Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gerhard Sandmann | + 1578 word(s) | 1578 | 2022-03-04 13:05:49 | | | |
| 2 | Vicky Zhou | -3 word(s) | 1575 | 2022-03-14 12:19:43 | | | | |
| 3 | Vicky Zhou | -3 word(s) | 1575 | 2022-03-14 12:22:11 | | | | |
| 4 | Vicky Zhou | -3 word(s) | 1575 | 2022-03-14 12:23:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sandmann, G. Fungal Carotenoids. Encyclopedia. Available online: https://encyclopedia.pub/entry/20547 (accessed on 07 February 2026).
Sandmann G. Fungal Carotenoids. Encyclopedia. Available at: https://encyclopedia.pub/entry/20547. Accessed February 07, 2026.
Sandmann, Gerhard. "Fungal Carotenoids" Encyclopedia, https://encyclopedia.pub/entry/20547 (accessed February 07, 2026).
Sandmann, G. (2022, March 14). Fungal Carotenoids. In Encyclopedia. https://encyclopedia.pub/entry/20547
Sandmann, Gerhard. "Fungal Carotenoids." Encyclopedia. Web. 14 March, 2022.
Copy Citation
Carotenoids represent a class of pigmented terpenoids. They are distributed in all taxonomic groups of fungi. Most of the fungal carotenoids differ in their chemical structures to those from other organisms. The general function of carotenoids in heterotrophic organisms is protection as antioxidants against reactive oxygen species generated by photosensitized reactions. Furthermore, carotenoids are metabolized to apocarotenoids by oxidative cleavage.
carotenoid biosynthesis
reaction mechanisms
trisporic acids
1. Introduction
Carotenoids are terpenoid pigments of yellow, orange, and red color. They are synthesized in species from all taxa with the exception of animals [1]. In contrast to photoautotrophic organism, carotenoids are not essential for fungi and are therefore accumulated in much lower concentrations than by plants and algae. However, fungal carotenoids are different to those found elsewhere, offering a structural diversity. In order to increase the levels of commercially interesting carotenoid in different fungal species and to match the concentrations of other organisms, classical and metabolic engineering procedures were established, which will be referred to. Carotenoids are found in all fungal groups along with non-carotenogenic species [2][3]. A general feature of carotenoids is their function as antioxidants. They are able to inactivate oxygen radicals and to quench singlet oxygen [4]. This reactive oxygen species is generated by photosensitized reactions. Protection from cell death caused by light and UV radiation were demonstrated with Microbotryum violaceum (formerly Ustilago violacea) and Neurospora crassa [5][6]. In several fungi, the synthesis of carotenoids is photo-regulated [7]. This light-dependent up-regulation corroborates the importance of antioxidative carotenoids. Another substantial role of carotenoids is their participation in the mating of mucoraceous fungi [8]. More details on the biological functions of carotenoids can be found in reference [9].
First attempts to elucidate the biosynthesis pathway were with mucoraceous fungi. Radioactivity incorporated by feeding acetate and mevalonate, both 14C-labelled at different positions [10], were recovered at individual positions of the β-carotene molecule [11]. The resulting labelling pattern were the first indication leading to the understanding of the reaction sequences in carotenoid biosynthesis. The establishment of the pathway was further substantiated by in vitro incorporation of mevalonate along the carotenoid pathway [12] and with different pigment mutants [13].
2. Formation of Carotenoid Structures and Their Distribution in Fungi
Table 1 gives an overview on the distribution of carotenoids in different fungal groups. Most of the carotenoids of this table were only structurally analyzed and identified without the determination of their concentrations. Only for a few species, the concentrations of their specific carotenoids were quantified. Their values range from 100 to 200 µg/g dw or were even lower [14][15][16][17][18]. The accumulating carotenoid in lower fungi, in the Chytridiomycota and Blastocladiomycota, is the monocyclic γ-carotene in Table 1. Its synthesis was due to a modified lycopene cyclase which catalyzed formation of only one ionone ring instead of two, as in the case of β-carotene formation. The bicyclic β-carotene is typical for species of the Mucoraceae. It was also found as the end product in a few other fungi of Ascomycota and Basidiomycota, but most carotenoids in both phyla were oxygenated derivatives (Figure 1). These oxy carotenoids were derived from acyclic or cyclic carotenes with a polyene system of at least 11 double bonds, and were synthesized as illustrated in Figure 2. Apart from the pathways to β-carotene, neurosporaxanthin, and astaxanthin, participating genes have not been identified yet and the enzymes involved in their synthesis are not known. Nevertheless, conceivable reaction mechanisms for the insertion of oxy groups into carotenoid molecules can be anticipated in analogy to those identified in bacterial carotenogenesis.
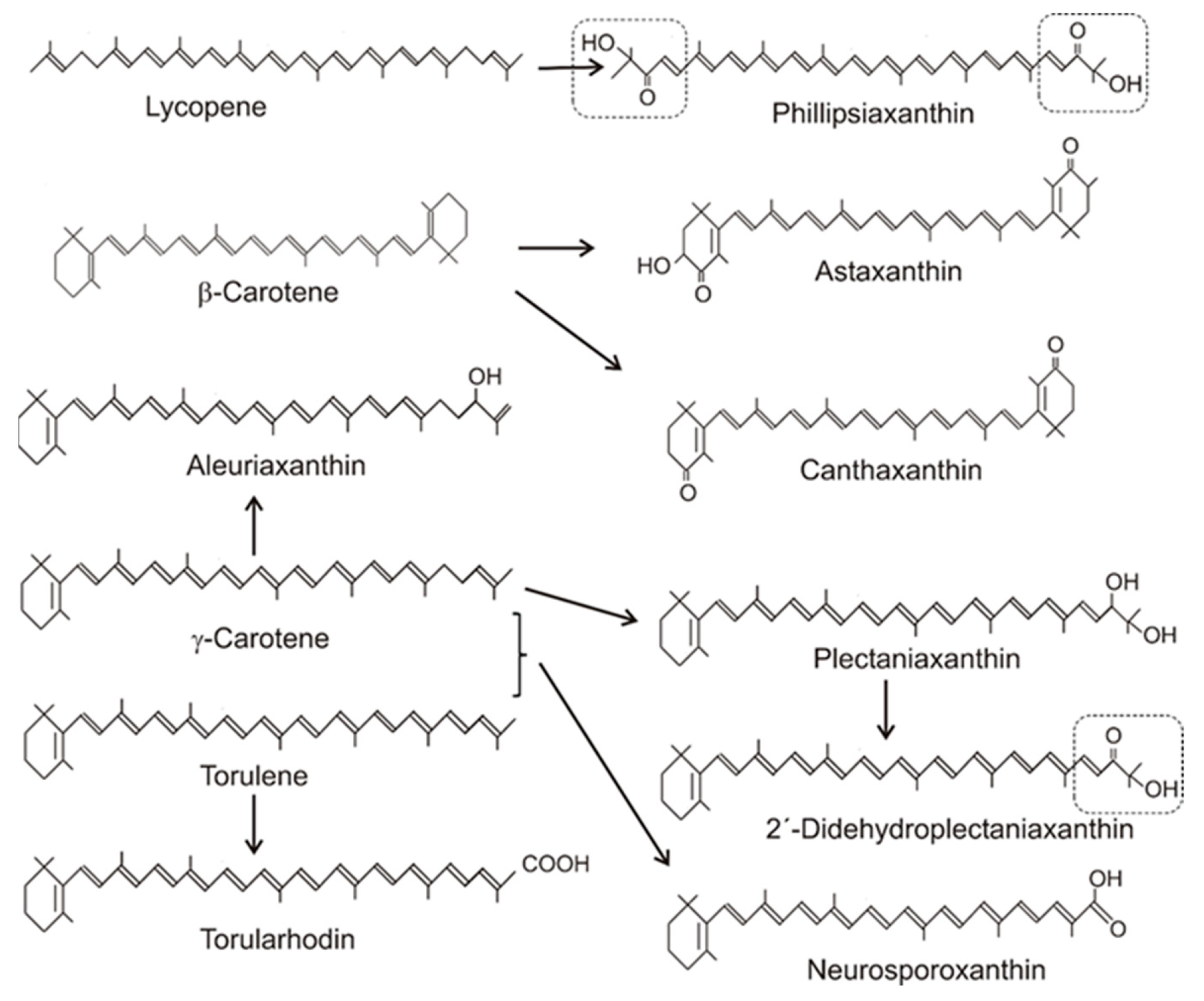 Figure 1. Precursors and structures of oxygenated fungal carotenoids. Identical 3′4′-didehydro-1′,2′-dihydro-1′-hydroxy-2′-one-ψ-end groups of different carotenoids are boxed.
Figure 1. Precursors and structures of oxygenated fungal carotenoids. Identical 3′4′-didehydro-1′,2′-dihydro-1′-hydroxy-2′-one-ψ-end groups of different carotenoids are boxed.Table 1. Fungal carotenoids and their direct carotene precursors in selected species.
| Carotenoids | Selected Species | References |
|---|---|---|
| Lycopene ---> γ-Carotene |
Chytridiomycota: | [2] |
| Cladochytrium replicatum | ||
| Blastocladiomycota | ||
| Allomyces arbuscula | ||
| Lycopene ---> β-Carotene |
Mucoromycotina: | [1] |
| Blakeslea trispora | ||
| Phycomyces blakesleanus | ||
| Ascomycota: | [19] | |
| Protomyces inundates | ||
| Basidiomycota: | [20] | |
| Tremella mesenterica | [21] | |
| Gymnosporangium juniperi-virginianae | ||
| Lycopene ---> Phillipsiaxanthin |
Ascomycota: | [2] |
| Phillipsia carminea | ||
| β-Carotene ---> Canthaxanthin |
Basidiomycota: | [22] |
| Cantharellus species | ||
| β-Carotene ---> Astaxanthin |
Basidiomycota: | [23] |
| Xanthophyllomyces dendrorhous | ||
| γ-Carotene ---> Aleuriaxanthin |
Ascomycota: | |
| Aleuria aurantiaca | [24] | |
| Scutellina umbrarum | [16] | |
| γ-Carotene and/or torulene ---> Neurosporaxanthin |
Ascomycota: | |
| Fusarium species | [25] | |
| Neurospora crassa | [26] | |
| γ-Carotene and/or torulene ---> Plectaniaxanthin and 2′-didehyroplectaniaxanthin |
Ascomycota: | |
| Plectania coccinea | [27] | |
| Sarcoscypha coccinea | [28] | |
| Basidiomycota: | ||
| Cryptococcus laurentii | [29] | |
| Dioszegia species | [17] | |
| Torulene ---> Torularhodin |
Ascomycota: | [30] |
| Cookeina sulcipes | ||
| Basidiomycota: | [31] | |
| Cystofilobasidium species | [18] | |
| Rhodotorula glutinis |
For structures, see Figure 1; for early steps, see Figure 2.
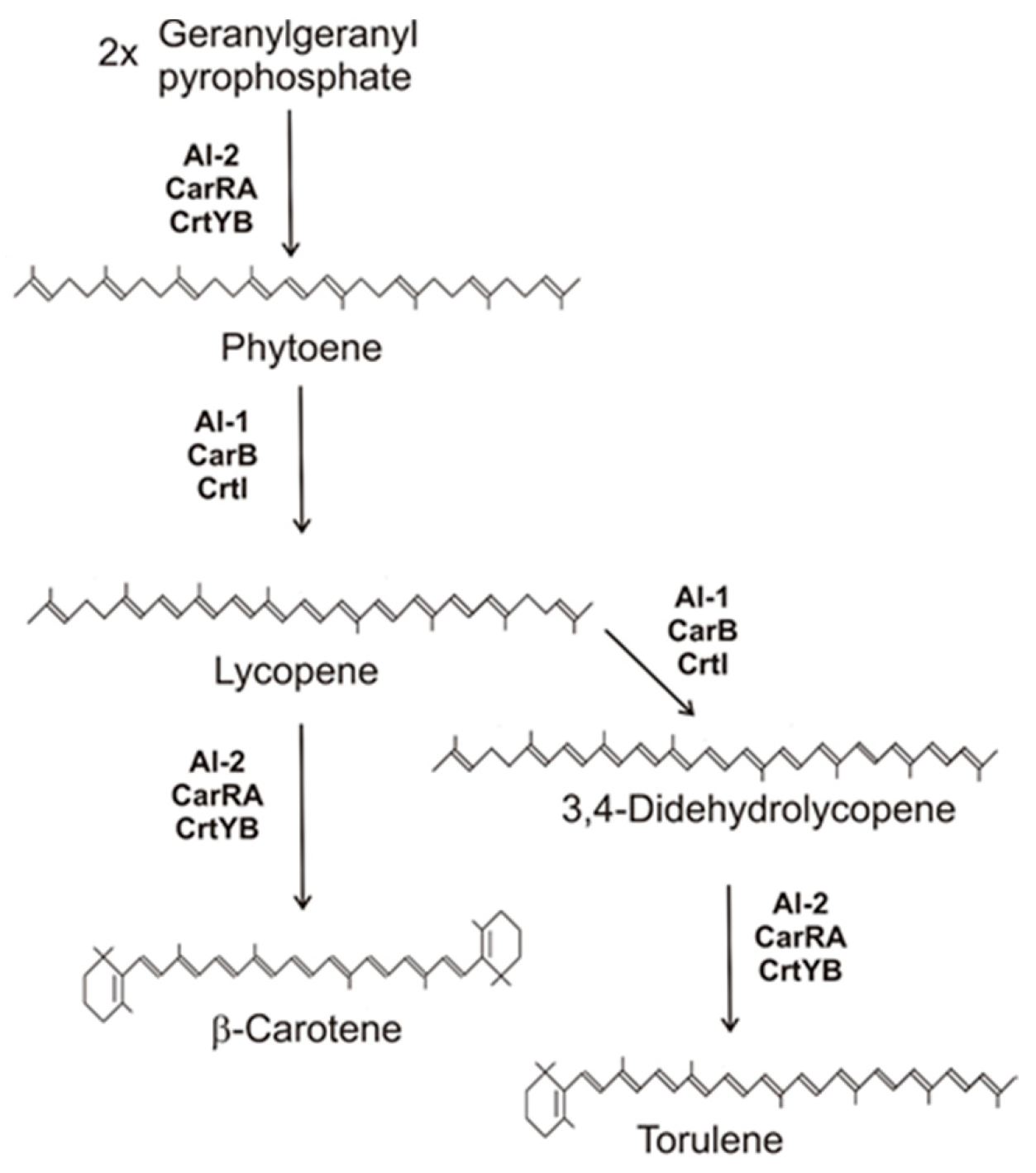 Figure 2. Carotenoid biosynthesis pathway of fungi to β-carotene and torulene with gene products involved.
Figure 2. Carotenoid biosynthesis pathway of fungi to β-carotene and torulene with gene products involved.3. Conversion of β-Carotene to Trisporic Acid in the Mucorales
Trisporic acids were first recognized by their stimulation of β-carotene synthesis in fungi from the order Mucorales. However, the significant function is the recognition of opposite mating types in heterothallic species for the initiation of zygospore formation. A comprehensive review on the early developments, the significance of trisporic acids, the role in communication between strains, their structures, and their origin from β-carotene as precursor can be found in reference [32]. Different trisporic acids and their precursors with slight chemical modifications have been identified since then [33]. The structures of the B and C types of trisporic acids first identified from B. trispora [34] are shown in Figure 3 with the exemplified biosynthesis pathway to trisporic acid B [8]. It starts from β-carotene by two successive cleavage reactions [35]. The products of the gene tsp3 from Rhizopus oryzae [36] or carS from P. blakesleeanus [37] cleave β-carotene at the C11′-C12′ double bond to β-apo-12′-carotenal in the initial reaction. The next cleavage step at the C13–C14 double bond by AcaA [37] leads to β-apo-13-carotenone, the C18 backbone of trisporoids. The following reactions to 4-dihydrotrisporin involve 4-hydroxylation and the saturation of the C11–C12 double bond (Figure 3A). For both reactions, the mechanisms have not been defined yet. The hydroxylation of the ionone ring may proceed via one of the steps described for 4-ketolation to canthaxanthin. A saturation reaction is very unusual for carotenoids or related compounds. This hydrogenation of C11-C12 shortens the polyene system and isolates the terminal keto group.
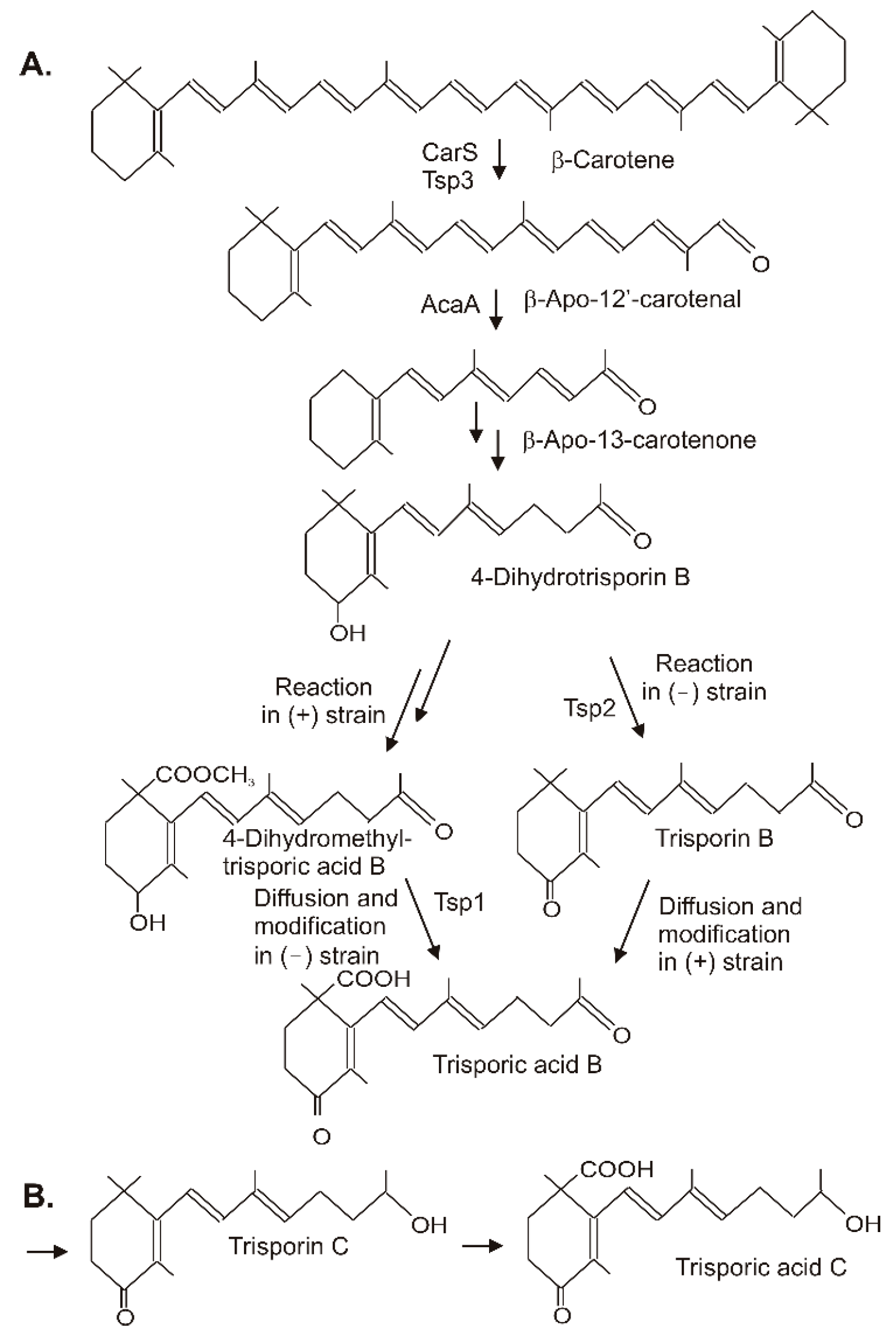 Figure 3. Synthesis of trisporic acids from β-carotene by interactions of different mating types in Mucoraceae. (A) Pathway to trisporic acid B; (B) Final step in trisporic acid C formation.
Figure 3. Synthesis of trisporic acids from β-carotene by interactions of different mating types in Mucoraceae. (A) Pathway to trisporic acid B; (B) Final step in trisporic acid C formation.The residual pathway continuing with the metabolization of 4-dihydrotrisporin relies on chemical interactions between the two mating partners [38]. Individual steps were catalyzed exclusively either by the (+) or the (−) strain. For pathway continuation, the reaction products from one strain must reach the other by diffusion. This metabolite exchange was necessary for the completion of the pathway to the trisporic acid end product. A 4-dihydrotrisporate dehydrogenase encoded by tsp2 was active in the (−) strain in the formation of trisporin [39]. It was upregulated in the (−) strain upon mating, but not in the (+) strain. Alternatively, the (+) strain exclusively converts 4-dihydrotrisporin to the C15 carboxylic acid, and further on to its methyl ester in B. trispora [40] (Figure 3). Obviously, this ester was transferred to the (−) mating partner where it was oxidized at C4 by NADP-dependent 4-dihydromethyltrisporate aldo/keto reductase, which was isolated from Mucor mucedo as the product of the tsp1 gene [41], and de-esterified to trisporic acid by a (−) strain-specific esterase [42]. In the (+) strain, trisporic acid finally accumulated from carboxylation of trisporin, which was taken up by diffusion from the (−) strain. The carboxylation reaction of the trisporins was not characterized. A possible mechanism may be similar to the carboxylation of a terminal C-atom in the synthesis of torularhodin. In the closely related pathway to trisporic acid D, C4 was further oxidized to a keto group, whereas the keto group at C13 was reduced (Figure 3B). The trisporic acids exert a positive feedback on their synthesis and on the carotenoid pathway by transcriptional up-regulation of the pathway genes [33].
4. Conclusions
Fungi are of biotechnological interest as biotechnological production platforms for commercially important metabolites. Furthermore, they have a high potential for genetic pathway engineering. In fungi, special carotenoids of unique structures exist. For some of them, the biosynthesis pathways have been established by mutant analysis and functionality studies of the pathway genes. However, for the majority of fungal carotenoids, very little is known about the reaction sequences, the enzymes, and the genes involved in their biosynthesis. By comparison to well-known bacterial carotenogenic reactions, mechanisms for the insertion of oxy groups into carotenoid molecules were proposed. They may give a clue on the expected types of genes involved in fungal carotenogenesis and may be helpful for their identification in the future.
Metabolic engineering of carotenoid pathway especially in yeasts, either carotenogenic or non-carotenogenic, has made considerable progress in recent years. Combination of mutagenesis, adaptive laboratory evolution of carotenogenic lines, genetic engineering of the central carbon metabolism to enhance acetyl-CoA supply, and modification of the triacylglycerol lipid pathway to improve carotenoid storage capacity led to the construction of high-yield carotenoid-producing strains. Apart from mass produced β-carotene with B. trispora which already made it into the market, astaxanthin from engineered X. dendrorhous, with a content of almost 1% of cell mass in non-optimized laboratory cultures [43], can compete with astaxanthin from Haematococcus species, especially as it is accumulating in a non-esterified form [44]. Metabolic engineering of Y. lipolytica [45], and particularly X. dendrorhous [46], resulted in synthesis of the rare carotenoid zeaxanthin matching the concentrations of the high-yield mutant of Dunaliella salina [47].
Microorganisms such as yeasts offer the advantage of a controlled fermentation process. Development of advancing conditions for customized fermentation and application of selected substrates can make these transgenic yeasts attractive for commercial utilization. This includes production of carotenoids by growing on agroindustrial waste materials. This recycling process may work with the original strains or with those further engineered for the utilization of a specific substrate.
References
- Goodwin, T.W. The Biochemistry of the Carotenoids, 2nd ed.; Chapman and Hall: London, UK; New York, NY, USA, 1980; Volumn I, Chapter 8; pp. 257–290.
- Valadon, L.R.G. Carotenoids as additional taxonomic characters in fungi: A review. Trans. Br. Mycol. Soc. 1976, 67, 1–15.
- Sandmann, G.; Misawa, N. Fungal carotenoids. In The Mycota X. Industrial Applications; Osiewacz, H.D., Ed.; Springer: Berlin, Germany, 2002; pp. 247–262.
- Sandmann, G. Antioxidant protection from UV- and light-stress related to carotenoid structures. Antioxidants 2019, 8, 219.
- Will, O.H., III; Jankowski, P.; Kowacs, A.; Rossing, W.; Schneider, P.; Newland, N.A. A comparison of photo-killing among carotene and cytochrome c accumulating strains of the smut fungus Ustilaga violacea at specific wavelengths from 400 to 600 nm. Photochem. Photobiol. 1987, 45, 609–615.
- Blanc, P.L.; Tuveson, R.W.; Sargent, M.L. Inactivation of carotenoid-producing and albino strains of Neurospora crassa by visible light, blacklight, and ultraviolet radiation. J. Bacteriol. 1976, 125, 616–625.
- Rau, W. Mechanism of photoregulation of carotenoid biosynthesis in plants. Pure Appl. Chem. 1985, 57, 777–784.
- Schimek, C.; Wöstemeyer, J. Carotene derivatives in sexual communication of zygomycete fungi. Phytochemistry 2009, 70, 1867–1875.
- Avalos, J.; Limón, M.C. Biological roles of fungal carotenoids. Curr. Genet. 2015, 61, 309–324.
- Braithwaite, G.D.; Goodwin, T.W. Studies in Carotenogenesis 26. The incorporation of acetate, mevalonate and 14CO2 into β-carotene by the fungus Phycomyces blakesleeanus. Biochem. J. 1960, 76, 5–10.
- Grob, C.; Butler, R. Über die Biosynthese des β-Carotins bei Mucor hiemalis Wehrner. Die Beteiligung der Essigsaure am Aufbau der Carotinmolekel, insbesondere in den Stellungen 3,4,6 bzw. 3’, 4’,6’, untersucht mit Hilfe von 14C-markierterEssigsäure. Helvet. Chim. Acta 1956, 39, 1775–1980.
- Lee, T.C.; Chichester, C.O. Geranylgeranyl pyrophosphate as the condensing unit for enzymatic synthesis of carotenes. Phytochemistry 1969, 8, 603–609.
- Bramley, P.M.; Davies, B. Carotene biosynthesis by cell extracts of mutants of Phycomyces blakesleeanus. Phytochemistry 1975, 14, 463–469.
- Almeida, E.R.A.; Cerdá-Olmedo, E. Gene expression in the regulation of carotene biosynthesis in Phycomyces. Curr. Genet. 2008, 53, 129–137.
- Sandmann, G.; Takaichi, S.; Fraser, P.D. Novel C35-apocarotenoids in the yellow mutant Neurospora crassa YLO. Phytochemistry 2008, 69, 2886–2890.
- Schrantz, J.P.; Lemoine, Y. Carotenoid composition of mycelium and apothecia in the discomycete Scutellinia umbrarum. Phytochemistry 1995, 40, 33–35.
- Madhour, A.; Anke, H.; Mucci, A.; Davoli, P.; Weber, R.W.S. Biosynthesis of the xanthophyll plectaniaxanthin as a stress response in the red yeast Dioszegia (Tremellales, Heterobasidiomycetes, Fungi). Phytochemistry 2005, 66, 2617–2626.
- Davoli, P.; Mierau, V.; Weber, R.W.S. Carotenoids and fatty acids in red yeasts Sporobolomyces roseus and Rhodotorula glutinis. Appl. Biochem. Microbiol. 2004, 40, 392–397.
- Valadon, L.R.G. Carotenoid pigments of Protomyces inundates, Dangerard. Phytochemistry 1963, 2, 71–73.
- Lederer, E. Sur les carotenoides des cryptogames. Bull. Soc. Chim. Fr. 1938, 20, 611–634.
- Smits, B.L.; Peterson, W.J. Carotenoids of telia galls of Gymnosporangium juniperi-virginianae LK. Science 1942, 96, 210–211.
- Haxo, F. Carotenoids of the mushroom Cantharellus cinnabarinus. Bot. Gaz. 1950, 112, 228–232.
- Andrewes, A.G.; Phaff, H.J.; Starr, M.P. Carotenoids of Phaffia rhodozyma, a red-pigmented fermenting yeast. Phytochemistry 1976, 15, 1003–1007.
- Arpin, N.; Kjφsen, H.; Francis, G.W.; Liaaen-Jensen, S. The structure of aleuriaxanthin. Phytochemistry 1973, 12, 2751–2758.
- Avalos, J.; Pardo-Medina, J.; Parra-Rivero, O.; Ruger-Herreros, M.; Rodríguez-Ortiz, R.; Hornero-Méndez, D.; Limón, M.C.J. Carotenoid biosynthesis in Fusarium. J. Fungi 2017, 3, 39.
- Goldie, A.H.; Subden, R.E. The neutral carotenoids of wild-type and mutant strains of Neurospora crassa. Biochem. Genet. 1973, 10, 275–284.
- Arpin, N.; Liaaen-Jensen, S. Recherches chimotaxinomiques sur les champignons. Fungal carotenoids. III-Nouveaux carotenoides, notamment sous forme d’esters tertiaires, isoles de Plectania coccinea (Scop. Ex Fr) Fuck. Phytochemistry 1967, 6, 995–1005.
- Molnár, P.; Ősz, E.; Turcsi, E.; Delia, J. Carotenoid composition of the mushroom Scarlet elf cup (Sarcoscypha coccinea). Heliyon 2019, 5, e01883.
- Bae, M.; Lee, T.H.; Yokoyama, H.; Böttger, H.G.; Chichester, C.O. The occurrence of plectaniaxanthin in Cryptococcus laurentii. Phytochemistry 1971, 10, 625–629.
- Arpin, N.; Liaaen-Jensen, S. Recherches chimiotaxinomiques sur le champignons. Sur la présence de l’ester méthylique de la torularhodine chez Cookeina sulcipes. C. R. Acad. Sci. Paris 1967, 265, 1083–1085.
- Herz, S.; Weber, R.W.S.; Anke, H.; Mucci, A.; Davoli, P. Intermediates in the oxidative pathway from torulene to torularhodin in the red yeasts Cystofilobasidium infirmominiatum and C. capitatum (Heterobasidiomycetes, Fungi). Phytochemistry 2007, 68, 2503–2511.
- Gooday, D.W.; Carlile, M.J. The discovery of fungal sex hormones. III. Trisporic acid and its precorsors. Mcologist 1997, 11, 126–130.
- Barreroa, A.F.; Herrador, M.M.; Arteaga, J.F.; González-Delgado, J.A. Occurrence and chemical synthesis of apocarotenoids from Mucorales: A review. Nat. Prod. Commun. 2017, 12, 733–741.
- Sutter, R.P.; Capage, D.A.; Harrison, T.L.; Keen, W.A. Trisporic acid biosynthesis in separate plus and minus cultures of Blakeslea trispora: Identification by Mucor assay of two mating-type-specific components. J. Bacteriol. 1973, 114, 1074–1082.
- Ahrazem, O.; Gómez-Gómez, L.; Rodrigo, M.R.; Avalos, J.; Limón, M.C. Carotenoid cleavage oxygenases from microbes and photosynthetic organisms: Features and functions. Int. J. Mol. Sci. 2016, 17, 1781.
- Burmester, A.; Richter, M.; Schultze, K.; Voelz, K.; Schachtschabel, D.; Boland, W.; Wöstemeyer, J.; Schimek, C. Cleavage of β-carotene as the first step in sexual hormone synthesis in zygomycetes is mediated by a trisporic acid regulated β-carotene oxygenase. Fungal Genet. Biol. 2007, 44, 1096–1108.
- Medina, H.R.; Cerdá-Olmedo, E.; Al-Babili, S. Cleavage oxygenases for the biosynthesis of trisporoids and other apocarotenoids in Phycomyces. Mol. Microbiol. 2011, 82, 199–208.
- Lee, S.C.; Heitman, J. Sex in the Mucoralean fungi. Mycoses 2014, 57, 18–24.
- Wetzel, J.; Scheibner, O.; Burmester, A.; Schimek, C.; Wöstemeyer, J. 4-Dihydrotrisporin-dehydrogenase, an enzyme of the sex hormone pathway of Mucor mucedo: Purification, cloning of the corresponding gene, and developmental expression. Eukaryot. Cell 2009, 8, 88–95.
- Schachtschabel, D.; David, A.; Menzel, K.-D.; Schimek, C.; Wöstemeyer, J.; Boland, W. Cooperative biosynthesis of trisporoids by the (+) and (-) mating types of the zygomycete Blakeslea trispora. ChemBioChem 2008, 9, 3004–3012.
- Czempinski, K.; Kruft, V.; Wöstemeyer, J.; Burmester, A. 4- Dihydromethyltrisporate dehydrogenase from Mucor mucedo, an enzyme of the sexual hormone pathway: Purification, and cloning of the corresponding gene. Microbiology 1966, 142, 2647–2654.
- Werkman, B. Localization and partial characterization of a sex-specific enzyme in homothallic and heterothallic Mucorales. Arch. Microbiol. 1976, 109, 209–213.
- Gassel, S.; Breitenbach, J.; Sandmann, G. Genetic engineering of the complete carotenoid pathway towards enhanced astaxanthin formation in Xanthophyllomyces dendrorhous starting from a high-yield mutant. Appl. Microbiol. Biotechnol. 2014, 98, 345–350.
- Sandmann, G. Carotenoids of biotechnological importance. In Biotechnology of Isoprenoids, Advances in Biochemical Engineering/Biotechnology; Schrader, J., Bohlmann, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 148, pp. 449–467.
- Xie, Y.; Chen, S.; Xiong, X. Metabolic engineering of non-carotenoid-producing yeast Yarrowia lipolytica for the biosynthesis of zeaxanthin. Front. Microbiol. 2021, 12, 699235.
- Breitenbach, J.; Pollmann, H.; Sandmann, G. Genetic modification of the carotenoid pathway in the red yeast Xanthophyllomyces dendrorhous: Engineering of a high-yield zeaxanthin strain. J. Biotechnol. 2019, 289, 112–117.
- Jin, E.; Feth, B.; Melis, A. A mutant of the green alga Dunaliella salina constitutively accumulates zeaxanthin under all growth conditions. Biotechnol. Bioeng. Symp. 2003, 81, 115–124.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
4 times
(View History)
Update Date:
14 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




