Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Abenaya Muralidharan | + 2112 word(s) | 2112 | 2022-02-15 07:20:34 | | | |
| 2 | Bruce Ren | -85 word(s) | 2027 | 2022-03-11 02:15:30 | | | | |
| 3 | Bruce Ren | Meta information modification | 2027 | 2022-03-14 06:44:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Muralidharan, A. SARS-CoV-2 Dysregulates Neutrophil Degranulation and Reduces Lymphocyte Counts. Encyclopedia. Available online: https://encyclopedia.pub/entry/20452 (accessed on 08 February 2026).
Muralidharan A. SARS-CoV-2 Dysregulates Neutrophil Degranulation and Reduces Lymphocyte Counts. Encyclopedia. Available at: https://encyclopedia.pub/entry/20452. Accessed February 08, 2026.
Muralidharan, Abenaya. "SARS-CoV-2 Dysregulates Neutrophil Degranulation and Reduces Lymphocyte Counts" Encyclopedia, https://encyclopedia.pub/entry/20452 (accessed February 08, 2026).
Muralidharan, A. (2022, March 10). SARS-CoV-2 Dysregulates Neutrophil Degranulation and Reduces Lymphocyte Counts. In Encyclopedia. https://encyclopedia.pub/entry/20452
Muralidharan, Abenaya. "SARS-CoV-2 Dysregulates Neutrophil Degranulation and Reduces Lymphocyte Counts." Encyclopedia. Web. 10 March, 2022.
Copy Citation
SARS-CoV-2, the virus that causes COVID-19, has given rise to one of the largest pandemics, affecting millions worldwide. High neutrophil-to-lymphocyte ratios have been identified as an important correlate to poor recovery rates in severe COVID-19 patients. However, the mechanisms underlying this clinical outcome and the reasons for its correlation to poor prognosis are unclear. Furthermore, the mechanisms involved in healthy neutrophils acquiring a SARS-CoV-2-mediated detrimental role are yet to be fully understood.
neutrophils
elastase
MPO
degranulation
SARS-CoV-2
lymphocytes
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a β coronavirus with a single-stranded RNA genome. It is the third virus in this group to show potential for causing large-scale pandemics, with SARS-CoV and MERS-CoV (Middle East respiratory syndrome coronavirus) causing outbreaks in 2003 and 2012, respectively [1][2][3][4][5]. In March 2020, the World Health Organization (WHO) declared SARS-CoV-2-infection-induced coronavirus disease 2019 (COVID-19) a pandemic. By November 2020, SARS-CoV-2 proved its tremendous infectivity and transmissibility by spreading to 216 countries and territories, infecting over 62 million individuals and killing over 1.5 million [6].
Although the majority of the infected individuals are asymptomatic or exhibit mild symptoms, about 15% develop pneumonia [7]. Infection in the higher-risk groups, such as the elderly and individuals with underlying chronic conditions, can cause acute respiratory distress syndrome and multiorgan failure resulting in death [7]. Many of the early symptoms resemble other respiratory viral infections, with high fever and dyspnea being the main difference between COVID-19 and the common cold [8]. Importantly, compared to infections by other commonly circulating viruses such as influenza, SARS-CoV-2 infection has higher chances of progressing to a critical state requiring oxygen therapy and ventilatory support. This suggests SARS-CoV-2 may have a systemic aspect to its infection that is accompanied by severe inflammation [9][10][11][12].
Hyperinflammation has been identified as one of the major causes of the morbidity and mortality observed in COVID-19. Specifically, neutrophilia correlates to COVID-19 disease severity, with increased blood neutrophil counts in severe patients compared to mild cases [13]. This, combined with lymphopenia, leading to elevated neutrophil-to-lymphocyte ratio (NLR), has been observed as a hallmark of severe COVID-19 suggestive of poor recovery rates [7][14][15].
Neutrophils make up 50–70% of all leukocytes and are the most abundant immune cells in human blood [16]. They serve as the first responders during infections and can shape cell-mediated responses. Although their role during bacterial and fungal infections have been well-studied, their role during viral infections remains to be fully understood [17][18][19]. Upon activation, neutrophils migrate to a target tissue where they defend against invading microbes. They can also interact with other immune cell populations and affect the microenvironment [18][20].
During SARS-CoV-2 infection, whole blood transcriptomics of patients who required intensive care showed increased neutrophil function and activation genes on the first day of hospitalization [21][22]. This indicates that neutrophil activation occurs before the onset of severe illness. Another study looking at 300 patients with confirmed COVID-19 observed that the presence of circulating activated neutrophils can serve as an independent predictor for mechanical ventilation and death [23].
Numerous clinical data collected during the pandemic, specifically regarding neutrophils and NLR, have immensely aided patient care in terms of predicting prognosis and devising a more informed treatment plan. However, in-depth mechanistic understanding of the neutrophilic dysregulation observed during SARS-CoV-2 infection is not fully understood. This knowledge is crucial for establishing better treatment strategies to allow for improved control of disease progression. As such, the researchers aimed to systematically delineate the effects of SARS-CoV-2 on neutrophil degranulation and subsequent lymphocyte numbers in vitro.
2. SARS-CoV-2 Dysregulates Neutrophil Degranulation and Reduces Lymphocyte Counts
2.1. Factors Secreted by SARS-CoV-2-Infected Epithelial Cells Diminish Myeloperoxidase Release While Modestly Increasing Elastase Release by Human Neutrophils In Vitro
To determine the effect of infected lung epithelial cells on neutrophil degranulation in vitro, the researchers infected Calu-3 cells with SARS-CoV-2 at two different MOIs for 48 h. To ensure effective cell entry and virus release, the researchers determined the viral titer in the cells and supernatant using qPCR (Figure 1A). At 48 h post infection, some cytopathic effect was observed at the higher MOI, leading to decreased viral load in the cells and the supernatant compared to MOI 0.01. The supernatant from the infected Calu-3 cells were then UV-irradiated for treatment of circulating human neutrophils isolated from healthy donors.
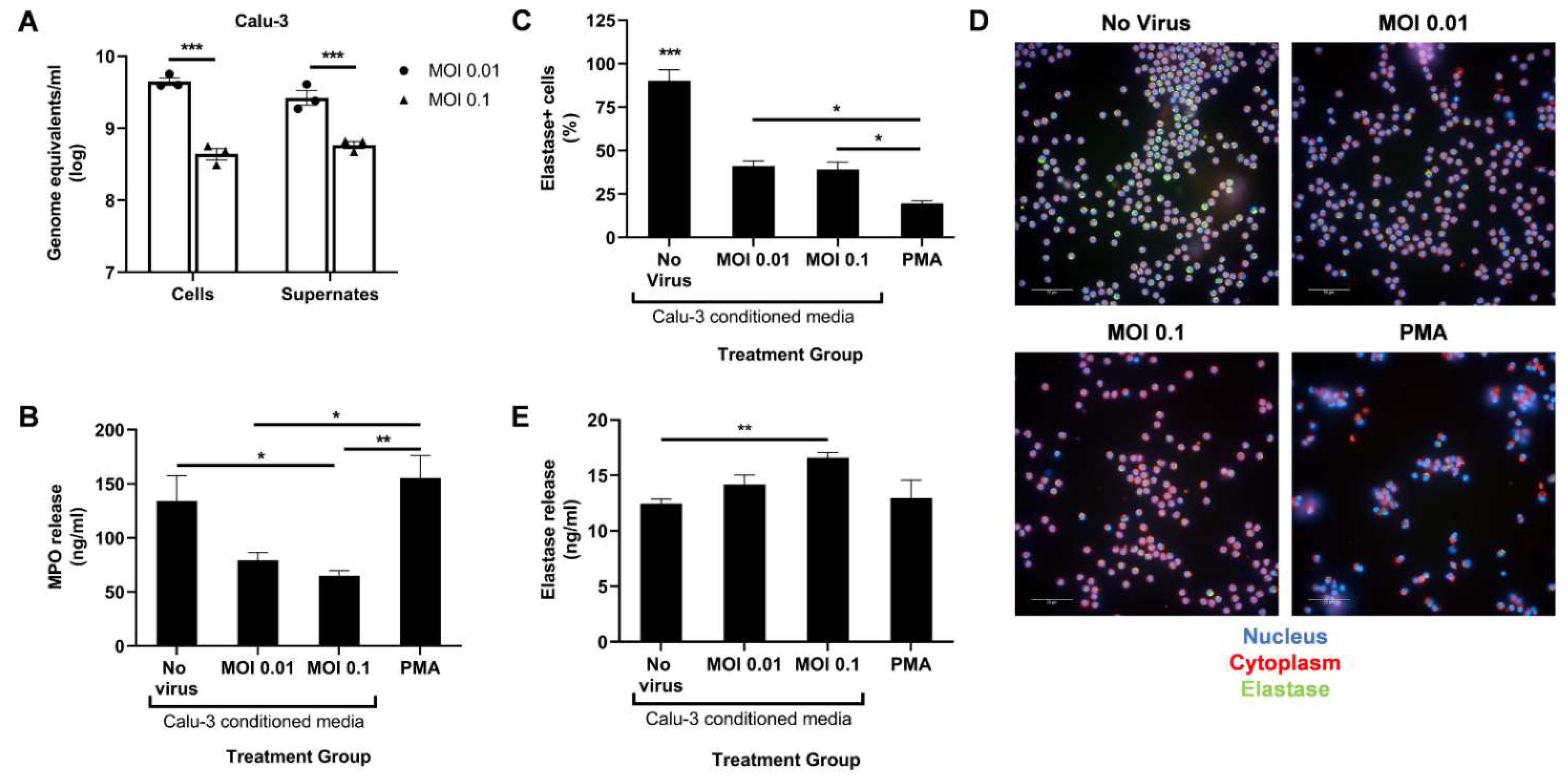
Figure 1. SARS-CoV-2-infected Calu-3 cells secrete factors that diminish neutrophil MPO release. (A) Viral titer in the cells and supernates of SARS-CoV-2-infected Calu-3 48 h post infection; UV-irradiated Calu-3 supernates, referred to as Calu-3-conditioned media, were used to treat human neutrophils. (B) MPO levels in the treated neutrophils supernatant media determined through ELISA; (C) Percentage of neutrophil elastase-positive cells among total cells; (D) Representative images at 40× magnification of neutrophils stained for their nucleus (blue), cytoplasm (red), and neutrophil elastase (green); (E) Neutrophil elastase levels in the treated neutrophils supernatant media determined through ELISA. Data shown are mean ± SEM; n = 3 per group in each experiment per donor; experiments were repeated twice with two different donors; * p < 0.05, ** p < 0.01, *** p < 0.001 (one-way ANOVA with Tukey’s post hoc test).
Following a 4 h treatment with Calu-3-conditioned media, the researchers measured the release of MPO and neutrophil elastase in the supernatant using ELISA. A PMA treatment control was added to serve as a positive control for potent neutrophil activation. Conditioned media from infected Calu-3 cells significantly reduced MPO release compared to the uninfected control media (Figure 1B). However, a dose–response relationship in MPO release with increasing infection was not observed. A MOI higher than 0.1 may be required to detect a significant drop in MPO release. As expected, a low dose of PMA induced high levels of MPO release (Figure 1B).
In contrast, levels of secreted neutrophil elastase slightly increased at the higher MOI compared to the uninfected control (Figure 1E). To determine if this increase correlated to lower intracellular elastase levels, the treated neutrophils were fixed, permeabilized, and stained with an antineutrophil elastase antibody. Lower percentage of elastase-positive cells were indeed seen after treatment with PMA and conditioned media from infected Calu-3 compared to the uninfected control (Figure 1C,D). However, the proportion between intracellular and released elastase seen with PMA treatment was not detected with infected conditioned media treatment (Figure 1C,E). Together, these data suggest that SARS-CoV-2-infected epithelial cells induce aberrant neutrophil degranulation, specifically in the azurophil granules, in vitro.
2.2. Direct Infection of Neutrophils with SARS-CoV-2 Promotes CD16 Shedding but Does Not Increase Release of Azurophil Granules
Next, the researchers sought to observe the direct effect of live SARS-CoV-2 on neutrophil degranulation. Circulating neutrophils isolated from healthy donors were infected with live virus for 2 h. The cells were then collected for qPCR to determine the viral load. A dose-dependent response was observed with a 10-fold increase in MOI resulting in a ~10-fold increase in viral genome detected (Figure 2A).
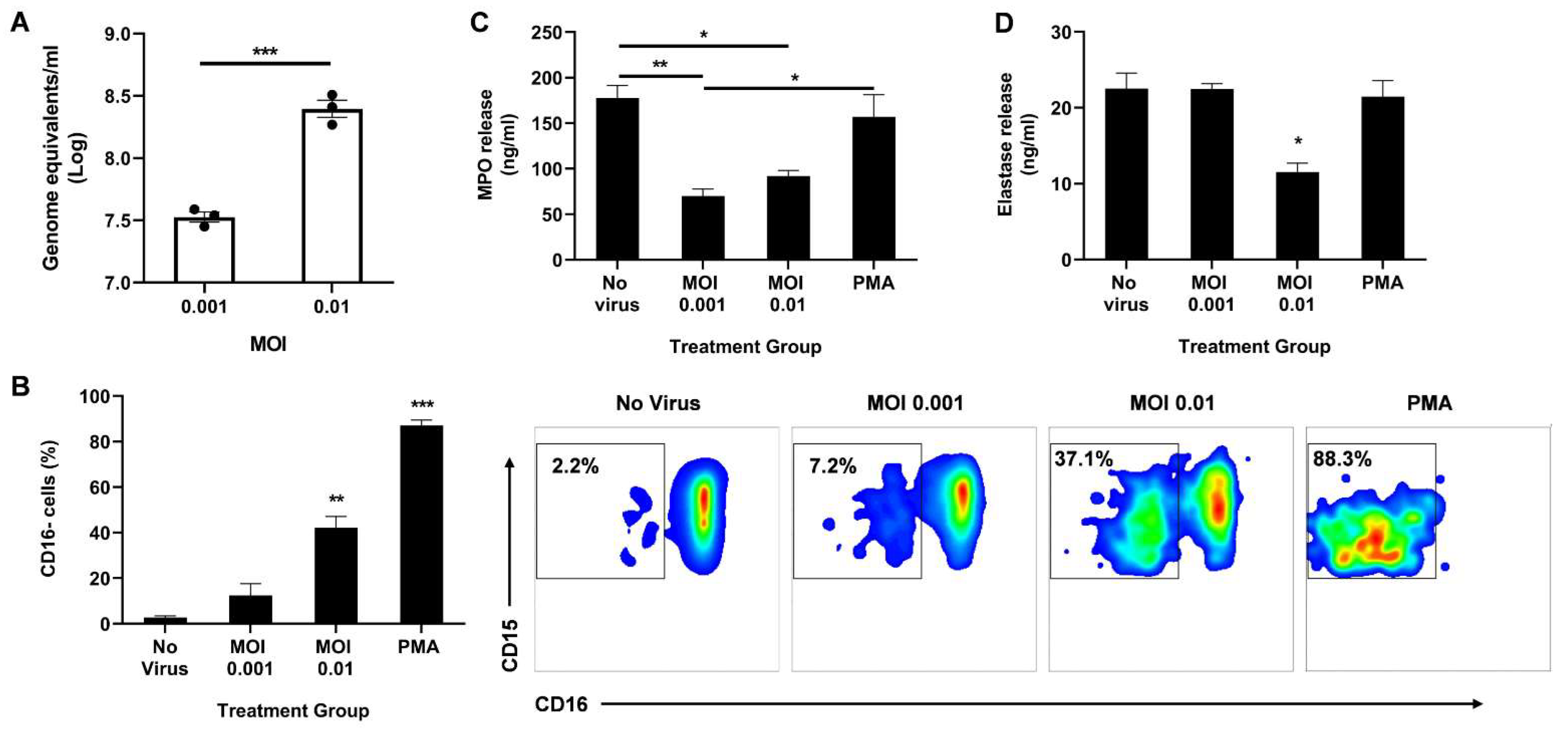
Figure 2. Direct infection of neutrophils with SARS-CoV-2 promotes CD16 shedding without increasing MPO or elastase release. (A) Viral titer in the neutrophils 2 h post infection; (B) Percentage of CD16 cells among CD11b+ CD66b+ CD14− CD15+ population (left). Representative flow cytometry density plots of CD11b+ CD66b+ CD14− CD15+ CD16− cells in different treatment groups (right); Levels of MPO (C) and neutrophil elastase (D) in the supernatant media determined through ELISA. Data shown are mean ± SEM; n = 3 per group in each experiment per donor; experiments were repeated three times with three different donors; * p < 0.05, ** p < 0.01, *** p < 0.001 (Student’s t-test or one-way ANOVA with Tukey’s post hoc test).
the researchers then analyzed the activation state of the neutrophils by measuring CD16 shedding. CD16, also known as FcγRIII, is the most abundant receptor on the surface of neutrophils [24]. During neutrophil activation, CD16 is shed, granting access to other activating receptors on the cell surface [24]. Using flow cytometry, the researchers determined the percentage of CD16-negative cells among the CD11b+ CD66b+ CD14− CD15+ population. Neutrophils infected at MOI 0.01 had a significant increase in CD16 shedding compared to uninfected controls (Figure 2B). As expected, stimulation with PMA resulted in the highest percentage of CD16 cells, indicating the highest activation state.
Since CD16 shedding is associated with enhanced degranulation [24][25], the researchers quantified secreted MPO and elastase in the supernatant 2 h post infection. Interestingly, infection significantly lowered the levels of released MPO (Figure 2C) and neutrophil elastase (Figure 2D). While both MOI 0.001 and 0.01 resulted in reduced MPO release, only the higher MOI decreased elastase release. It is important to note that there is some MPO and elastase release even with very low CD16 shedding as seen in the ‘No virus’ control, suggesting that the neutrophils are not at resting state in ex vivo conditions, as anticipated. Moreover, as expected from the high CD16 shedding, a low-dose PMA treatment induced effective MPO and elastase release, albeit to the same levels as the uninfected control (Figure 2C,D).
2.3. SARS-CoV-2-Infected Neutrophils Reduce B Cell, CD8+ T Cell, and CD4+ T Cell Counts In Vitro
As part of contributing to the inflammatory milieu, the release of granules by neutrophils has a significant effect on lymphocyte numbers, and in turn, on their function [26]. Therefore, the researchers ascertained the effect of infected neutrophils on B- and T-cell populations in vitro. Peripheral blood lymphocytes (PBLs) were isolated from healthy donors and either treated directly with PMA or LPS, or with UV-irradiated conditioned media from neutrophils treated with live virus, PMA or LPS two hours post-treatment. The PBLs were also co-cultured with treated neutrophils. Following an 18 h treatment, the PBLs were analyzed using flow cytometry. The number of CD3− CD19+ B cells (Figure 3A), CD19− CD3+ CD8+ T cells (Figure 3C), and CD19− CD3+ CD4+ T cells (Figure 3E) significantly decreased with infected conditioned media, while media from LPS-treated neutrophils had comparable numbers to the uninfected control. A similar trend was observed when treated neutrophils were co-cultured with PBLs (Figure 3B,D,F,G).
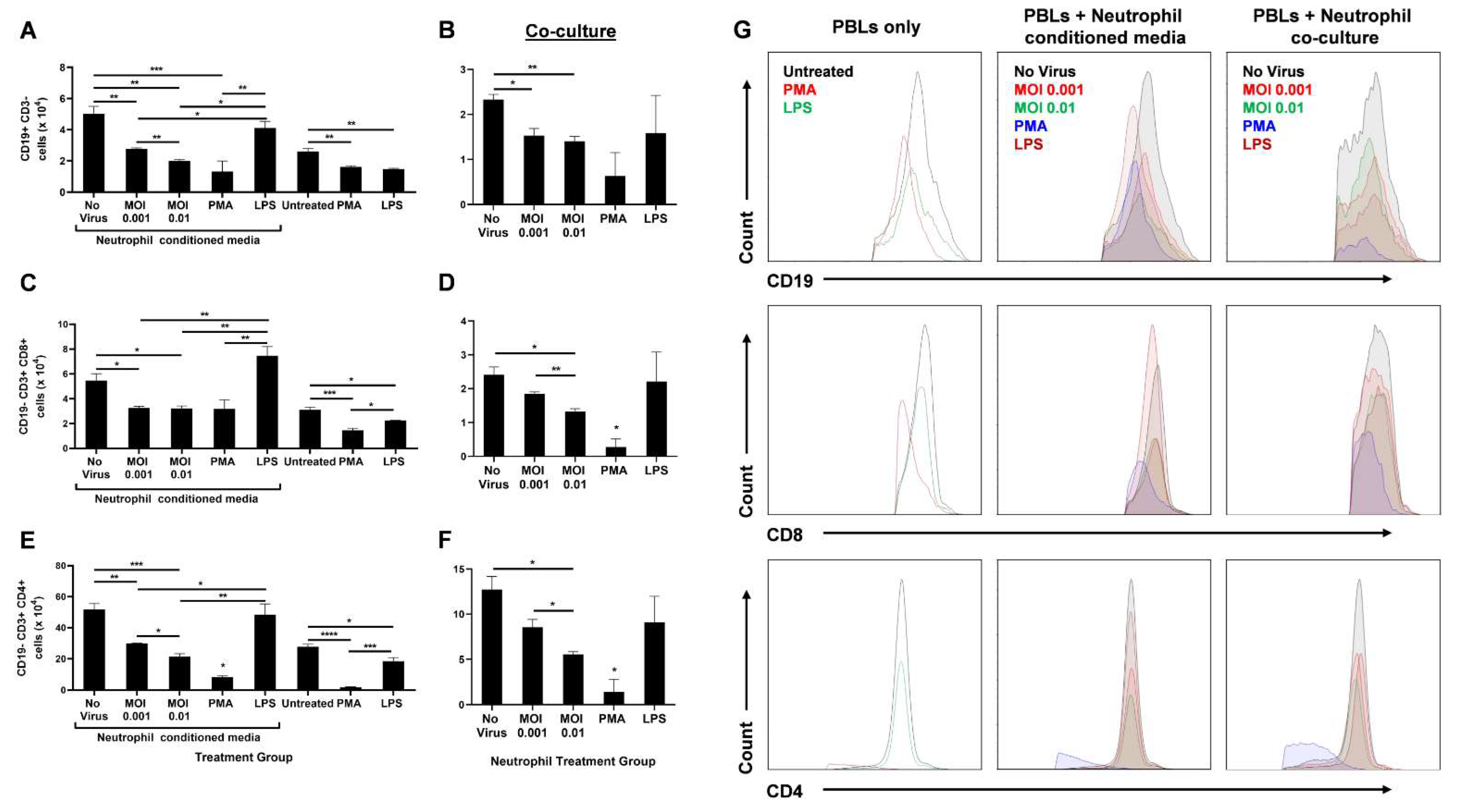
Figure 3. SARS-CoV-2-infected neutrophils reduce lymphocyte counts in vitro. After 18 h treatment of PBLs with conditioned media from infected neutrophils or direct stimulation with PMA or LPS, the number of CD19+ CD3- B cells (A), CD19− CD3+ CD8+ T cells (C), and CD19− CD3+ CD4+ T cells are shown (E). Following 18 h coculture of PBLs with infected neutrophils, the number of CD19+ CD3− B cells (B), CD19− CD3+ CD8+ T cells (D), and CD19− CD3+ CD4+ T cells are shown (F); (G) Representative flow cytometry histograms of B and T cells in different treatment groups of PBLs alone, PBLs treated with neutrophil-conditioned media, and PBL/neutrophil co-culture. Data shown are mean ± SEM; n = 3 per group in each experiment per donor; experiments were repeated three times with three different donors; * p < 0.05, ** p < 0.01, *** p < 0.001 (one-way ANOVA with Tukey’s post hoc test).
Since no other stimulants were added to induce active proliferation of these lymphocytes, the researchers did not expect any differences between ‘No virus’ and LPS-conditioned media or co-culture groups for B- and T-cell populations. Furthermore, PBLs either directly treated with PMA or treated with PMA-stimulated neutrophils were drastically diminished in number. This may be due to a possible toxic effect of PMA on lymphocytes.
The lack of other stimulants also resulted in lower B- and T-cell counts in the untreated (PBLs only) group than the uninfected neutrophil-conditioned media treatment. Similarly, direct LPS treatment of PBLs resulted in decreased lymphocyte numbers compared to treatment with media from LPS-stimulated neutrophils. This may be due to the higher expression of Toll-like receptor 4 (TLR4) on neutrophils than unstimulated PBLs [27][28]. Higher TLR4 levels leads to higher LPS-induced activation of neutrophils that can further release lymphocyte-activating factors. In addition, lymphocytes upregulate TLR4 expression upon CD3/CD28 activation [28]. Since there were no such activators present, direct LPS treatment of PBLs did not augment the lymphocyte counts (Figure 3).
Although infection of neutrophils had a significant impact on lymphocyte numbers, an increase in MOI only had a mild effect during neutrophil-conditioned media treatment. However, when the PBLs were co-cultured with the infected neutrophils, a substantial decrease in CD8+ (Figure 3D) and CD4+ (Figure 3F) T cells was seen with increasing MOI, even though the overall counts were lower compared to conditioned media treatment. This may be due to additional effects neutrophils can have on lymphocytes through direct contact. Together, these data highlight the significant role neutrophils have on B- and T-cell counts during SARS-CoV-2 infection.
References
- Morens, D.M.; Breman, J.G.; Calisher, C.H.; Doherty, P.C.; Hahn, B.H.; Keusch, G.T.; Kramer, L.D.; LeDuc, J.W.; Monath, T.P.; Taubenberger, J.K. The Origin of COVID-19 and Why It Matters. Am. J. Trop. Med. Hyg. 2020, 103, 955–959.
- Zhong, N.S.; Zheng, B.J.; Li, Y.M.; Poon, L.L.M.; Xie, Z.H.; Chan, K.H.; Li, P.H.; Tan, S.Y.; Chang, Q.; Xie, J.P.; et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February 2003. Lancet 2003, 362, 1353–1358.
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W.; et al. A Novel Coronavirus Associated with Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003, 348, 1953–1966.
- Reina, J.; Reina, N. The Middle East respiratory syndrome coronavirus. Med. Clin. 2015, 145, 529–531.
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820.
- Ganesh, B.; Rajakumar, T.; Malathi, M.; Manikandan, N.; Nagaraj, J.; Santhakumar, A.; Elangovan, A.; Malik, Y.S. Epidemiology and pathobiology of SARS-CoV-2 (COVID-19) in comparison with SARS, MERS: An updated overview of current knowledge and future perspectives. Clin. Epidemiol. Glob. Health 2021, 10, 100694.
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720.
- He, D.; Gao, D.; Li, Y.; Zhuang, Z.; Cao, P.; Lou, Y.; Yang, L. An Updated Comparison of COVID-19 and Influenza. SSRN Electron. J. 2020, 90, 107233.
- Yi, Y.; Lagniton, P.N.P.; Ye, S.; Li, E.; Xu, R.H. COVID-19: What has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020, 16, 1753–1766.
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069.
- Wu, J.T.; Leung, K.; Bushman, M.; Kishore, N.; Niehus, R.; de Salazar, P.M.; Cowling, B.J.; Lipsitch, M.; Leung, G.M. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat. Med. 2020, 26, 506–510.
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020, 5, 128.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768.
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017, 17, 248–261.
- Tomar, B.; Anders, H.J.; Desai, J.; Mulay, S.R. Neutrophils and Neutrophil Extracellular Traps Drive Necroinflammation in COVID-19. Cells 2020, 9, 1383.
- Galani, I.E.; Andreakos, E. Neutrophils in viral infections: Current concepts and caveats. J. Leukoc. Biol. 2015, 98, 557–564.
- Cavalcante-Silva, L.H.A.; Carvalho, D.C.M.; de Almeida Lima, É.; Galvão, J.G.F.M.; de França da Silva, J.S.; de Sales-Neto, J.M.; Rodrigues-Mascarenhas, S. Neutrophils and COVID-19: The road so far. Int. Immunopharmacol. 2021, 90, 107233.
- Naumenko, V.; Turk, M.; Jenne, C.N.; Kim, S.J. Neutrophils in viral infection. Cell Tissue Res. 2018, 371, 505–516.
- Johansson, C.; Kirsebom, F.C.M. Neutrophils in respiratory viral infections. Mucosal Immunol. 2021, 14, 815–827.
- Meizlish, M.L.; Pine, A.B.; Bishai, J.D.; Goshua, G.; Nadelmann, E.R.; Simonov, M.; Chang, C.H.; Zhang, H.; Shallow, M.; Bahel, P.; et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv. 2021, 5, 1164–1177.
- Dennison, D.; Al Khabori, M.; Al Mamari, S.; Aurelio, A.; Al Hinai, H.; Al Maamari, K.; Alshekaili, J.; Al Khadouri, G. Circulating activated neutrophils in COVID-19: An independent predictor for mechanical ventilation and death. Int. J. Infect. Dis. 2021, 106, 155–159.
- Wang, Y.; Jönsson, F. Expression, Role, and Regulation of Neutrophil Fcγ Receptors. Front. Immunol. 2019, 10, 1958.
- Zhang, Y.; Boesen, C.C.; Radaev, S.; Brooks, A.G.; Fridman, W.H.; Sautes-Fridman, C.; Sun, P.D. Crystal Structure of the Extracellular Domain of a Human FcγRIII. Immunity 2000, 13, 387–395.
- Leliefeld, P.H.C.; Koenderman, L.; Pillay, J. How neutrophils shape adaptive immune responses. Front. Immunol. 2015, 6, 471.
- McCracken, J.M.; Allen, L.A.H. Regulation of Human Neutrophil Apoptosis and Lifespan in Health and Disease. J. Cell Death 2014, 7, 15.
- Lawlor, N.; Nehar-Belaid, D.; Grassmann, J.D.S.; Stoeckius, M.; Smibert, P.; Stitzel, M.L.; Pascual, V.; Banchereau, J.; Williams, A.; Ucar, D. Single Cell Analysis of Blood Mononuclear Cells Stimulated Through Either LPS or Anti-CD3 and Anti-CD28. Front. Immunol. 2021, 12, 636720.
More
Information
Subjects:
Immunology; Virology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
958
Entry Collection:
COVID-19
Revisions:
3 times
(View History)
Update Date:
14 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




