pH = 2), Si3N4, RT, 2.8 × 10−5–7 × 10−5 mm3·N−1·m−1 |
|
Liu et al. [418] |
AlCrCuFeNi2 |
Ball-on-block, dry, simulated rainwater & deionized water, Si3N4, RT, 2.163 × 10−3–0.23 × 10−3 mm3·N−1·m−1 |
|
|
Kong et al. [419] |
Al1.8CrCuFeNi2 |
BCC |
MMS-2A roller friction wear tester, dry, -, RT |
|
Malatji et al. [263] |
AlCrCuFeNi |
FCC + BCC |
Ball-on-disk, dry, SiC, RT |
|
Wang et al. [420] |
Al1.3CoCuFeNi2 |
FCC + BCC |
Ball-on block, dry, deionized water & acid rain (pH = 2), Si3N4, RT, 1 × 10−4–12 × 10−4 mm3·N−1·m−1 |
|
Xiao et al. [421] |
AlxCoCrFeNiSi (x = 0.5–1.5) |
FCC + BCC |
Ball-on-flat, distilled water, WC-12Co, RT, 6.7 × 10−6–5.5 × 10−5 mm3·N−1·m−1 |
|
Liu et al. [422] |
AlCoCrFeNiSix (0–0.5) |
BCC |
Pin-on-disk, dry, ZrO2, RT, 1.3 × 10−4–5.1 × 10−4 mm3·N−1·m−1 |
|
Hsu et al. [423] |
Al0.5CoCrFeNiCuBx (x = 0–1) |
FCC + boride precipitates |
Pin-on-disk, dry, Al2O3, RT |
|
Chen et al. [424] |
Al0.5CoCrFeNiCuTix (x = 0–0.2) Al0.5CoCrFeNiCuTix (x = 0.4–1) Al0.5CoCrFeNiCuTix (x = 1.2–2) |
FCC FCC + BCC FCC + BCC + Ti2N |
Pin-on-disk, dry, Al2O3, RT |
|
Lobel et al. [425] |
AlCoCrFeNiTi |
BCC |
Ball-on-disc, dry, Al2O3, RT |
|
Lobel et al. [426] |
AlCoCrFeNiTi |
BCC |
Ball-on-plate, dry, 100Cr6 Steel, RT |
|
Wu et al. [427] |
AlCoCrFeNiTix (x = 0.5–1) AlCoCrFeNiTix (x = 1.5) AlCoCrFeNiTix (x = 2) |
FCC + BCC FCC + BCC + Ti2Ni FCC + BCC + Ti2Ni + ordered BCC |
Cavitation erosion tests, Distilled water+ 3.5 wt% NaCl, RT |
|
Erdogan et al. [428] |
AlxCoCrFeNiTiy (x = 0–0.5, y = 0–0.5) |
FCC + BCC |
Ball-on-disc, dry, WC, RT, 0.25 × 10−4–1.78 × 10−4 mm3·N−1·m−1, 0.25 × 10−4–1.78 × 10−4 mm3·N−1·m−1 |
|
Xin et al. [429] |
Al0.2Co1.5CrFeNi1.5Ti0.5 + TiC |
FCC |
Ball-on-disc, dry, Si3N4, RT, 0.3 × 10−5–12.6 × 10−5 mm3·N−1·m−1 |
|
Gouvea et al. [430] |
Al0.2Co1.5CrFeNi1.5Ti |
FCC |
Ball-on-plate, dry, AISI 52,100 steel, RT, 1.6 × 10−8–7.5 × 10−5 mm2·N−1 |
|
Chuang et al. [431] |
AlxCo1.5CrFeNi1.5Tiy (x = 0–0.2, y = 0.5–1) |
FCC |
Pin-on-disk, dry, SKH51 steel, RT, ~4 × 10−4–1.8 × 10−4 mm3·N−1·m−1 |
|
Liu et al. [432] |
AlCoCrFeNiTi0.8 |
BCC + B2 |
Ball-on-disc, dry, Si3N4, RT, 1.36 × 10−6–6.96 × 10−6 mm3·N−1·m−1, 0.7 × 10−4–6 × 10−4 mm3·N−1·m−1 |
|
Yu et al. [433] |
AlCoCrFeNiTi0.5 |
BCC1 + BCC2 |
Pin-on-disk, H2O2, SiC & ZrO2, RT |
|
Lobel et al. [434] |
AlCoCrFeNiTi0.5 |
BCC (A2 + B2) |
SRV-Tribometer, dry, Al2O3, 22–900 °C |
|
Chen et al. [435] |
Al0.6CoCrFeNiTi |
BCC |
Pin-on-disc, Dry, Al2O3 RT-500 °C |
|
Yu et al. [436] |
AlCoCrFeNiTi0.5 AlCoCrFeNiCu |
Pin-on-disc, dry, Si3N4 |
|
|
Yu et al. [437] |
AlCoCrFeNiCu AlCoCrFeNiTi0.5 |
FCC + BCC1 BCC1 + BCC2 |
Pin-on-disk, H2O2, 1Cr18Ni9Ti steel & ZrO2/SiC ceramic, RT |
|
Jin et al. [438] |
AlCoFeNiCu |
FCC + BCC |
Ball-on-disk, dry, WC, 200–800 °C |
|
Zhu et al. [439] |
AlCoFeNiCu + TiC (10–30 wt%) |
FCC + BCC |
Ball-on-disk, dry, Si3N420–600 °C, ~0.1 × 10−5–6.5 × 10−5 mm3·N−1·m−1 |
|
Wu et al. [440] |
Al0.5CoCrFeNiCu Al1.0CoCrFeNiCu Al2.0CoCrFeNiCu |
FCC FCC + BCC BCC |
Pin-on-disk, dry, SKH-51 steel, RT |
|
Yan et al. [441] |
AlCoCrFeNiSi + Ti (C, N) |
BCC + FCC |
Ball-on-disc, dry, GCr15, RT, - |
|
Li et al. [442] |
AlCoCrFeNi + Ti (C,N) + TiB2 |
FCC |
Ball-on-disc, dry, WC-6Co, 200–800 °C, 2.69 × 10−5–8.66 × 0−5 mm3·N−1·m−1 |
|
Kumar et al. [443] |
AlCoCrCuFeNiSi0.3 AlCoCrCuFeNiSi0.6 |
FCC + BCC FCC + BCC + σ |
Pin-on-disk, dry, -, RT, - |
|
Xin et al. [444] |
Al0.2Co1.5CrFeNi1.5Ti0.5 |
FCC |
Pin-on-disk, dry, Si3N4, 25–800 °C, 1.21 × 10−5–6.7 × 10−5 mm3·N−1·m−1 |
|
Karakaş et al. [445] |
Al0.07Co1.26Cr1.80Fe1.42Mn1.35Ni1.1 |
FCC |
Ball-on-disc, 3.5%NaCl & 5%H2SO4, -, RT, 16.26 × 10−9–77.84 × 10−8 mm3·N−1·m−1 |
|
Xin et al. [446] |
Al0.2Co1.5CrFeNi1.5Ti (0.5+x) + Cx (x = 0) |
FCC |
Pin-on-disk, dry, Si3N4, 25–800 °C, 3.12 × 10−6–12.59 × 10−5 mm3·N−1·m−1 |
|
Zhao et al. [447] |
AlCrCoFeNiCTax (x = 0–1) |
BCC |
Pin-on-disk, 3.5%NaCl & air, Si3N4, RT, 1.67 × 10−6–2.22 × 10−5 mm3·N−1·m−1 |
|
Ghanbariha et al. [448] |
AlCoCrFeNi + ZrO2 |
FCC + BCC |
Pin-on-disk, dry, WC, RT, 1.11 × 10−3–2.52 × 10−3 mm3·N−1·m−1 |
|
Li et al. [449] |
AlxCrFeCoNiCu (x = 0–0.5) AlxCrFeCoNiCu (x = 0.5–2) |
FCC FCC + BCC |
-, dry, GCr15, RT, 6.64 × 10−7–2.26 × 10−4 mm3·N−1·m−1 |
|
Cai et al. [450] |
AlCrTiV, AlCrTiVSi |
BCC |
Nanoindenter G200, dry, CGr15 &Al2O3, RT, - |
|
Chandrakar et al. [451] |
AlCoCrCuFeNiSix (x = 0–0.9) |
BCC |
Pin-on-disk, dry, -, RT, - |
|
Erdogan et al. [452] |
AlCrFeNiSi AlCrFeNix (x = Cu,Co) |
BCC BCC + FCC |
Ball-on-disc, dry, WC, RT, - |
|
Duan et al. [453] |
AlCoCrFeNiCu |
- |
Pin-on-disc, H2O2, Si3N4, RT |
|
Chen et al. [454] |
Al0.5CoCrFeNiCuVx (x = 0–0.2) Al0.5CoCrFeNiCuVx (x = 0.4–0.8) Al0.5CoCrFeNiCuVx (x = 1–2) |
FCC FCC + BCC BCC |
Pin-on-disk, dry, Al2O3, RT, 1 × 10−4–2.7 × 10−4 mm3·N−1·m−1 |
|
Gu et al. [455] |
AlxMo0.5NbFeTiMn2 (x = 1–2) |
BCC |
Pin-on-disk, dry, Al2O3, RT |
|
Hsu et al. [456] |
AlCoCrFexNiMo0.5 (x = 0.6–2) |
BCC + σ |
Pin-on-disk, dry, SKH51 steel, RT |
|
Liang et al. [457] |
AlCrFe2Ni2W0.2Mo0.75 |
BCC |
Ball-on-disc, deionized water, Al2O3, RT, ~5 × 10−6–22 × 10−6 mm3·N−1·m−1 |
|
Qui et al. [458] |
Al2CoCrFeCuTiNix (x = 0–2) |
FCC + BCC |
Tribometer, -, -, RT |
|
Kanyane et al. [459] |
AlTiSiMoW |
BCC + TiSi2 (ordered FCC) |
Ball-on-disc, dry, stainless steel, RT |
|
Huang et al. [460] |
AlTiSiVCr |
BCC+ (Ti,V)5Si3 precipitates |
Ball-on-disc, dry, GCr15 steel, RT, 2 × 10−5–2.5 × 10−5 mm3·N−1·m−1 |
|
Zhang et al. [461] |
AlTiSiVNi |
B2 (NiAl) + (Ti,V)5Si3 + TiN |
Ball-on-disc, dry, Si3N4, RT & 800 °C |
|
Lin et al. [462] |
AlCoCrNiW AlCoCrNiSi |
W + AlNi + Cr15.58Fe7.42C6 BCC |
Pin-on-disc, dry, AISI 52100, RT |
|
Yadav et al. [463] |
AlCrFeMnV (AlCrFeMnV)90Bi10 (AlCrFeMnV)90Bi10 + 10 wt% TiB2 (AlCrFeMnV)90Bi10 + 15 wt% TiB2 |
BCC BCC + AlV3 + Bi BCC + AlV3 + Bi + TiB2 BCC + AlV3 + Bi + TiB2 |
Ball-on-disk, dry, SAE 52,100 steel, RT, 1.02 × 10−5–7.02 × 10−5 mm3·N−1·m−1 |
|
Bhardwaj et al. [464] |
AlTiZrNbHf |
BCC |
Pin-on-disk, dry, CGr15, RT, - |
|
Zhao et al. [465] |
AlNbTaZrx (x = 0.2–1) |
BCC + HCP |
Ball-on-disc, dry, Si3N4, RT, 1.85 × 10−4–2.41 × 10−4 mm3·N−1·m−1 |
|
Tuten et al. [466] |
TiZrHfNbTa |
Amorphous |
Ball-on-disc, dry, Al2O3, RT |
|
Pole et al. [467] |
TiZrHfTaV, TiZrTaVW |
BCC |
Ball-on-disk, dry, Si3N4, RT-500 °C, ~1 × 10−4–8 × 10−4 mm3·N−1·m−1 |
|
Ye et al. [468] |
TiZrHfNb |
BCC |
Nano-scratch, dry, diamond indenter, RT |
|
Pogrebnjak et al. [469] |
(TiZrHfNbV)N |
FCC |
Ball-on-disc, dry, Al2O3, 20 °C |
|
Gong et al. [470] |
TiZrHfBeCu TiZrHfBeNi Ti20Zr20Hf20Be20Cu10Ni10 Ti13.8Zr41.2Ni10Be22.5Cu12.5 |
Amorphous |
Nano-scratch, dry, diamond indenter, RT |
|
Zhao et al. [471] |
TiZrNiBeCu |
Amorphous |
Nano-scratch, dry, diamond indenter, RT |
|
Jhong et al. [472] |
(TiZrNbCrSi)Cx (x = 36.7–87.8 at.%) |
FCC |
Ball-on-disc, dry, 100Cr6 steel, RT, 0.2 × 10–3.3 × 10−6 mm3·N−1·m−1 |
|
Mathiou et al. [473] |
TiZrNbMoTa |
BCC + HCP |
Ball-on disc, dry, 100Cr6 steel, Al2O3, RT, 0.154 × 10−1–0.199 × 10−1 mm3·N−1·m−1 |
|
Petroglou et al. [474] |
MoTaxNbVTi (x = 0.25–1) |
BCC |
Ball-on-disk, dry, 100Cr6 steel, RT, 0.19 × 10−6–0.38 × 10−6 g·N−1·m−1 |
|
Poulia et al. [475] |
MoTaNbVW |
BCC |
Ball-on-disc, dry, 100Cr6 steel & Al2O3, RT |
|
Poulia et al. [476] |
MoTaNbVW |
BCC |
Ball-on-disc, dry, 100Cr6 steel & Al2O3, RT, 1.05 × 10−4–4.89 × 10−4 mm3·N−1·m−1 |
|
Poulia et al. [477] |
MoTaNbVTi |
BCC + hexagonal C14 Laves + cubic C15 laves |
Ball-on disc, dry, 100Cr6 steel, Al2O3, RT |
|
Alvi et al. [478] |
MoTaWVCu |
BCC |
Ball-on-disc, dry, E52100 steel & Si3N4, RT-600 °C, 2.3 × 10−2–5 × 10−2 mm3·N−1·m−1 |
|
Hua et al. [479] |
TixZrNbTaMo (x = 0.5–2) |
BCC |
HSR-2M tester, dry, Si3N4, RT, 2.22 × 10−7–2.42 × 10−7 mm3·N−1·m−1 |
|
Gu et al. [480] |
Ni1.5CrFeTi2.0.5Mox (x = 0–0.25) Ni1.5CrFeTi2.0.5Mox (x = 0.5–0.25) |
BCC BCC + FCC |
Ball-on-disc, dry, Al2O3, RT, 7.99 × 107–2.7 × 107 µm3 |
This entry is adapted from the peer-reviewed paper 10.3390/met11121980
