
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | J. Douglas Bremner | + 2299 word(s) | 2299 | 2020-09-16 11:23:54 | | | |
| 2 | Vivi Li | -174 word(s) | 2125 | 2020-09-17 10:27:33 | | | | |
| 3 | Vivi Li | Meta information modification | 2125 | 2020-09-18 05:53:33 | | |
Video Upload Options
Neuromodulation is a promising new area with treatment applications for psychiatry. Electrical stimulation of the vagus nerve is associated with a reduction in peripheral sympathetic and inflammatory function and modulation of brain areas mediating fear and the stress response, and thus has potential applications to patients with stress-related psychiatric disorders, including posttraumatic stress disorder (PTSD) and depression. Non-invasive Vagal Nerve Stimulation (nVNS) can be applied to vagus nerve locations at the neck (transcutaneous cervical, or tcVNS) or ear (transcutaneous auricular, or taVNS), intervening at the level of the underlying psychobiology with potential beneficial treatment effects.
1. Introduction
Stress-related psychiatric disorders, including depression and posttraumatic stress disorder (PTSD), are important public health problems. Early life stress increases the risk of development of depression in adulthood [1][2], and stressful life events are associated with an increased risk for depressive episodes [3], while PTSD requires exposure to a traumatic stressor as part of the diagnosis [4]. At any given time, 10% of the United States population meets the criteria for major depression or other mood disorders based on Diagnostic and Statistical Manual (DSM) criteria [5], with an annual cost of lost productivity of USD 44 billion [6]. Similarly, PTSD affects 6% of the population at some time in their lives [7]. The cost of treating PTSD and comorbid depression in soldiers returning from the wars in Iraq and Afghanistan has been estimated to be USD 6.2 billion [8], and since PTSD affects a larger total number of civilians in the United States than military personnel, the costs for society as a whole are likely much higher [9]. The most common cause of PTSD in women is sexual abuse and assault in childhood, while, for men, it is physical assault [10]. On average, women have higher occurrence of PTSD compared to men in the civilian population [11][12]. PTSD is characterized by intrusive thoughts, nightmares, avoidance, emotional blunting, negative cognitions, hypervigilance, and hyperarousal [13]. Depression is associated with depressed mood, loss of appetite, decreased psychomotor activity, and, in extreme cases, suicidal ideation. Other symptoms, such as poor sleep and concentration, negative cognitions, loss of interest in things, and anhedonia, are common to both conditions. In fact, there is a degree of comorbidity between the two conditions [14][15][16][17][18][19]. Furthermore, patients with comorbid disorders have a worse clinical course, with, for instance, a higher risk of suicidal ideation [20][21].
The standard of care for both PTSD and depression includes psychotherapy and/or medication [22][23]. Psychotherapy treatments for PTSD, however, have dropout rates as high as 50%, which limit their applicability [24][25]. First-line medication treatments for stress-related psychiatric disorders involves the Selective Serotonin Reuptake Inhibitor (SSRI) antidepressants [26][27]. However, as highlighted by a report from the Institute of Medicine, there is not sufficient evidence to conclude that they are effective for PTSD [28]. In fact, only one-third of those suffering from PTSD are able to achieve full remission with the current standard of care [26]. Similar limitations exist for treatment of major depression. As illustrated by the STAR*D study, only one-third of patients with major depression remitted to first-line therapy with antidepressants and only about two-thirds of patients met remission criteria after multiple algorithms that included psychotherapy, switching classes, and multiple heroic augmentation trials [29]. Given limitations of current treatment options, new paradigms are clearly needed for the management of stress-related psychiatric disorders.
2. Noninvasive Vagal Nerve Stimulation: Application to Stress-Related Psychiatric Disorders
The requirement for direct VNS to be surgically implanted has limited widespread implementation in stress-related psychiatric disorders to date due to cost and inconvenience [30][31]. These forms of VNS are also limited by the fact that true sham-controlled trials cannot be performed due to ethical reasons, which has led to questions about the true efficacy of these devices [32]. Since devices are only implanted in patients who have not responded to multiple antidepressants, the patient populations are also not necessarily representative of those typically seen in clinical psychiatry practices, which may explain why VNS, although yielding statistically significant improvements, did not lead to complete remission in all patients [33]. Additionally, treatments have not been reimbursed by Medicare or other insurance companies, which has further limited implementation [34]. Studies have shown the utility of both tcVNS and taVNS for various psychiatric disorders, including schizophrenia [35] and obsessive-compulsive disorder [36], as well as major depression [37]. Human studies also suggest that noninvasive VNS improves hyperarousal in PTSD patients with mild traumatic brain injury [38] and reduces symptoms in treatment-resistant anxiety disorders [39].
As proven by their cost and convenience, noninvasive VNS technologies have widespread applicability to patients with stress-related psychiatric disorders.
Neck-based tcVNS was recently approved by the FDA for the treatment of intractable cluster headache [40][41][42][43]. We have implemeneted this device in healthy human subjects with a history of exposure to traumatic stressful events since 2017, and have found it to be safe and feasible [44][45][46]. In our studies, we compared active tcVNS to a sham control in a randomized trial (Figure 1). Both were handheld devices that were applied to the left neck for stimulation with identical placement and operation (GammaCore, ElectroCore, Basking Ridge, New Jersey). nVNS or sham were applied using collar electrodes on the left side of the neck in order to permit placement while subjects were in the research-dedicated brain scanner, which had a small aperture. The treatment area on the neck was located by finding the carotid artery pulsation. An electrically conductive gel was applied on the stimulation surfaces and device is placed on the located treatment area. Active tcVNS devices produced a 5-kHz sine wave burst lasting for 1 millisecond (five sine waves, each lasting 200 microseconds), repeated one in every 40 milliseconds (25 Hz), generating 30-V peak voltage and 60-mA peak output current. The final stimulation intensity depended on the subject’s verbal feedback: The researcher was instructed to increase the intensity gradually until the subject voiced discomfort, at which point the intensity was reduced slightly below that threshold. Sham devices produced a nearly direct voltage signal, whose polarity was slowly varied (0.2-Hz biphasic voltage), in contrast to the higher-frequency, alternating current used for the active nVNS (25 Hz with 5-kHz bursts). The sham device delivered a biphasic signal generating a 14-V peak voltage and 60-mA peak output current, consisting of pulses repeating every 5 s (0.2 Hz). High-frequency voltage signals (such as the active stimulus) pass through the skin with minimal power dissipation due to the low skin-electrode impedance at kHz frequencies. In contrast, lower-frequency signals (such as the sham stimulus) are mainly attenuated at the skin-electrode interface due to the high impedance [47]. Accordingly, the active tcVNS can deliver substantial energy to the vagus nerve to facilitate stimulation, while the voltage levels appearing at the vagus would be expected to be orders of magnitude lower for the sham device and thus vagal stimulation is unlikely. Nevertheless, since the sham device does deliver relatively high voltage and current levels directly to the skin, it activates skin nociceptors, causing a similar feeling to a pinch. This sensation is necessary for blinding of the participants and is thought as a critical detail by the investigators for the valuation of the potential treatment in psychiatric populations. Both active and sham interventions lasted for two minutes. The subject, research staff, and investigators were all blind to the device category, and the key was kept in a locked office by an individual not involved in the research in two locations. The specific details are summarized as follows: The manufacturer sent the active and sham devices to an individual who was not involved in research, and the individual randomized patients to the devices prior to patient recruitment. In addition, every subject was given a different, dedicated device, hence the number of patients was equal to the number of devices. Every week when a new patient arrived, the individual not involved in research delivered a different device to the research staff for use for that subject.
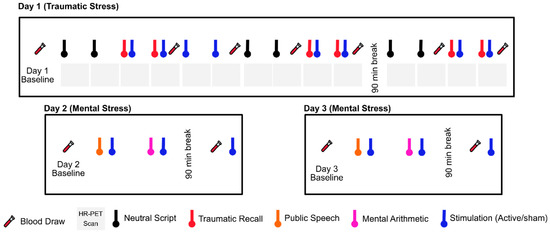
Figure 1. Study protocol undergoing since 2017. Physiological sensing data is collected continuously throughout three study days. The protocol timeline depicts neutral and trauma scripts, HR-PET scans (first day), mental stress tasks of public speech and mental arithmetic (second and third day), stimulation with active tcVNS or sham, and blood draws (all days).
In our study with physically healthy traumatized subjects and patients with PTSD, we constructed a multisignal dataset that include physiological signals related to cardiovascular and peripheral activity. The signals included electrocardiography (ECG), respiration (RSP), seismocardiography (SCG), photoplethysmography (PPG), electrodermal activity (EDA), and blood pressure (BP). Upon beat-by-beat signal processing, we extracted parameters related to autonomic tone with a beat-by-beat resolution. These parameters included both standard and nonstandard indices of psychophysiological reactivity, such as heart rate (HR), pre-ejection period of the heart (PEP), amplitude of the peripheral photoplethysmogram (PPG), pulse arrival time (PAT), properties of respiration signal (respiration rate, RR, width, RW, prominence, RP), frequency- and time- domain heart rate variability indices including low- and high-frequency heart rate variability (LF HRV, HF HRV), Poincare-based nonlinear heart rate variability (SD1, SD2), acceleration and deceleration capacity (AC, DC), and skin conductance level and response (SCL, SCR). In our healthy cohort, PEP, PPG amplitude, skin conductance, and respiratory indices resulted in marked differences between active and sham groups, indicating a blunted sympathetic response with tcVNS [48][49]. We later used this blunted physiological reactivity pattern to devise a machine learning based method that could indicate stimulation presence [45][46]. Brain imaging using High-Resolution Positron Emission Tomography (HR-PET) in traumatized participants without PTSD exposed to personalized traumatic scripts showed that tcVNS compared to sham stimulation blocked activations in the medial prefrontal cortex, parahippocampal gyrus, and insula, brain areas that play key roles in emotion and response to stress [50].
We also studied the effects of tcVNS on inflammatory markers in traumatized individuals with and without PTSD. We found that tcVNS paired with personalized traumatic scripts blocked stress-induced increases in proinflammatory biomarkers IL-6 and IFN-γ, and showed a pattern of decreased anger responses to scripts [51]. Increases in IL-6 and IFN-γ likely occur multiple times a day with minor stressors and triggers in PTSD patients, so tcVNS could result in a decrease in symptoms driven by inflammation and lead to improvements in clinical course. The reduction in subjective anger, in addition to improved mental health, also likely have beneficial health effects, for instance, in patients with comorbid PTSD and coronary artery disease (CAD), where we found not only an increase in mental stress-induced IL-6 in those with comorbid PTSD [52], but also that anger, PTSD, and other symptoms of psychological distress were associated with long-term adverse cardiovascular outcomes [53] and an increase in mental stress-induced myocardial ischemia [54][55].
Studies are ongoing with patients with PTSD, paired with assessment of the brain with High Resolution Positron Emission Tomography (HR-PET), and assessment of inflammatory and other blood biomarkers [56]. Due to low cost, increased convenience, limited side effects, feasibility for use at home or in the field for military medicine applications, and the ability to assess efficacy with true sham control comparison, tcVNS and taVNS show great promise in our opinion for the treatment of patients with stress-related psychiatric disorders and enhancement of human performance [32].
3. Conclusions
Current treatments for PTSD, major depression, and other stress-related psychiatric disorders, including medications and psychotherapy, have limitations and are not efficacious for all patients. Neuromodulation is an important alternative treatment, and noninvasive forms of VNS have the advantages of cost and noninvasiveness and can potentially be widely implemented for these patients. Both tcVNS and taVNS show promise for intervening at the level of the underlying neurobiology of these disorders.
PTSD is triggered by experiencing or witnessing exposure to traumatic events and leads to uncontrollable thoughts about the events. Our results from traumatized subjects without PTSD demonstrate decreased sympathetic and increased parasympathetic tone during tcVNS following acute traumatic stress, suggesting possible translation of this treatment to patients with PTSD, in the clinic or at home, as an acute treatment for these recurrent memories [44][45][46][57]. tcVNS has potential promise for enhancing recovery from acute traumatic stress by means of modulation of autonomic response in PTSD populations. As patients with PTSD show exaggerated responsivity to reminders of traumatic memories, the physiological changes induced by tcVNS observed in traumatized individuals without PTSD may be similarly observed in PTSD populations. Moreover, recent studies have shown that invasive VNS enhances the extinction of conditioned fear in rats [58]. Additionally, taVNS was shown to lead to improvement in vagal tone in patients with PTSD [38] and to inhibit long-term fear responses during extinction training in healthy human subjects [59]. Implanted VNS has already been approved by the FDA as a treatment for treatment resistant depression and epilepsy, but its cost and the intrusive nature of the surgery have limited its use. Noninvasive VNS technologies would be a significant addition to both facilitate further research into the circuitry of PTSD and treatment resistant depression, and would provide a new and highly acceptable treatment option for patients suffering from both severe and recurrent depression and PTSD [60][32].
References
- Anda, R.F.; Felitti, V.J.; Walker, J.; Whitfield, C.; Bremner, J.D.; Perry, B.D.; Dube, S.R.; Giles, W.H. The enduring effects of childhood abuse and related experiences in childhood: A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 174–186.
- Kessler, R.C.; Magee, W.J. Childhood adversities and adult depression: Basic patterns of association in a US national survey. Psychol. Med. 1993, 23, 679–690.
- Kenneth S. Kendler; Laura M. Thornton; Charles O. Gardner; Stressful Life Events and Previous Episodes in the Etiology of Major Depression in Women: An Evaluation of the “Kindling” Hypothesis. American Journal of Psychiatry 2000, 157, 1243-1251, 10.1176/appi.ajp.157.8.1243.
- Frank W. Weathers; Michelle J. Bovin; Daniel J. Lee; Denise M. Sloan; Paula P. Schnurr; Danny G. Kaloupek; Terence M. Keane; Brian P. Marx; The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans.. Psychological Assessment 2018, 30, 383-395, 10.1037/pas0000486.
- Ronald C. Kessler; Katherine A. McGonagle; ShanYang Zhao; Christopher B. Nelson; Michael Hughes; Suzann Eshleman; Hans-Ulrich Wittchen; Kenneth S. Kendler; Lifetime and 12-Month Prevalence of DSM-III-R Psychiatric Disorders in the United States. Archives of General Psychiatry 1994, 51, 8-19, 10.1001/archpsyc.1994.03950010008002.
- Stewart, W.F.; Ricci, J.A.; Chee, E.; Hahn, S.R.; Morganstein, D.; Cost of Lost Productive Work Time Among US Workers With Depression—Correction. JAMA 2003, 290, 2128, 10.1001/jama.290.16.2128.
- R. H. Pietrzak; Risë B. Goldstein; Steven M. Southwick; Bridget F. Grant; Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: Results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Anxiety Disorders 2011, 25, 456-465, 10.1016/j.janxdis.2010.11.010.
- Eibner, C. The Invisible Wounds of War: Quantifying the Societal Costs of Psychological and Cognitive Injuries; RAND Corporation: Santa Monica, CA, USA, 2008.
- McCauley, J.; Kern, D.E.; Kolodner, K.; Dill, L.; Schroeder, A.F.; DeChant, H.K.; Ryden, J.; Derogatis, L.R.; Bass, E.G. Clinical characteristics of women with a history of childhood abuse: Unhealed wounds. J. Am. Med. Assoc. 1997, 277, 1362–1368.
- MacMillan, H.L.; Fleming, J.E.; Trocme, N.; Boyle, M.H.; Wong, M.; Racine, Y.A.; Beardslee, W.R.; Offord, D.R. Prevalence of child physical and sexual abuse in the community: Results from the Ontario Health Supplement. J. Am. Med. Assoc.
- Kessler, R.C.; Sonnega, A.; Bromet, E.; Hughes, M.; Nelson, C.B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 1995, 52, 1048–1060.
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602.
- Bremner, J.D. (Ed.) Posttraumatic Stress Disorder: From Neurobiology to Treatment, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016.
- Blanchard, E.B.; Buckley, T.C.; Hickling, E.J.; Taylor, A.E. Posttraumatic stress disorder and comorbid major depression: Is the correlation an illusion? J. Anxiety Disord. 1998, 12, 1–37.
- Franklin, C.L.; Zimmerman, M. Posttraumatic stress disorder and major depressive disorder: Investigating the role of overlapping symptoms in diagnostic comorbidity. J. Nerv. Ment. Dis. 2001, 189, 548–551.
- Flory, J.D.; Yehuda, R. Comorbidity between post-traumatic stress disorder and major depressive disorder: Alternative explanations and treatment considerations. Dialogues Clin. Neurosci. 2015, 17, 141–150.
- Nijdam, M.J.; Gersons, B.P.R.; Olff, M. The role of major depression in neurocognitive functioning in patients with posttraumatic stress disorder. Eur. J. Psychotraumatol. 2013, 4, 19979.
- Shalev, A.Y.; Freedman, S.; Peri, T. Prospective study of post-traumatic stress disorder and depression following trauma. Am. J. Psychiatry 1988, 155, 630–637.
- Rytwinski, N.K.; Scur, M.D.; Feeny, N.C.; Youngstrom, E.A. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: A meta-analysis. J. Trauma. Stress 2013, 26, 299–309.
- Oquendo, M.; Brent, D.A.; Birmaher, B.; Greenhill, L.; Kolko, D.; Stanley, B.; Zelazny, J.; Burke, A.K.; Firinciogullari, S.; Ellis, S.P.; et al. Posttraumatic stress disorder comorbid with major depression: Factors mediating the association with suicidal behavior. Am. J. Psychiatry 2005, 162, 560–566.
- Ramsawh, H.J.; Fullerton, C.S.; Mash, H.B.H.; Ng, T.H.H.; Kessler, R.C.; Stein, M.B.; Ursano, R.J. Risk for suicidal behaviors associated with PTSD, depression, and their comorbidity in the U.S. Army. J. Affect. Disord. 2014, 161, 116–122.
- Ballenger, J.C.; Davidson, J.R.; Lecrubier, Y.; Nutt, D.J.; Foa, E.B.; Kessler, R.C.; McFarlane, A.C.; Shalev, A.Y. Consensus statement on posttraumatic stress disorder from the International Consensus Group on Depression and Anxiety. J. Clin. Psychiatry 2000, 61, 60–66.
- Foa, E.B.; Davidson, J.R.T.; Frances, A.; Culpepper, L.; Ross, R.; Ross, D. The expert consensus guideline series: Treatment of posttraumatic stress disorder. J. Clin. Psychiatry 1999, 60, 4–76.
- Schottenbauer, M.A.; Glass, C.R.; Arnkoff, D.B.; Tendick, V.; Gray, S.H. Nonresponse and dropout rates in outcome studies on PTSD: Review and methodological considerations. Psychiatry 2008, 71, 134–168.
- Hembree, E.A.; Foa, E.B.; Dorfan, N.M.; Street, G.P.; Kowalski, J.; Tu, X. Do patients drop out prematurely from exposure therapy for PTSD? J. Trauma. Stress 2003, 16, 555–562.
- Ballenger, J.C.; Davidson, J.R.; Lecrubier, Y.; Nutt, D.J.; Marshall, R.D.; Nemeroff, C.B.; Shalev, A.Y.; Yehuda, R. Consensus statement update on posttraumatic stress disorder from the international consensus group on depression and anxiety. J. Clin. Psychiatry 2004, 65 (Suppl. 1), 55–62.
- Davis, L.; Hamner, M.; Bremner, J.D. Pharmacotherapy for PTSD: Effects on PTSD symptoms and the brain. In Posttraumatic Stress Disorder: From Neurobiology to Treatment; Bremner, J.D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 389–412.
- Institute of Medicine of the National Academies. Treatment for Posttraumatic Stress Disorder in Military and Veteran Populations: Final Assessment; National Academies of Science, Engineering and Medicine, Health and Medicine Division: Washington, DC, USA, 2014.
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1907.
- Lauren B Marangell; A. John Rush; Mark S George; Harold A Sackeim; Christopher R Johnson; Mustafa M Husain; Ziad Nahas; Sarah H Lisanby; Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biological Psychiatry 2002, 51, 280-287, 10.1016/s0006-3223(01)01343-9.
- H Sackeim; Harold A Sackeim; A. John Rush; Mark S. George; Lauren B. Marangell; Mustafa M. Husain; Ziad Nahas; Christopher R Johnson; Stuart Seidman; Cole Giller; et al.Stephen J HainesRichard K Simpson JrRobert R Goodman Vagus Nerve Stimulation (VNS™) for Treatment-Resistant Depression Efficacy, Side Effects, and Predictors of Outcome. Neuropsychopharmacology 2001, 25, 713-728, 10.1016/s0893-133x(01)00271-8.
- J. Douglas Bremner; Mark Hyman Rapaport; Vagus Nerve Stimulation: Back to the Future.. American Journal of Psychiatry 2017, 174, 609-610, 10.1176/appi.ajp.2017.17040422.
- Roumen Milev; Peter Giacobbe; Sidney H. Kennedy; Daniel M. Blumberger; Zafiris J. Daskalakis; Jonathan Downar; Mandana M. Modirrousta; Simon Patry; Fidel Vila-Rodriguez; Raymond W. Lam; et al.Glenda M. MacQueenSagar V. ParikhArun V. Ravindranthe CANMAT Depression Work Group Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder. The Canadian Journal of Psychiatry 2016, 61, 561-575, 10.1177/0706743716660033.
- Rachel Feldman; David L. Dunner; James S. Muller; Devin A. Stone; Medicare patient experience with vagus nerve stimulation for treatment-resistant depression. Journal of Medical Economics 2012, 16, 62-74, 10.3111/13696998.2012.724745.
- Alkomiet Hasan; Claus Wolff-Menzler; Sebastian Pfeiffer; Peter Falkai; Elif Weidinger; Andrea Jobst; Imke Hoell; Berend Malchow; Peyman Yeganeh-Doost; Wolfgang Strube; et al.Silke QuastNorbert MüllerThomas Wobrock Transcutaneous noninvasive vagus nerve stimulation (tVNS) in the treatment of schizophrenia: a bicentric randomized controlled pilot study. European Archives of Psychiatry and Clinical Neuroscience 2015, 265, 589-600, 10.1007/s00406-015-0618-9.
- Giordano D’Urso; Andre R. Brunoni; Maria Pia Mazzaferro; Annalisa Anastasia; Andrea De Bartolomeis; Antonio Mantovani; Transcranial direct current stimulation for obsessive-compulsive disorder: A randomized, controlled, partial crossover trial. Depression and Anxiety 2016, 33, 1132-1140, 10.1002/da.22578.
- Peijing Rong; Jun Liu; Liping Wang; Rupeng Liu; Ji-Liang Fang; Jingjun Zhao; Yufeng Zhao; Honghong Wang; Mark Vangel; Sharon Sun; et al.Hui BenJoel ParkShaoyuan LiHong MengBing ZhuJian Kong Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: A nonrandomized controlled pilot study.. Journal of Affective Disorders 2016, 195, 172-9, 10.1016/j.jad.2016.02.031.
- Damon G. Lamb; Eric C. Porges; Greg F. Lewis; John B. Williamson; Non-invasive Vagal Nerve Stimulation Effects on Hyperarousal and Autonomic State in Patients with Posttraumatic Stress Disorder and History of Mild Traumatic Brain Injury: Preliminary Evidence. Frontiers in Medicine 2017, 4, 124, 10.3389/fmed.2017.00124.
- Mark S. George; Herbert E. Ward; Philip T. Ninan; Mark Pollack; Ziad Nahas; Berry Anderson; Samet Kose; Robert H. Howland; Wayne K. Goodman; James C. Ballenger; et al. A pilot study of vagus nerve stimulation (VNS) for treatment-resistant anxiety disorders. Brain Stimulation 2008, 1, 112-121, 10.1016/j.brs.2008.02.001.
- Elinor Ben-Menachem; David Révész; B. J. Simon; S. Silberstein; Surgically implanted and non‐invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. European Journal of Neurology 2015, 22, 1260-1268, 10.1111/ene.12629.
- Barbanti, P.; Grazzi, L.; Egeo, G.; Padovan, A.; Liebler, E.; Bussone, G. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: An open-label study. J. Headache Pain 2015, 16, 61.
- Nesbitt, A.D.; Marin, J.C.A.; Tomkins, E.; Ruttledge, M.H.; Goadsby, P.J. Non-invasive vagus nerve stimulation for the treatment of cluster headache: A case series. J. Headache Pain 2013, 14.
- Gaul, C.; Magis, D.; Liebler, E.J.; Straube, A. Effects of non-invasive vagus nerve stimulation on attack frequency over time and expanded response rates in patients with chronic cluster headache: A post hoc analysis of the randomized, controlled PREVA Study. J. Headache Pain 2017, 18, 22.
- Gurel, N.Z.; Huang, M.; Wittbrodt, M.T.; Jung, H.; Ladd, S.L.; Shandhi, M.H.; Ko, Y.-A.; Shallenberger, L.; Nye, J.A.; Pearce, B.; et al. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul. 2020, 13, 47–59.
- Gurel, N.Z.; Gazi, A.H.; Scott, K.L.; Wittbrodt, M.T.; Shah, A.J.; Vaccarino, V.; Bremner, J.D.; Inan, O.T. Timing considerations for noninvasive Vagal Nerve Stimulation in clinical studies. AMIA Annu. Symp. Proc. 2020, 2019, 1061–1070.
- Gurel, N.Z.; Wittbrodt, W.T.; Jung, H.; Ladd, S.L.; Shah, A.J.; Vaccarino, V.; Bremner, J.D.; Inan, O.T. Automatic detection of target engagement in transcutaneous cervical Vagal Nerve Stimulation for traumatic stress triggers. IEEE J. Biomed. Health Inform. 2020, 24, 1917–1925.
- Javier Rosell-Ferrer; J. Colominas; P. Riu; R. Pallas-Areny; J. G. Webster; Skin impedance from 1 Hz to 1 MHz. IEEE Transactions on Biomedical Engineering 1988, 35, 649-651, 10.1109/10.4599.
- Nil Z. Gurel; Minxuan Huang; Matthew T. Wittbrodt; Hewon Jung; Stacy L. Ladd; Mobashir H. Shandhi; Yi-An Ko; Lucy Shallenberger; Jonathon A. Nye; Bradley Pearce; et al.Viola VaccarinoAmit J ShahJ. Douglas BremnerOmer T. Inan Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimulation 2020, 13, 47-59, 10.1016/j.brs.2019.08.002.
- Gazi, A.H.; Gurel, N.Z.; Richardson, J.L.S.; Wittbrodt, M.T.; Shah, A.J.; Vaccarino, V.; Bremner, J.D.; Inan, O.T. Investigating digital cardiovascular biomarker responses to transcutaneous cervical vagus nerve stimulation: State-space modeling, prediction, and simulation. JMIR hHealth uHealth 2020.
- Matthew T. Wittbrodt; Nil Z. Gurel; Jonathon A. Nye; Stacy Ladd; Mobashir H. Shandhi; Minxuan Huang; Amit J. Shah; Bradley D. Pearce; Zuhayr S. Alam; Mark H. Rapaport; et al.Nancy MurrahYi-An KoAmmer A. HafferLucy H. ShallenbergerViola VaccarinoOmer T. InanJ. Douglas Bremner Non-Invasive Vagal Nerve Stimulation Decreases Brain Activity During Trauma Scripts. Brain Stimulation 2020, 13, 1333–1348, 10.1016/j.brs.2020.07.002.
- J. Douglas Bremner; Nil Z. Gurel; Yunshen Jiao; Matthew T. Wittbrodt; Oleksiy M. Levantsevych; Minxuan Huang; Hewon Jung; MdMobashir H. Shandhi; Joy Beckwith; Isaias Herring; et al.Mark H. RapaportNancy MurrahEmily DriggersYi-An KoMhmtJamil L. AlkhalafMajd SoudanJiawei SongBenson S. KuLucy ShallenbergerAllison N. HankusJonathon A. NyeJeanie ParkViola VaccarinoAmit J. ShahOmer T. InanBradley D. Pearce Transcutaneous Vagal Nerve Stimulation Blocks Stress-Induced Activation of Interleukin-6 and Interferon-γ in Posttraumatic Stress Disorder: A Double-Blind, Randomized, Sham-Controlled Trial. Brain, Behavior, & Immunity - Health 2020, -, 100138, 10.1016/j.bbih.2020.100138.
- Bruno B. Lima; Muhammad Hammadah; Kobina Wilmot; Brad D. Pearce; Amit J Shah; Oleksiy Levantsevych; Belal Kaseer; Malik Obideen; Mohamad Mazen Gafeer; Jeong Hwang Kim; et al.Samaah SullivanTené T. LewisLei WengLisa ElonLian LiJ. Douglas BremnerPaolo RaggiArshed QuyyumiViola Vaccarino Posttraumatic stress disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain, Behavior, and Immunity 2019, 75, 26-33, 10.1016/j.bbi.2018.08.015.
- Pratik Pimple; Bruno B. Lima; Muhammad Hammadah; Kobina Wilmot; Ronnie Ramadan; Oleksiy Levantsevych; Samaah Sullivan; Jeong Hwan Kim; Belal Kaseer; Amit J Shah; et al.Laura WardPaolo RaggiJ. Douglas BremnerJohn HanfeltTene LewisArshed A. QuyyumiViola Vaccarino Psychological Distress and Subsequent Cardiovascular Events in Individuals With Coronary Artery Disease.. Journal of the American Heart Association 2019, 8, e011866, 10.1161/JAHA.118.011866.
- Lima, B.B.; Hammadah, M.; Pearce, B.D.; Shah, A.; Moazzami, K.; Kim, J.H.; Sullivan, S.; Levantsevych, O.; Lewis, T.T.; Weng, L.; et al. Association of posttraumatic stress disorder with mental stress-induced myocardial ischemia in adults after myocardial infarction. JAMA Netw. Open 2020, 3, e202734.
- Pimple, P.; Shah, A.; Rooks, C.; Bremner, J.D.; Nye, J.; Ibeanu, I.; Murrah, N.; Shallenberger, L.; Kelley, M.; Raggi, P.; et al. Association between anger and mental stress-induced myocardial ischemia. Am. Heart J. 2015, 169, 115–121.
- Nil Z. Gurel; Asim H. Gazi; Kristine L. Scott; Matthew T. Wittbrodt; Amit J. Shah; Viola Vaccarino; J. Douglas Bremner; Omer T. Inan; Timing Considerations for Noninvasive Vagal Nerve Stimulation in Clinical Studies.. null 2020, 2019, 1061-1070.
- Nil Z. Gurel; Mobashir H. Shandhi; J. Douglas Bremner; Viola Vaccarino; Stacy L. Ladd; Lucy Shallenberger; Amit J Shah; Omer T. Inan; Toward closed-loop transcutaneous vagus nerve stimulation using peripheral cardiovascular physiological biomarkers: A proof-of-concept study. 2018 IEEE 15th International Conference on Wearable and Implantable Body Sensor Networks (BSN) 2018, -, 78-81, 10.1109/bsn.2018.8329663.
- David Frausto Peã±A; Jessica E. Childs; Shawn Willett; Analicia Vital; Christa K. McIntyre; Sven Kröner; David Frausto Peña; Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Frontiers in Behavioral Neuroscience 2014, 8, 1-8, 10.3389/fnbeh.2014.00327.
- Christoph Szeska; Jan Richter; Julia Wendt; Mathias Weymar; Alfons O. Hamm; Promoting long-term inhibition of human fear responses by non-invasive transcutaneous vagus nerve stimulation during extinction training. Scientific Reports 2020, 10, 1-16, 10.1038/s41598-020-58412-w.
- Scott T. Aaronson; Peter Sears; Francis Ruvuna; Mark Bunker; Darin D. Dougherty; Frederick W. Reimherr; Thomas L. Schwartz; John M. Zajecka; Charles R. Conway; A 5-Year Observational Study of Patients With Treatment-Resistant Depression Treated With Vagus Nerve Stimulation or Treatment as Usual: Comparison of Response, Remission, and Suicidality. American Journal of Psychiatry 2017, 174, 640-648, 10.1176/appi.ajp.2017.16010034.




