Silver nanoparticles (AgNPs) are widely used for their bacteriostatic or antimicrobial properties
[5]. The antibacterial effects of AgNPs have been demonstrated on many bacteria, including bacteria resistant to common antibiotics
[10]. They prevent the adhesion and proliferation of bacteria to the surface of materials and suppress microorganisms already adhered
[6]. This property is used in the medical field where these materials protect equipment prone to biofilm formation, such as catheters and implants
[11]. AgNPs are also used in the treatment of wounds, where they accelerate the conversion of fibroblasts into myofibroblasts (collagen-producing connective tissue cells)
[12].
Catalysis is another important application area where metal nanoparticles play a crucial role
[13][14]. Especially in the chemical industry, catalysis has become a key part of the production of chemicals by reducing the activation energy of reactions and accelerating their course
[15]. The development of nanomaterials has contributed to heterogeneous catalysts with a large surface area
[16]. Silver nanoparticles provide excellent results in dye reduction and removal
[17]. Iqbal et al.
[18] developed a hydrogel containing AgNPs capable of catalytically decomposing various types of azo dyes into less toxic products. Gels with immobilized AgNPs also serve to reduce amino groups. Begum et al.
[19] created gels with AgNPs that remained functional for several months and contributed to the rapid reduction of 4-nitrophenol to 4-aminophenol in an aqueous medium with NaBH
4.
2. Laser-Promoted Immobilization of Ag Nanoparticles
The shape and the morphology of prepared nanoparticles were characterized by TEM. Figure 1 shows that synthetized silver nanoparticles were mostly spherical with a minimal incidence of rods. The average size of spherical AgNPs was around 20 nm.
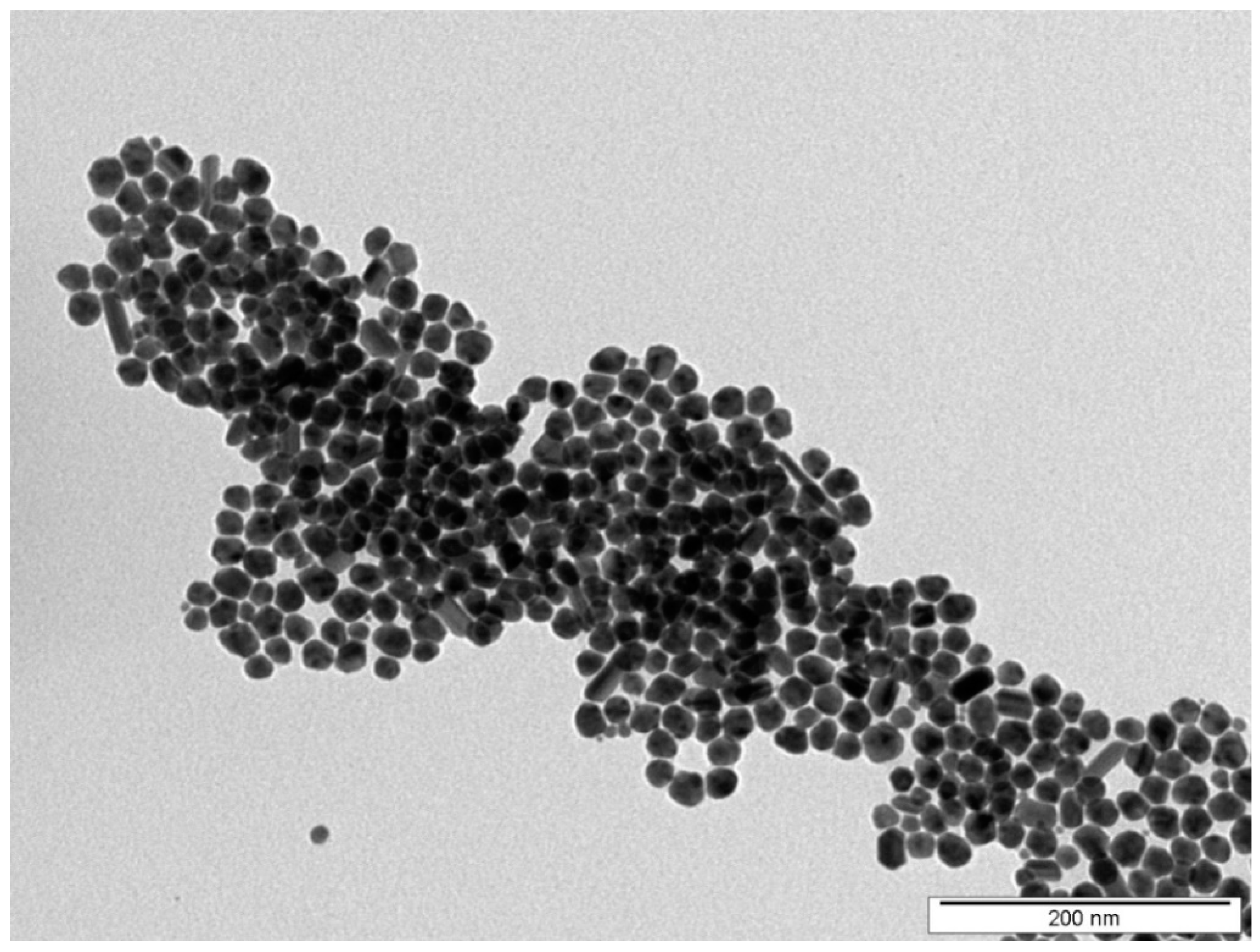 Figure 1.
Figure 1. TEM image of synthesized AgNPs used in immobilization process.
The surface morphology of pristine PET, laser pre-treated PET with LIPSS structure and PET with immobilized silver NPs was measured by AFM and is depicted in
Figure 2. Related surface parameters derived from AFM measurements such as surface roughness
Ra and surface area difference SAD (the ratio of the difference between the measured area and the scanned area to the scanned area) are summarized in
Table 1. It is evident from
Figure 2A that pristine PET exhibited pretty flat surface morphology, which turned into coherent ripple patterns once the PET was irradiated with laser light (
Figure 2B). These structures are referred to as LIPSS
[8].
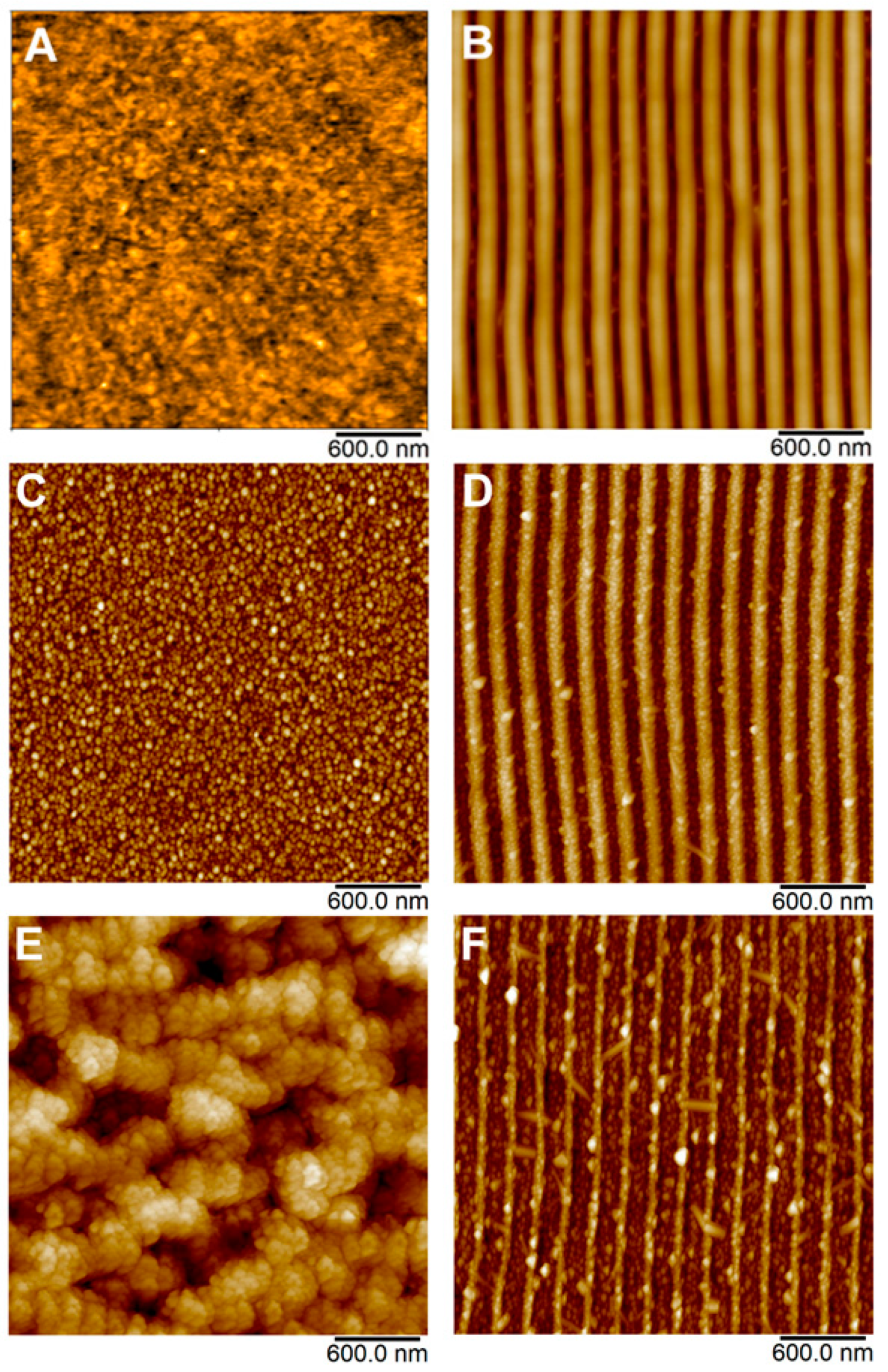 Figure 2.
Figure 2. AFM images of pristine PET (
A); laser pre-treated PET with LIPSS structure (
B); and PET with immobilized AgNPs on pristine PET (
C,
E) and PET with LIPSS structure (
D,
F).
Table 1. Image characteristics (average surface roughness Ra and surface area difference SAD) derived from AFM analysis. The designation of the samples corresponds to the Figure 2.
| Sample |
Laser Fluence
(mJ cm−2) |
Ra (nm) |
SAD (%) |
| PET (A) |
- |
0.78 |
4.93 |
| LIPSS/PET (B) |
- |
10.5 |
27.2 |
| AgNPs/PET (C) |
10 |
4.59 |
16.1 |
| AgNPs/PET (D) |
24 |
27.5 |
26.2 |
| AgNPs/LIPSS/PET (E) |
10 |
10.3 |
26.7 |
| AgNPs/LIPSS/PET (F) |
24 |
20.9 |
32.9 |
It is evident from
Table 1 that once the LIPSS were formed, both surface roughness and SAD increased significantly compared to new PET, which is typical for rugged surfaces with complicated morphology
[20].
Figure 2C–F shows the development of surface morphology of PET after immobilization of silver nanoparticles from AgNPs colloids. In addition to the specific morphology changes of the underlying polymer, which will be discussed in detail below, these AFM images clearly show that the polymer surface was evenly decorated by AgNPs, regardless of particular fluence applied or polymer pre-treatment (pristine or LIPSS). Obviously, lower immobilization fluence did not cause any visible changes in the morphology of underlying PET in the case of both pristine and laser-modified samples (
Figure 2C,D), which was quantified by a similar
Ra and SAD, especially in the case of LIPSS/PET and AgNPs/LIPSS/PET samples (see
Table 1). However, when higher laser fluence was applied, the surface morphology of pristine PET was dramatically changed (worm-like structure appeared, accompanied by a significant increase of
Ra and SAD, see
Table 1) due to considerable material ablation. In the case of AgNPs immobilization on PET with LIPSS, higher fluence degraded the structures in terms of their narrowing; however, the LIPSS were preserved.
A significant difference between the effect of higher fluence applied on pristine PET a PET with LIPSS in the view of the change of surface morphology may be attributed to the reorganization of macromolecular domains and the recrystallization of the polymer typical in the process of LIPSS formation
[21][20]. As a result, considerably higher energy is needed to perturb the organized structure of the LIPSS than in the case of pristine PET. Therefore, the observed changes in morphology during the immobilization process at elevated laser fluence (24 mJ cm
−2) are much more pronounced in the case of pristine PET when compared to PET with LIPSS structure.
The surface morphology of AgNPs decorated polymers was observed also by FEGSEM microscopy (Figure 3) to better visualize the AgNPs through the different material contrast (metal/polymer). Figure 3A,B show smooth and coherently patterned PET (LIPSS) with immobilized Ag nanoparticles under the fluencies of 10 mJ cm−2. Similarly to AFM analysis, it follows from SEM micrographs that under the lower immobilization fluence, the morphology of the underlying polymer remained fully preserved and AgNPs evenly decorated the polymer. When the immobilization fluence increased to 24 mJ cm−2, the most pronounced change in the surface morphology occurred at originally planar polymer, while PET with LIPSS structure exhibited only minor disturbances of the periodic structure (see Figure 3C,D). The cause of this different behavior was discussed in the previous paragraph and most probably was related to the presence of the LIPSS themselves. It is also evident that regardless of the nature of the polymer surface after nanoparticle immobilization (smooth, rough, or with LIPSS structure), the AgNPs were always located at the same surface area of the polymer.
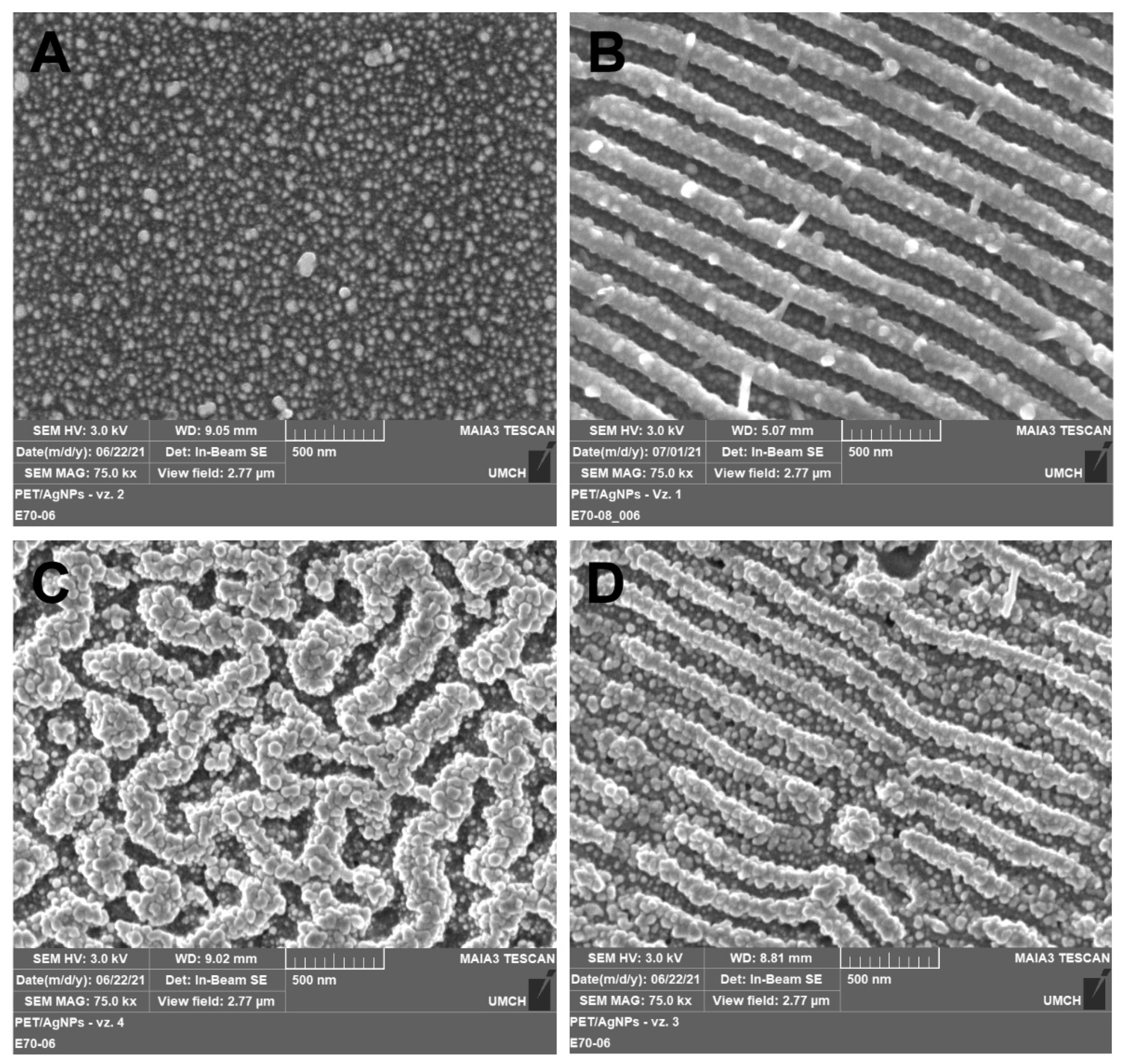 Figure 3.
Figure 3. FEGSEM micrographs showing the surface morphology of PET immobilized with AgNPs at laser fluencies of 10 (
A,
B) and 24 mJ cm
−2 (
C,
D) on pristine PET (
A,
C) and PET with LIPSS structure (
B,
D). Micrographs were recorded with an in-beam SE detector at accelerating voltage 3 kV.
To track the changes in the chemical composition of the polymer surface, researchers performed the XPS analysis. Atomic concentrations of elements are summarized in
Table 2. Atomic concentrations of C and O in pristine PET correspond well with the theoretical content based on polymer stoichiometry
[22], 71.4 and 28.6 for C and O, respectively. Once the LIPSS structures were formed, the elemental composition was shifted towards oxygen at the expense of carbon, the effect of which was due to rearrangement of polymer macromolecules, their reorientation, and partial oxidation during the process of ripple patterns formation
[20]. After the AgNPs immobilization, Ag was detected on all samples in concentrations exceeding 10 at.%, regardless on both specific laser fluence applied and input polymer treatment; however, one can see slightly increased Ag content on PET with LIPSS structure. This may probably be caused by the increased affinity of silver nanoparticles (which usually carry positive charge
[23]) to the LIPSS surface, which exhibits a higher concentration of oxygen than pristine PET and thus carries a partial negative charge. It is evident that the higher the immobilization fluence, the higher the Ag content, regardless of whether or not the LIPSS structure is present.
Table 2. Concentrations of silver Ag3d, carbon C1s, and oxygen O1s (in at.%) in pristine, LIPSS modified, and AgNPs immobilized PET, derived from XPS analysis. The designation of the samples corresponds to Figure 2.
| Sample |
Laser Fluence
(mJ cm−2) |
Ag |
C |
O |
| PET (A) |
- |
- |
71.0 |
29.0 |
| LIPSS/PET (B) |
- |
- |
67.2 |
32.8 |
| AgNPs/PET (C) |
10 |
10.7 |
67.7 |
21.6 |
| AgNPs/PET (D) |
24 |
11.3 |
66.5 |
22.2 |
| AgNPs/LIPSS/PET (E) |
10 |
13.1 |
65.8 |
21.1 |
| AgNPs/LIPSS/PET (F) |
24 |
15.1 |
63.5 |
21.4 |
In
Figure 4, the absorption spectra of the samples after irradiation in a colloidal solution of AgNPs are shown. Researchers present the data in the range of wavelengths from 330 to 580 nm because the maximum of the LSPR band of silver nanoparticles is located in this area (typically 400–410 nm
[24]). The coalescence of NPs did not cause the extension of the absorbance bands during immobilization into the PET surface (see SEM images,
Figure 3), but rather by the change of dielectric environment, the essence of which is the shortening of polymer chains, the formation of volatile substances and their crosslinking, the formation of carbon clusters, and in some cases, the formation of multiple bonds
[25].
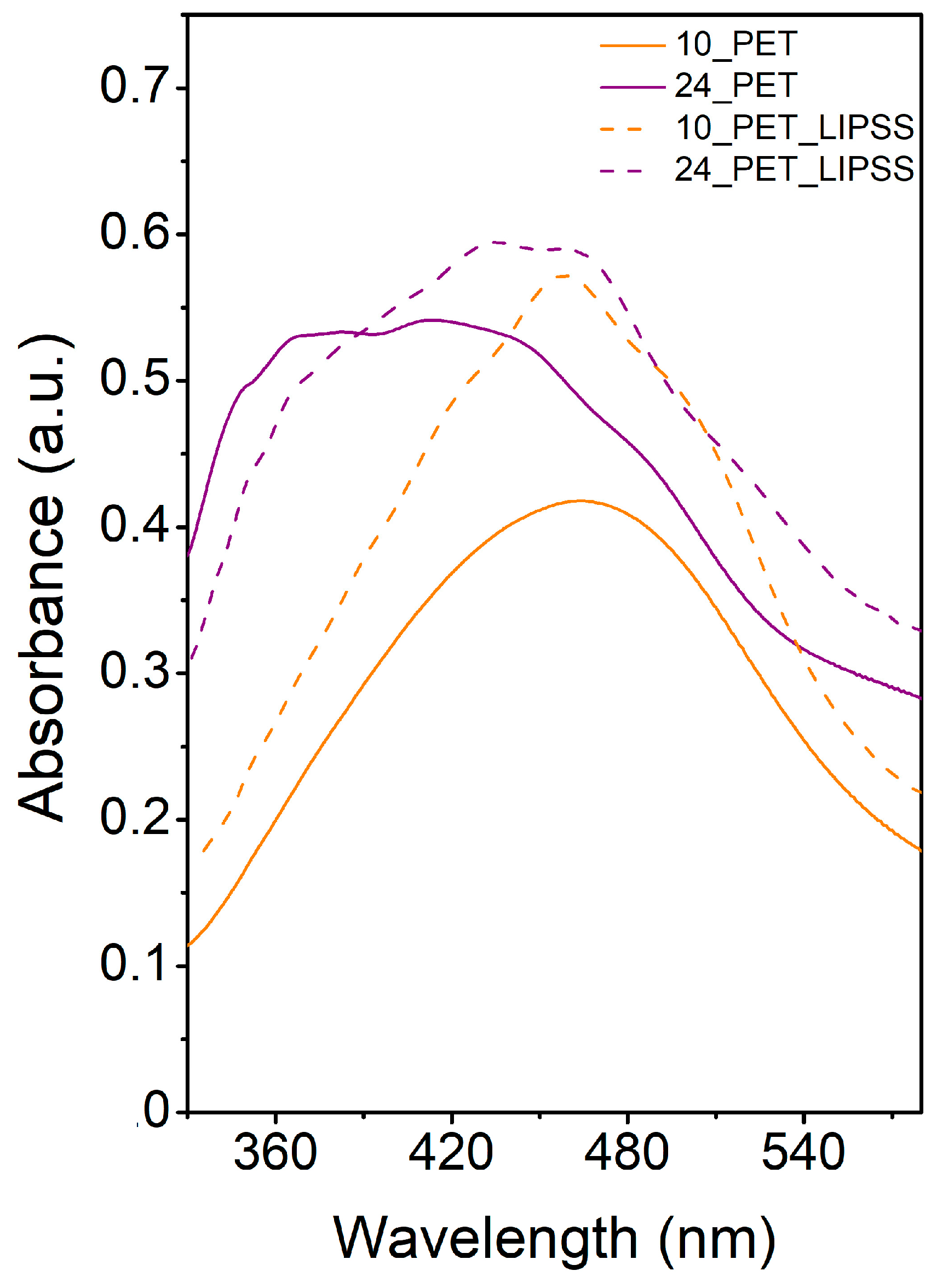 Figure 4.
Figure 4. UV-Vis absorption spectra of AgNPs immobilized with the fluence of 10 mJ cm
−2 on pristine PET (10_PET) and PET with LIPSS structure (10_PET_LIPSS) and with the fluence of 24 mJ cm
−2 on pristine PET (24_PET) and PET with LIPSS structure (24_PET_LIPSS).
However, the change of dielectric conditions is not the only phenomenon observed in the spectra. The multimodality of the absorption bands in the samples with LIPSS structure was caused by the coupling effect
[26]. The increase in absorbance is due to the excitation of the waveguide modes of the structure. These modes are related to the electromagnetic distribution inside the material; they oscillate inside the waveguide and partially penetrate the outer area. The rate of coupling size affects the refractive index, wavelength, and shorter structure size (width)
[27]. The formation of LIPSS changed the refractive index on the PET surface
[28]. The change in the refractive index likely manifested itself also in the sample with a worm-like structure (see curve 24_PET). Coupling was observed in the spectra of these samples as small peaks at 350, 370, 430, 460 nm. LSPR, therefore, influenced the measured UV-Vis spectra in silver nanoparticles (410 nm), the change of dielectric environment due to polymer modification, and electromagnetic coupling. Generally, the higher absorbance of the structures formed with the fluence of 24 mJ cm
−2 (compared to their 10 mJ cm
−2 counterparts) is in accordance with the increase of multiple double bonds as a consequence of cross-linking of macromolecules after the interaction of intense light with the polymer.

 Figure 1. TEM image of synthesized AgNPs used in immobilization process.
Figure 1. TEM image of synthesized AgNPs used in immobilization process. Figure 2. AFM images of pristine PET (A); laser pre-treated PET with LIPSS structure (B); and PET with immobilized AgNPs on pristine PET (C,E) and PET with LIPSS structure (D,F).
Figure 2. AFM images of pristine PET (A); laser pre-treated PET with LIPSS structure (B); and PET with immobilized AgNPs on pristine PET (C,E) and PET with LIPSS structure (D,F). Figure 3. FEGSEM micrographs showing the surface morphology of PET immobilized with AgNPs at laser fluencies of 10 (A,B) and 24 mJ cm−2 (C,D) on pristine PET (A,C) and PET with LIPSS structure (B,D). Micrographs were recorded with an in-beam SE detector at accelerating voltage 3 kV.
Figure 3. FEGSEM micrographs showing the surface morphology of PET immobilized with AgNPs at laser fluencies of 10 (A,B) and 24 mJ cm−2 (C,D) on pristine PET (A,C) and PET with LIPSS structure (B,D). Micrographs were recorded with an in-beam SE detector at accelerating voltage 3 kV. Figure 4. UV-Vis absorption spectra of AgNPs immobilized with the fluence of 10 mJ cm−2 on pristine PET (10_PET) and PET with LIPSS structure (10_PET_LIPSS) and with the fluence of 24 mJ cm−2 on pristine PET (24_PET) and PET with LIPSS structure (24_PET_LIPSS).
Figure 4. UV-Vis absorption spectra of AgNPs immobilized with the fluence of 10 mJ cm−2 on pristine PET (10_PET) and PET with LIPSS structure (10_PET_LIPSS) and with the fluence of 24 mJ cm−2 on pristine PET (24_PET) and PET with LIPSS structure (24_PET_LIPSS).