
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kentaro Hanada | + 1378 word(s) | 1378 | 2022-03-04 12:07:58 | | | |
| 2 | Vicky Zhou | -1 word(s) | 1377 | 2022-03-14 11:18:27 | | | | |
| 3 | Vicky Zhou | -3 word(s) | 1375 | 2022-03-14 11:28:25 | | |
Video Upload Options
Lipid transfer proteins (LTPs) are recognized as key players in the inter-organelle trafficking of lipids and are rapidly gaining attention as a novel molecular target for medicinal products. In mammalian cells, ceramide is newly synthesized in the endoplasmic reticulum (ER) and converted to sphingomyelin in the trans-Golgi regions. The ceramide transport protein CERT, a typical LTP, mediates the ER-to-Golgi transport of ceramide at an ER-distal Golgi membrane contact zone. A potent inhibitor of CERT, named (1R,3S)-HPA-12, was found by coincidence among ceramide analogs. Since then, various ceramide-resembling compounds have been found to act as CERT inhibitors. Nevertheless, the inevitable issue remains that natural ligand-mimetic compounds might directly bind both to the desired target and to various undesired targets that share the same natural ligand. To resolve this issue, a ceramide-unrelated compound named E16A, or (1S,2R)-HPCB-5, that potently inhibits the function of CERT has been developed, employing a series of in silico docking simulations, efficient chemical synthesis, quantitative affinity analysis, protein–ligand co-crystallography, and various in vivo assays. (1R,3S)-HPA-12 and E16A together provide a robust tool to discriminate on-target effects on CERT from off-target effects.
1. Introduction
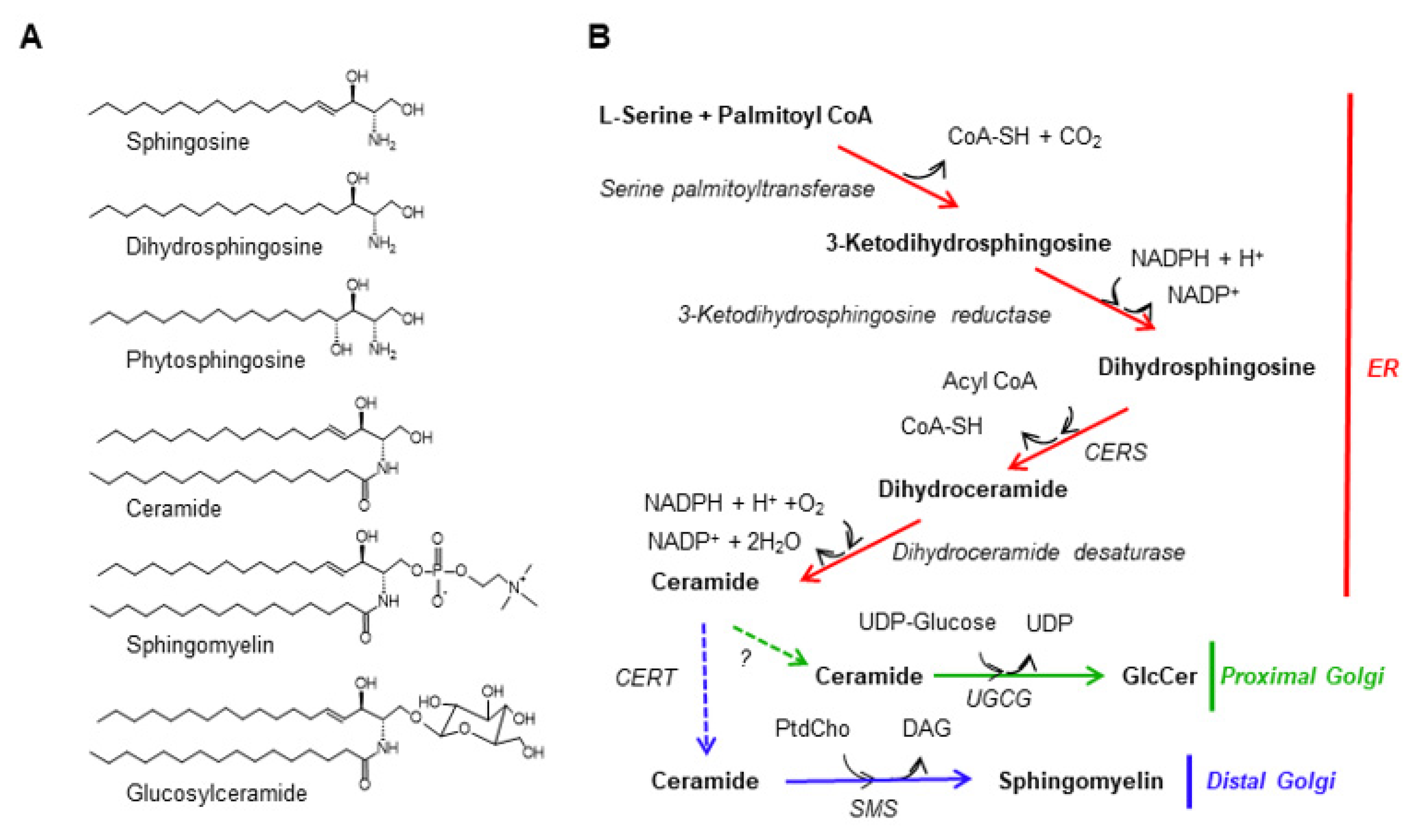 Figure 1. The de novo synthetic pathway for sphingomyelin. (A) Structure of mammalian sphingolipids. The chemical structures of three major sphingoid bases and several complex sphingolipids in mammalian cells are shown. Ceramide, sphingomyelin, and GlcCer are depicted as the N-palmitoyl sphingosine type, although there are various combinations of sphingoid bases and N-acyl chains in cells. (B) The de novo synthetic pathways for sphingomyelin and GlcCer in mammalian cells are shown. Solid arrows represent enzymatic reactions while dotted arrows represent inter-organelle transport processes. PtdCho, phosphatidylcholine.
Figure 1. The de novo synthetic pathway for sphingomyelin. (A) Structure of mammalian sphingolipids. The chemical structures of three major sphingoid bases and several complex sphingolipids in mammalian cells are shown. Ceramide, sphingomyelin, and GlcCer are depicted as the N-palmitoyl sphingosine type, although there are various combinations of sphingoid bases and N-acyl chains in cells. (B) The de novo synthetic pathways for sphingomyelin and GlcCer in mammalian cells are shown. Solid arrows represent enzymatic reactions while dotted arrows represent inter-organelle transport processes. PtdCho, phosphatidylcholine.2. Possible Applications of CERT Inhibitors
References
- Hanada, K. Lipid transfer proteins rectify inter-organelle flux and accurately deliver lipids at membrane contact sites. J. Lipid Res. 2018, 59, 1341–1366. Correction in. J. Lipid Res. 2018, 59, 2034.
- Holthuis, J.C.; Menon, A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature 2014, 510, 48–57.
- Wong, L.H.; Gatta, A.T.; Levine, T.P. Lipid transfer proteins: The lipid commute via shuttles, bridges and tubes. Nat. Rev. Mol. Cell Biol. 2019, 20, 85–101.
- Bohnert, M. Tether Me, Tether me not—Dynamic organelle contact sites in metabolic rewiring. Dev. Cell 2020, 54, 212–225.
- Reinisch, K.M.; Prinz, W.A. Mechanisms of nonvesicular lipid transport. J. Cell Biol. 2021, 220, e202012058.
- Cockcroft, S. Mammalian lipids: Structure, synthesis and function. Essays Biochem. 2021, 65, 813–845.
- Arita, M. Phosphatidylinositol-4 kinase III beta and oxysterol-binding protein accumulate unesterified cholesterol on poliovirus-induced membrane structure. Microbiol. Immunol. 2014, 58, 239–256.
- Bley, H.; Schöbel, A.; Herker, E. Whole Lotta Lipids—From HCV RNA Replication to the Mature Viral Particle. Int. J. Mol. Sci. 2020, 21, 2888.
- Derre, I.; Swiss, R.; Agaisse, H. The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites. PLoS Pathog. 2011, 7, e1002092.
- Kumagai, K.; Elwell, C.A.; Ando, S.; Engel, J.N.; Hanada, K. Both the N- and C- terminal regions of the Chlamydial inclusion protein D (IncD) are required for interaction with the pleckstrin homology domain of the ceramide transport protein CERT. Biochem. Biophys. Res. Commun. 2018, 505, 1070–1076.
- Hanada, K. Co-evolution of sphingomyelin and the ceramide transport protein CERT. Biochim. Biophys. Acta 2014, 1841, 704–719. Correction in. Biochim. Biophys. Acta 2014, 1841, 1561–1562.
- Harrison, P.J.; Dunn, T.M.; Campopiano, D.J. Sphingolipid biosynthesis in man and microbes. Nat. Prod. Rep. 2018, 35, 921–954.
- Olea-Ozuna, R.J.; Poggio, S.; Quiroz-Rocha, E.; García-Soriano, D.A.; Sahonero-Canavesi, D.X.; Padilla-Gómez, J.; Martínez-Aguilar, L.; López-Lara, I.M.; Thomas-Oates, J.; Geiger, O. Five structural genes required for ceramide synthesis in Caulobacter and for bacterial survival. Environ. Microbiol. 2021, 23, 143–159.
- Stankeviciute, G.; Tang, P.; Ashley, B.; Chamberlain, J.D.; Hansen, M.E.; Coleman, A.; D’emilia, R.; Fu, L.; Mohan, E.C.; Nguyen, H. Convergent evolution of bacterial ceramide synthesis. Nat. Chem. Biol. 2021, 1–8.
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191.
- Dunn, T.M.; Tifft, C.J.; Proia, R.L. A perilous path: The inborn errors of sphingolipid metabolism. J. Lipid Res. 2019, 60, 475–483.
- Keenan, T.W.; Morre, D.J. Phospholipid class and fatty acid composition of Golgi apparatus isolated from rat liver and comparison with other cell fractions. Biochemistry 1970, 9, 19–25.
- Yachi, R.; Uchida, Y.; Balakrishna, B.H.; Anderluh, G.; Kobayashi, T.; Taguchi, T.; Arai, H. Subcellular localization of sphingomyelin revealed by two toxin-based probes in mammalian cells. Genes Cells 2012, 17, 720–727.
- Abe, M.; Makino, A.; Murate, M.; Hullin-Matsuda, F.; Yanagawa, M.; Sako, Y.; Kobayashi, T. PMP2/FABP8 induces PI (4, 5) P2-dependent transbilayer reorganization of sphingomyelin in the plasma membrane. Cell Rep. 2021, 37, 109935.
- Wang, H.-Y.; Bharti, D.; Levental, I. Membrane heterogeneity beyond the plasma membrane. Front. Cell Dev. Biol. 2020, 8, 580814.
- Sviridov, D.; Miller, Y.I. Biology of Lipid Rafts: Introduction to the Thematic Review Series. J. Lipid Res. 2020, 61, 598–600.
- Turpin-Nolan, S.M.; Brüning, J.C. The role of ceramides in metabolic disorders: When size and localization matters. Nat. Rev. Endocrinol. 2020, 16, 224–233.
- Tallima, H.; Azzazy, H.M.; El Ridi, R. Cell surface sphingomyelin: Key role in cancer initiation, progression, and immune evasion. Lipids Health Dis. 2021, 20, 150.
- Taniguchi, M.; Okazaki, T. Role of ceramide/sphingomyelin (SM) balance regulated through “SM cycle” in cancer. Cell. Signal. 2021, 87, 110119.
- Hanada, K.; Kumagai, K.; Yasuda, S.; Miura, Y.; Kawano, M.; Fukasawa, M.; Nishijima, M. Molecular machinery for non-vesicular trafficking of ceramide. Nature 2003, 426, 803–809.
- Ueno, M.; Kitagawa, H.; Ishitani, H.; Yasuda, S.; Hanada, K.; Kobayashi, S. Catalytic enantioselective synthesis of a novel inhibitor of ceramide trafficking,(1R, 3R)-N-(3-hydroxy-1-hydroxymethyl-3-phenylpropyl) dodecanamide (HPA-12). Tetrahedron Lett. 2001, 42, 7863–7865.
- Kumagai, K.; Yasuda, S.; Okemoto, K.; Nishijima, M.; Kobayashi, S.; Hanada, K. CERT mediates intermembrane transfer of various molecular species of ceramides. J. Biol. Chem. 2005, 280, 6488–6495.
- Nakao, N.; Ueno, M.; Sakai, S.; Egawa, D.; Hanzawa, H.; Kawasaki, S.; Kumagai, K.; Suzuki, M.; Kobayashi, S.; Hanada, K. Natural ligand-nonmimetic inhibitors of the lipid-transfer protein CERT. Commun. Chem. 2019, 2, 20.
- Swanton, C.; Marani, M.; Pardo, O.; Warne, P.H.; Kelly, G.; Sahai, E.; Elustondo, F.; Chang, J.; Temple, J.; Ahmed, A.A.; et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell 2007, 11, 498–512.
- Heering, J.; Weis, N.; Holeiter, M.; Neugart, F.; Staebler, A.; Fehm, T.N.; Bischoff, A.; Schiller, J.; Duss, S.; Schmid, S.; et al. Loss of the ceramide transfer protein augments EGF receptor signaling in breast cancer. Cancer Res. 2012, 72, 2855–2866.
- Guo, J.; Zhu, J.X.; Deng, X.H.; Hu, X.H.; Zhao, J.; Sun, Y.J.; Han, X. Palmitate-induced inhibition of insulin gene expression in rat islet beta-cells involves the ceramide transport protein. Cell. Physiol. Biochem. 2010, 26, 717–728.
- Gjoni, E.; Brioschi, L.; Cinque, A.; Coant, N.; Islam, M.N.; Ng, C.K.; Verderio, C.; Magnan, C.; Riboni, L.; Viani, P.; et al. Glucolipotoxicity impairs ceramide flow from the endoplasmic reticulum to the Golgi apparatus in INS-1 beta-cells. PLoS ONE 2014, 9, e110875.
- Bandet, C.L.; Mahfouz, R.; Veret, J.; Sotiropoulos, A.; Poirier, M.; Giussani, P.; Campana, M.; Philippe, E.; Blachnio-Zabielska, A.; Ballaire, R.; et al. Ceramide Transporter CERT Is Involved in Muscle Insulin Signaling Defects Under Lipotoxic Conditions. Diabetes 2018, 67, 1258–1271.
- Revert-Ros, F.; López-Pascual, E.; Granero-Moltó, F.; Macías, J.; Breyer, R.; Zent, R.; Hudson, B.G.; Saadeddin, A.; Revert, F.; Blasco, R. Goodpasture antigen-binding protein (GPBP) directs myofibril formation: Identification of intracellular downstream effector 130-kDa GPBP-interacting protein (GIP130). J. Biol. Chem. 2011, 286, 35030–35043.
- Revert, F.; Merino, R.; Monteagudo, C.; Macias, J.; Peydró, A.; Alcácer, J.; Muniesa, P.; Marquina, R.; Blanco, M.; Iglesias, M. Increased Goodpasture antigen-binding protein expression induces type IV collagen disorganization and deposit of immunoglobulin A in glomerular basement membrane. Am. J. Pathol. 2007, 171, 1419–1430.
- Hernandez-Tiedra, S.; Fabrias, G.; Davila, D.; Salanueva, I.J.; Casas, J.; Montes, L.R.; Anton, Z.; Garcia-Taboada, E.; Salazar-Roa, M.; Lorente, M.; et al. Dihydroceramide accumulation mediates cytotoxic autophagy of cancer cells via autolysosome destabilization. Autophagy 2016, 12, 2213–2229.
- Wang, X.; Rao, R.P.; Kosakowska-Cholody, T.; Masood, M.A.; Southon, E.; Zhang, H.; Berthet, C.; Nagashim, K.; Veenstra, T.K.; Tessarollo, L.; et al. Mitochondrial degeneration and not apoptosis is the primary cause of embryonic lethality in ceramide transfer protein mutant mice. J. Cell Biol. 2009, 184, 143–158.
- Rao, R.P.; Scheffer, L.; Srideshikan, S.M.; Parthibane, V.; Kosakowska-Cholody, T.; Masood, M.A.; Nagashima, K.; Gudla, P.; Lockett, S.; Acharya, U.; et al. Ceramide transfer protein deficiency compromises organelle function and leads to senescence in primary cells. PLoS ONE 2014, 9, e92142.
- Chung, L.H.; Liu, D.; Liu, X.T.; Qi, Y. Ceramide Transfer Protein (CERT): An Overlooked Molecular Player in Cancer. Int. J. Mol. Sci. 2021, 22, 13184.
- de Ligt, J.; Willemsen, M.H.; van Bon, B.W.; Kleefstra, T.; Yntema, H.G.; Kroes, T.; Vulto-van Silfhout, A.T.; Koolen, D.A.; de Vries, P.; Gilissen, C.; et al. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 2012, 367, 1921–1929.
- Hamdan, F.F.; Srour, M.; Capo-Chichi, J.M.; Daoud, H.; Nassif, C.; Patry, L.; Massicotte, C.; Ambalavanan, A.; Spiegelman, D.; Diallo, O.; et al. De novo mutations in moderate or severe intellectual disability. PLoS Genet. 2014, 10, e1004772.
- The Deciphering Developmental Disorders Study. Large-scale discovery of novel genetic causes of developmental disorders. Nature 2015, 519, 223–228.
- Lelieveld, S.H.; Wiel, L.; Venselaar, H.; Pfundt, R.; Vriend, G.; Veltman, J.A.; Brunner, H.G.; Vissers, L.; Gilissen, C. Spatial Clustering of de Novo Missense Mutations Identifies Candidate Neurodevelopmental Disorder-Associated Genes. Am. J. Hum. Genet. 2017, 101, 478–484.
- Murakami, H.; Tamura, N.; Enomoto, Y.; Shimasaki, K.; Kurosawa, K.; Hanada, K. Intellectual disability-associated gain-of-function mutations in CERT1 that encodes the ceramide transport protein CERT. PLoS ONE 2020, 15, e0243980.
- Tamura, N.; Sakai, S.; Martorell, L.; Colome, R.; Mizuike, A.; Goto, A.; Ortigoza-Escobar, J.D.; Hanada, K. Intellectual-disability-associated mutations in the ceramide transport protein gene CERT1 lead to aberrant function and subcellular distribution. J. Biol. Chem. 2021, 297, 101338.
- Revert, F.; Ventura, I.; Martinez-Martinez, P.; Granero-Molto, F.; Revert-Ros, F.; Macias, J.; Saus, J. Goodpasture antigen-binding protein is a soluble exportable protein that interacts with type IV collagen. Identification of novel membrane-bound isoforms. J. Biol. Chem. 2008, 283, 30246–30255.
- Raya, A.; Revert, F.; Navarro, S.; Saus, J. Characterization of a novel type of serine/threonine kinase that specifically phosphorylates the human goodpasture antigen. J. Biol. Chem. 1999, 274, 12642–12649.
- Mencarelli, C.; Bode, G.H.; Losen, M.; Kulharia, M.; Molenaar, P.C.; Veerhuis, R.; Steinbusch, H.W.; De Baets, M.H.; Nicolaes, G.A.; Martinez-Martinez, P. Goodpasture antigen-binding protein/ceramide transporter binds to human serum amyloid P-component and is present in brain amyloid plaques. J. Biol. Chem. 2012, 287, 14897–14911.
- Bode, G.H.; Losen, M.; Buurman, W.A.; Veerhuis, R.; Molenaar, P.C.; Steinbusch, H.W.; De Baets, M.H.; Daha, M.R.; Martinez-Martinez, P. Complement activation by ceramide transporter proteins. J. Immunol. 2014, 192, 1154–1161.
- Crivelli, S.M.; Luo, Q.; Stevens, J.A.A.; Giovagnoni, C.; van Kruining, D.; Bode, G.; den Hoedt, S.; Hobo, B.; Scheithauer, A.L.; Walter, J.; et al. CERTL reduces C16 ceramide, amyloid-beta levels, and inflammation in a model of Alzheimer’s disease. Alzheimers Res. Ther. 2021, 13, 45.
- Aizaki, H.; Morikawa, K.; Fukasawa, M.; Hara, H.; Inoue, Y.; Tani, H.; Saito, K.; Nishijima, M.; Hanada, K.; Matsuura, Y.; et al. Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection. J. Virol. 2008, 82, 5715–5724.
- Amako, Y.; Syed, G.H.; Siddiqui, A. Protein kinase D negatively regulates hepatitis C virus secretion through phosphorylation of oxysterol-binding protein and ceramide transfer protein. J. Biol. Chem. 2011, 286, 11265–11274.
- Gewaid, H.; Aoyagi, H.; Arita, M.; Watashi, K.; Suzuki, R.; Sakai, S.; Kumagai, K.; Yamaji, T.; Fukasawa, M.; Kato, F. Sphingomyelin is essential for the structure and function of the double-membrane vesicles in hepatitis C virus RNA replication factories. J. Virol. 2020, 94, e01080-20.
- Otsuki, N.; Sakata, M.; Saito, K.; Okamoto, K.; Mori, Y.; Hanada, K.; Takeda, M. Both sphingomyelin and cholesterol in the host cell membrane are essential for Rubella virus entry. J. Virol. 2017, 92, e01130-17.
- Elwell, C.A.; Jiang, S.; Kim, J.H.; Lee, A.; Wittmann, T.; Hanada, K.; Melancon, P.; Engel, J.N. Chlamydia trachomatis co-opts GBF1 and CERT to acquire host sphingomyelin for distinct roles during intracellular development. PLoS Pathog. 2011, 7, e1002198. Correction in. PLoS Pathog. 2013, 9.
- Tachida, Y.; Kumagai, K.; Sakai, S.; Ando, S.; Yamaji, T.; Hanada, K. Chlamydia trachomatis-infected human cells convert ceramide to sphingomyelin without sphingomyelin synthases 1 and 2. FEBS Lett. 2020, 594, 519–529.




